Abstract
Myeloperoxidase (MPO) is one of the major target antigens of antineutrophil cytoplasmic antibodies (ANCA) in primary systemic vasculitis. It is known that propylthiouracil (PTU) could induce MPO–ANCA-positive vasculitis. The production of anti-MPO antibodies in patients with PTU-induced vasculitis may be different from that in patients with primary microscopic polyangiitis (MPA). One possible reason for this may be differences in epitope recognition. The aim of this study is to compare the epitopes of antibodies to MPO in sera from patients with PTU-induced vasculitis (n = 10) and MPA (n = 10). The sera were collected and used to inhibit monoclonal antibodies against human MPO (3D8 and 6B9) and affinity purified, horseradish peroxidase conjugated human anti-MPO antibodies (Pab1-HRP, Pab2-HRP) in a competitive inhibition enzyme-linked immunosorbent assay (ELISA) system using soluble human MPO as solid phase ligand. The Pab1-HRP and Pab2-HRP were affinity purified from plasma exchanges of a patient with PTU-induced vasculitis and a patient with MPA, respectively. The inhibition rates were evaluated and compared between the PTU and primary MPA groups. In the PTU group all 10 sera could inhibit 3D8: the average inhibition rate was 44·7% ± 5·0%; 9/10 sera could inhibit 6B9: the average inhibition rate was 35·6% ± 6·0%. However, in the MPA group all 10 sera could inhibit 3D8 and 6B9; the average inhibition rates were 68·4% ± 16·1% (P < 0·01) and 62·2% ± 17·2% (P < 0·01), respectively. Sera in both the PTU and MPA groups could inhibit Pab1-HRP and the inhibition rates were 81·4% ± 9·4%versus 86·6% ± 17·2% (P > 0·05). However, the average inhibition rate for Pab2-HRP in the MPA group was significantly higher than that in the PTU group (76·3% ± 7·8%versus 58·9% ± 15·5%, P < 0·01). We conclude that anti-MPO antibodies from patients with PTU-induced vasculitis and from patients with primary MPA could recognize more than one epitope on the native MPO molecule. Although the epitopes overlapped between the two groups, the epitopes of anti-MPO antibodies from patients with PTU-induced vasculitis might be more restricted.
Keywords: antibody, epitope mapping, myeloperoxidase, microscopic polyangiitis, propylthiouracil
INTRODUCTION
Anti-neutrophil cytoplasmic antibodies (ANCA) are important serological markers of certain small vessel vasculitides. Anti-proteinase 3 (PR3) antibodies produce cytoplasmic fluorescence and are found in patients with Wegener's granulomatosis (WG); antimyeloperoxidase (MPO) antibodies produce perinuclear fluorescence and are found in microscopic polyangiitis (MPA). Propylthiouracil (PTU) is a common antithyroid drug, which has been known to induce ANCA-positive vasculitis [1–3]. In most of the cases of PTU-induced ANCA-associated vasculitis, a higher prevalence of positive MPO–ANCA was reported. After withdrawal of PTU and immunosuppressant treatment, the clinical symptoms could resolve rapidly and ANCA titres could decline. The patients with PTU-induced vasculitis had a better prognosis than those with primary vasculitis [4]. It was suggested that the mechanism involved in the synthesis of PTU-induced anti-MPO antibodies might be different from that in patients with MPA, and we speculated that the epitope recognition of anti-MPO antibodies might be different. In the current study, we mapped the epitopes of anti-MPO antibodies in sera from both patients with PTU-induced vasculitis and MPA and compared the difference of the epitope recognition.
MATERIALS AND METHODS
Materials
Ten patients with PTU-induced vasculitis and 10 patients with primary MPA diagnosed in Peking University First Hospital, during December 1999 to December 2002 were enrolled in this study; all patients were selected according to the 1994 Chapel Hill Consensus Conference definition for MPA and with a positive p-ANCA and a positive in MPO–ANCA. Patients’ sera at presentation were stored at −20·C until use.
