Abstract
The association of psoriasis with Streptococcus pyogenes throat infections suggests a potential antigenic target for the T cells that are known to infiltrate psoriatic skin. Streptococcal M protein share an extensive sequence homology with the human epidermal keratins. Keratin 17 (K17), while being mostly absent from uninvolved skin, is up-regulated in psoriatic lesions. Consequentially, M-protein-primed T cells may recognize up-regulated keratin epitopes via molecular mimicry. Using in vitro lymphocyte culture and cytokine flow cytometry we demonstrate that HLA-Cw*0602+ psoriasis patients had significant CD8+ T cell interferon (IFN)-γ responses to peptides from the K17 and M6 protein selected on the basis of sequence homology and predicted HLA-Cw*0602 binding. These responses were about 10 times more frequent in the skin-homing cutaneous lymphocyte-associated antigen-expressing (CLA+) subset of CD8+ T cells. CD4+ T cells showed only borderline responses. CLA+ CD8+ T cells from Cw6+ non-psoriatic individuals responded to some M6 peptides but rarely to K17 peptides. Cw6– psoriasis patients showed a response that was intermediate between Cw6+ patients and controls. These findings indicate that psoriatic individuals have CD8+ T cells that recognize keratin self-antigens and that epitopes shared by streptococcal M proteins and human keratins may be targets for the CD8+ T cells that infiltrate psoriatic skin lesions.
Keywords: CLA, HLA-Cw6, keratin, M protein, psoriasis
INTRODUCTION
Psoriasis is a chronic inflammatory skin disease affecting all major human populations with a maximal prevalence of 2–3% in northern Europeans [1]. The disease is clinically characterized by persistent, erythematous, indurated and scaly plaques, reflecting the infiltration of inflammatory cells and increased proliferation of keratinocytes. Compelling evidence has accumulated indicating that psoriasis is a T cell-mediated disease [2], and a distinct pattern of T cell localization is apparent in the dermal and epidermal skin compartments, in that CD4+ T cells predominate in the dermis, while most lesional epidermal T cells are CD8+[3,4]. These epidermal CD8+ T cells are likely to be effector cells although they probably require help from the dermal CD4+ T cells, which may respond to different peptide antigens. The identities of the antigens recognized by T cells infiltrating the dermis and epidermis of psoriatic skin remain to be determined. These epitopes, however, are likely to be provided by the hyperproliferating keratinocytes. The case for the involvement of CD8+ T cells in the disease pathogenesis is strengthened by the observation that about two-thirds of psoriatic individuals carry the HLA-Cw*0602 allele which is a strong candidate susceptibility allele [5], encoding a protein (hereby referred to as Cw6) which may be integral to psoriasis in these individuals due to its role in antigen presentation to CD8+ T cells.
It is well established that infection by M-protein-positive β-haemolytic streptococci can trigger the onset and exacerbation of psoriasis [6,7]. M protein is a major antigenic determinant of groups A, C and G streptococci [8,9] and its variable N-terminal half defines more than 100 different serotypes. However, the ability of Streptococcus pyogenes to trigger psoriasis has not been demonstrated to be serotype-specific [6], therefore the more conserved C-terminal half, proximal to the bacterial cell wall, may potentially contain T cell epitopes important in the pathogenesis of psoriasis. Indeed, an increased peripheral blood mononuclear cells (PBMC) response by psoriatic individuals to peptides from the C-terminal portion of M protein has been demonstrated previously [10]. Thus, the M protein peptides selected for analysis in this study were derived from the more conserved C-terminal portion of the M protein.
Streptococcal M proteins share an extensive amino acid sequence homology with Type I human epidermal keratins [11] and as a consequence of this, M-protein-primed T cells may recognize keratin epitopes via molecular mimicry. The keratin heterodimers K16/K6a and K17/K6b are mostly absent from uninvolved skin, but their expression is up-regulated in psoriatic lesions [12] and this abnormal keratin expression provides a potential antigenic target for the cross-reactive T cells. Such a scheme has been postulated, whereby psoriasis is mediated by T cells which recognize epitopes that are common to streptococcal M proteins and the keratins that are up-regulated in psoriatic lesions [13]. Indeed, in psoriatic individuals there is an increase in the frequency of interferon (IFN)-γ-producing T cells specific for keratin-derived peptides that share sequences with M proteins [14].
The keratin peptides used herein are present in two coil-forming regions of the K17 sequence. Such conserved regions of coiled-coil proteins have previously been investigated for potential cross-reactivity and antigen mimicry, as the constraints required for secondary structure formation lead to a high degree of sequence similarity in the coiled-coil domains of these proteins. Investigations into other diseases have attempted to exploit this phenomenon, including both the identification of cross-reactive B and T cell epitopes of streptococcal M protein [15]. In addition, human T cell lines specific for M peptides cultured from patients with rheumatic fever were shown to respond strongly to peptides representing homologous regions from within the coiled-coil protein myosin from both cardiac and skeletal muscle [16]. It is interesting in this context that K17, which is mostly absent from normal epidermis, is present at a low level in normal hair follicles, which fits well with the fact that the scalp is most commonly the first site to be affected by psoriasis [17]. K17 is the only keratin that is know to be up-regulated by IFN-γ[18,19] and intradermal injection of IFN-γ can induce psoriatic lesions [20], thus IFN-γ is believed to play a key role in the pathogenesis of psoriasis [21,22].
Cytokine flow cytometry has previously been shown to be