The mouse monoclonal anti-MPO antibodies (3D8 and 6B9) were kindly provided by Dr A. K. Short, who used to be at the School of Clinical Medicine, University of Cambridge, UK.
Detection of ANCA
All sera were screened for ANCA by indirect immunofluorescence technique using precooled ethanol-fixed normal peripheral neutrophils as substrate according to the manufacturer's instructions (Euroimmun, Germany) and MPO–ANCA were measured by enzyme-linked immunosorbent assay (ELISA). In brief, highly purified human native MPO [5] were coated to Dynatech microtitre plates (Dynatech Laboratory Inc., Chantilly, VA, USA) 2·0 µg/ml in coating buffer (0·05 mol/l bicarbonate buffer, pH 9·6). The volume in each well was 100 µl in this step and subsequent steps and every sample was added in duplication, all incubations were carried out at 37°C for 1 h, and the plates were washed three times with phosphate buffered solution (PBS) containing 0·1% Tween-20 (PBST) between stages. Sera from patients were diluted at 1 : 50 with PBST and were incubated for 1 h at 37°C and the binding was revealed with a horseradish peroxidase-conjugated goat antihuman IgG (Jackson Immunoresearch, USA) diluted at 1 : 10000, followed by addition of diaminobenzidine. The absorbance was recorded at 490 nm. Every plate contained a positive control, a negative control and blank controls.
Preparation of affinity-purified anti-MPO antibodies
Two MPO affinity columns (5 ml each) were prepared by coupling 5 mg highly purified native MPO from human neutrophils to 2 g cyanogens bromide-activated Sepharose 4B beads (Amersham, Sweden), according to the manufacturer's instructions [6].
One hundred ml plasma exchange from a patient with PTU-induced vasculitis and a patient with primary MPA were passed over a Protein G column (Amersham, Sweden) and 300 mg IgG was collected, respectively. The IgG was then applied to the MPO affinity column and the bound anti-MPO antibodies were eluted with 0·1 m glycine/HCl buffer (pH 2·8). After neutralization, the affinity-purified antibodies were dialysed against PBS and were stored at 4°C.
Preparation of horseradish peroxidase conjugated affinity purified anti-MPO antibodies
Two mg of affinity purified anti-MPO antibodies were conjugated with horseradish peroxidase (HRP) (Sigma, UK) as described previously [7]. In brief, 1 ml of 3 mg/ml HRP solution was incubated with 0·2 ml 0·05 m sodium periodate solution for 20 min at room temperature, followed by adding 0·1 ml ethylene glycol to remove periodate. The solution was mixed with 2 mg affinity purified anti-MPO antibodies and 20 µl 0·2 m sodium carbonate buffer (pH 9·5), and then incubated for 2 h at room temperature, followed by adding 0·1 ml fresh sodium borohydride solution and incubating for 2 h at 4°C. At the end of incubation, 0·1 ml 0·2 m glycine–PBS was added and mixed for 2 h at room temperature. The HRP-conjugated anti-MPO antibodies were dialysed against PBS and stored at 4°C. Pab1-HRP was from the patient with PTU-induced vasculitis and Pab2-HRP was from the patient with MPA. The two patients were from the two groups, respectively. The conjugation rates of the anti-MPO antibodies were both more than 70%.
Competitive inhibition ELISA
The MPO ELISA was set up to allow the anti-MPO antibodies from patients’ sera in excess over MPO [8]. The optimal concentration of MPO was determined from dilution curves. Highly purifed MPO was diluted at 0·05 µg/ml. Sera from patients were diluted from 1 : 25 to 1 : 50 with PBST. Each diluted serum was added in duplicate; two wells were added to PBST acting only as controls without serum inhibition. For the competitive inhibition assay with mouse monoclonal anti-MPO antibodies, both 3D8 and 6B9 diluted 1 : 80000 with PBST were added. The binding was revealed using a HRP-conjugated goat antimouse IgG (Jackson Immunoresearch, USA) diluted 1 : 5000 with PBST, followed by addition of diaminobenzidine. The absorbance was recorded at 490 nm. The inhibition rate of a serum was calculated as (1-B/A) × 100%, where A represented the average absorbance of a monoclonal anti-MPO antibody without a serum blocking and B represented the average absorbance of a monoclonal anti-MPO antibody with a serum blocking. For the competitive inhibition assay with human anti-MPO probes, Pab1-HRP and Pab2-HRP were diluted 1 : 800 and 1 : 200 with PBST, respectively. After incubation and washing, the binding was revealed by adding diaminobenzidine and the absorbance was recorded at 490 nm. The inhibition rate was calculated as above. If the inhibition rate was over 30%, the probe was considered as inhibited by the patient sera.
Statistical analysis
The average inhibition rates between groups were examined by Student's t-test, significant difference was considered as P < 0·05.
RESULTS
Competitive inhibition ELISA
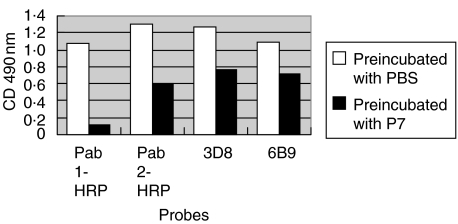
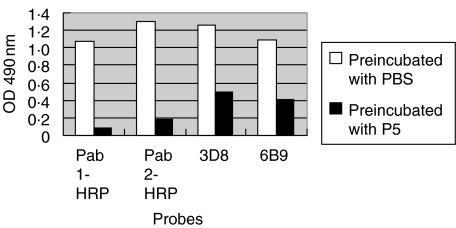
The anti-MPO levels of the two affinity purified antibodies and two monoclonal antibodies after preincubation with serum from patient 7 of the PTU group and PBS are shown in Fig. 1. The anti-MPO levels of the two affinity purified antibodies and two monoclonal antibodies after preincubation with serum from patient 5 of the MPA group and PBS are shown in Fig. 2.
Fig. 1.
The anti-MPO levels of the two affinity purified antibodies and two monoclonal antibodies after preincubation with serum from patient 7 of the PTU group and PBS (control).
Fig. 2.
The anti-MPO levels of the two affinity purified antibodies and two monoclonal antibodies after preincubation with serum from patient 5 of the MPA group and PBS (control).
In the PTU group, all 10 sera could inhibit the binding of the mouse monoclonal antibody 3D8 from 39·6% to 52·1%; the average inhibition rate was 44·7% ± 5·0%, 9/10 sera could inhibit 6B9 from 31·2% to 90·3% and the average inhibition rate was 35·6% ± 6·0% (Table 1). In the MPA group all 10 sera could inhibit 3D8 and 6B9; the average inhibition rates were 68·4% ± 16·1% and 62·2% ± 17·2%, respectively (Table 2). The inhibition rates of sera from patients in the MPA group were significantly higher than those of the PTU group (P < 0·01, respectively) (Table 3).
Table 1.
Competitive inhibition rates of sera from patients with PTU-induced vasculites
| No | Age | Sex | Pab1-HRP | Pab2-HRP | 3D8 | 6B9 |
|---|---|---|---|---|---|---|
| 1 | 20 | F | 90·7% | 84·3% | 42·5% | 39·3% |
| 2 | 51 | F | 85·2% | 68·4% | 48·9% | 35·7% |
| 3 | 37 | M | 82·7% | 67·2% | 52·1% | 41·8% |
| 4 | 51 | F | 84·3% | 62·8% | 45·5% | 31·2% |
| 5 | 61 | F | 64·9% | 30·2% | 39·6% | 22·1% |
| 6 | 20 | F | 87·4% | 63·5% | 40·7% | 43·9% |
| 7 | 20 | F | 90·0% | 53·7% | 39·6% | 34·8% |
| 8 | 44 | F | 75·6% | 63·7% | 40·2% | 36·9% |
| 9 | 27 | F | 87·4% | 37·9% | 45·7% | 34·7% |
| 10 | 42 | F | 66·0% | 57·5% | 52·1% | 36·0% |
Table 2.
Competitive inhibition rates of sera from patients with MPA
| No | Age | Sex | Pab1-HRP | Pab2-HRP | 3D8 | 6B9 |
|---|---|---|---|---|---|---|
| 1 | 64 | M | 92·0% | 68·4% | 54·0% | 45·6% |
| 2 | 63 | M | 94·2% | 76·3% | 59·2% | 49·4% |
| 3 | 43 | F | 71·3% | 83·0% | 83·7% | 81·7% |
| 4 | 17 | F | 87·4% | 77·9% | 46·4% | 48·0% |
| 5 | 70 | M | 91·7% | 86·5% | 61·2% | 62·1% |
| 6 | 50 | F | 96·9% | 76·3% | 51·4% | 37·0% |
| 7 | 56 | M | 90·4% | 88·4% | 90·3% | 84·8% |
| 8 | 45 | F | 98·1% | 69·7% | 70·3% | 66·1% |
| 9 | 55 | F | 70·9% | 66·4% | 86·5% | 83·9% |
| 10 | 44 | M | 73·0% | 69·8% | 81·0% | 63·3% |
Table 3.
Comparison of competitive inhibition rates between the PTU and MPA groups
| Group | Pab1-HRP | Pab2-HRP | 3D8 | 6B9 |
|---|---|---|---|---|
| PTU | 81·4% ± 9·4% | 58·9% ± 15·5% | 44·7% ± 5·0% | 35·6% ± 6·0% |
| MPA | 86·6% ± 17·2% | 76·3% ± 7·8%* | 68·4 ± 16·1%* | 62·2% ± 17·2%* |
Compared to the PTU group, P < 0·01.
Sera in both groups could inhibit Pab1-HRP and the inhibition rates were 81·4% ± 9·4%versus 86·6% ± 17·2% (P > 0·05); however, the average inhibition rate for Pab2-HRP in the MPA group was significantly higher than that in the PTU group (76·3% ± 7·8%versus 58·9% ± 15·5%, P < 0·01) (Tables 1, 2 and 3).
DISCUSSION
MPO is a 120–150 kDa dimer composed of one heavy (55–60 kDa) and one light (14–15 kDa) chain and carries two identical prosthetic haeme groups. The two heavy chains are joined by a disulphide link. The correlation of anti-MPO antibody with disease pathogenesis is still unknown. Several hypotheses have been proposed to explain their action, of which the most widely accepted is that the binding of MPO–ANCA and the MPO molecules on the neutrophil surface leading to the neutrophil activation, such as oxygen radical production and degranulation [9], which induces glomerular capillary wall necrosis. It has been reported that the titre of MPO–ANCA correlated with activity of vasculitis [10,11]. However, high MPO–ANCA titres in the remission phase or low MPO–ANCA titres in the active phase have still been observed in a small number of patients [11,12]. This may be attributed to the differences in epitope recognition, antibody subclasses and antibody affinity.
MPO epitopes recognized by human sera were mainly conformational [13], but some were linear peptides [14]. MPO–ANCA reacted with recombinants of MPO heavy chain, but not light chain. Short et al. have suggested that the same or similar epitopes were recognized by sera from individual patients with vasculitis, and there was an immunodominant region on the surface of the MPO molecule [8]. Audrain et al. have shown that the human MPO autoantibodies response to at least four different epitopes [15]. Fujii et al. have reported that the most common sites were regions on the upstream of Met341and Met409 near the N-terminus of the MPO heavy chain and a region on the downstream of Gly598 near the C-terminus. The incidence of alveolar haemorrhage and pulmonary fibrosis in patients whose MPO–ANCA antibodies recognized one or two major epitopes was significantly higher than those whose MPO–ANCA antibodies recognized three major epitopes. The epitope recognition profiles were correlated with clinical features, suggesting a pathogenetic role of MPO–ANCA in vasculitis [16]. However, the exact epitopes of native MPO molecule still remain unknown.
The pathogenesis of PTU-induced vasculitis is not yet clear. Lee et al. demonstrated that the structure of MPO could be partially changed by the repeated administration of PTU [17]. Jiang et al. suggested that PTU could serve as MPO substrates and the metabolites might induce autoimmunity by exposing autoreactive lymphocytes to abnormal forms of self-material [18]. It is reasonable to speculate that the pathogenesis of PTU-induced vasculitis might be different from that of primary vasculitis and the mechanism of production of PTU-induced ANCA is different from that in primary vasculitis, and this could be reflected in the difference in epitope recognition.
The current study mapped epitopes of MPO–ANCA on native MPO molecules and compared patients with PTU-induced vasculitis and patients with primary MPA. The two mouse monoclonal antibodies to MPO could be inhibited by most sera, but the inhibition rates in the MPA group were significantly higher than those in the PTU group, suggesting that the epitopes of anti-MPO antibodies from patients with PTU-induced vasculitis might be more restricted, although the epitopes overlapped between the two groups. Pab1-HRP, from a patient with PTU-induced vasculitis, could be inhibited heavily by sera from both groups; this suggested that the epitopes recognized by MPO–ANCA from the patients with PTU-induced vasculitis could almost be covered by MPO–ANCA from patients with MPA. However, Pab2-HRP from a patient with MPA could also be inhibited by sera from both groups, but the average inhibition rate in the MPA group was significantly higher than that in PTU group (76·3%versus 58·9%, P < 0·01), which also indicated that epitopes recognized by patients with PTU-induced vasculitis might be more restricted. Nevertheless, the numbers of epitopes and the detailed fine specificities of epitopes could not be deduced from the current study.
We concluded that antimyeloperoxidase antibodies from patients with PTU-induced vasculitis and primary MPA could recognize more than one epitope on native MPO molecule. Although the epitopes overlapped between the two groups, the epitopes of anti-MPO antibodies from patients with PTU-induced vasculitis might be more restricted.
REFERENCES
- 1.Dolman KM, Gans RO, Rervaat TJ, et al. Vasculitis and antineutrophil cytoplasmic autoantibodies associated with propylthiouracil therapy. Lancet. 1993;342:651–2. doi: 10.1016/0140-6736(93)91761-a. [DOI] [PubMed] [Google Scholar]
- 2.Choi HK, Merkel PA, Walker AM, Niles JL. Drug-associated antineutrophil cytoplasmic antibody-positive vasculitis: prevalence among patients with high titers of antimyeloperoxidase antibodies. Arthritis Rheum. 2000;43:405–13. doi: 10.1002/1529-0131(200002)43:2<405::AID-ANR22>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 3.Gao Y, Zhao MH, Guo XH, Xin G, Gao Y, Wang HY. The prevalence and target antigens of antithyroid drugs induced antineutrophil cytoplasmic antibodies (ANCA) in Chinese patients with hyperthyroidism. Endocr Res. 2004;31:1–9. doi: 10.1081/erc-120037729. [DOI] [PubMed] [Google Scholar]
- 4.Fujieda M, Hattori M, Kurayama H, Koitabashi Y. Clinical features and outcomes in children with antineutrophil cytoplasmic autoantibody-positive glomerulonephritis associated with propylthiouracil treatment. J Am Soc Nephrol. 2002;13:437–45. doi: 10.1681/ASN.V132437. [DOI] [PubMed] [Google Scholar]
- 5.Zhao MH, Lockwood CM. A comprehensive method to purify three major ANCA antigens: proteinase 3, myeloperoxidase and bactericidal/permeability-increasing protein from human neutrophil granule acid extract. J Immunol Meth. 1996;197:121–30. doi: 10.1016/0022-1759(96)00123-8. [DOI] [PubMed] [Google Scholar]
- 6.Pharmacia LKB Biotechnology. Affinity chromatography principles and methods. Pharmacia LKB Biotechnology; [Google Scholar]
- 7.Claassen E. Immunocytochemical antigen-specific detection of antibodies. In: Lefkovits I, editor. Immunology methods manual. UK: Academic Press Limited; 1997. pp. 1051–2. [Google Scholar]
- 8.Short AK, Lockwood CM. Studies of epitope restriction on myeloperoxidase (MPO), an important antigen in systemic vasculitis. Clin Exp Immunol. 1997;110:270–6. doi: 10.1111/j.1365-2249.1997.tb08327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Falk RJ, Terrell RS, Charles LA, Jennette JC. Anti-neutrophil cytoplasmic autoantibodies induce nertrophils to degranulate and produce oxygen radicals in vitro. Proc Natl Acad Sci USA. 1990;87:4115–9. doi: 10.1073/pnas.87.11.4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arimura Y, Minoshima S, Nakabayashi K, et al. Serum myeloperoxidase and serum cytokines in antimyeloperoxidase antibody-associated glomerulonephritis. Clin Nephrol. 1993;40:256–64. [PubMed] [Google Scholar]
- 11.Jayne DR, Gaskin G, Pusey CD, Lockwood CM. ANCA and predicting relapse in systemic vasculitis. Q J Med. 1995;88:127–33. [PubMed] [Google Scholar]
- 12.Cohen P, Guillevin L, Baril L, Lhote F, Noel LH, Lesavre P. Persistence of antineutropil cytoplasmic antibodies (ANCA) in asymptomatic patients with systemic polyarteritis nodosa or Churg–Strauss syndrome: follow-up of 53 patients. Clin Exp Rheumatol. 1995;13:193–8. [PubMed] [Google Scholar]
- 13.Falk RJ, Becker M, Terrell R, Jennette JC. Anti-myeloperoxidase autoantibodies react with native but not denatured myeloperoxidase. Clin Exp Immunol. 1992;89:274–8. doi: 10.1111/j.1365-2249.1992.tb06944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tomizawa K, Mine E, Fujii A, et al. A panel set for epitope analysis of myeloperoxidase (MPO)-specific antineutrophil cytoplasmic antibody MPO–ANCA using recombinant hexamer histidine-tagged MPO deletion mutants. J Clin Immunol. 1998;18:142–52. doi: 10.1023/a:1023251001261. [DOI] [PubMed] [Google Scholar]
- 15.Audrain MA, Baranger TA, Moguilevski N, et al. Anti-native and recombinant myeloperoxidase monoclonals and human autoantibodies. Clin Exp Immunol. 1997;107:127–34. doi: 10.1046/j.1365-2249.1997.d01-895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fujii A, Tomizawa K, Arimura Y, et al. Epitope analysis of myeloperoxidase (MPO) specific anti-neutrophil cytoplasmic autoantibodies (ANCA) in MPO–ANCA-associated glomerulonephritis. Clin Nephrol. 2000;53:242–52. [PubMed] [Google Scholar]
- 17.Lee E, Hirouchi M, Hosokawa M, Sayo H, Kohno M, Kariya K. Inactivation of peroxidases of rat bone marrow by repeated administration of propylthiouracil is accompanied by a change in the heme structure. Biochem Pharmacol. 1988;37:2151–3. doi: 10.1016/0006-2952(88)90574-6. [DOI] [PubMed] [Google Scholar]
- 18.Jiang X, Khursigara G, Rubin RL. Transformation of lupus-inducing drugs to cytotoxic products by activated neutrophils. Science. 1994;266:810–3. doi: 10.1126/science.7973636. [DOI] [PubMed] [Google Scholar]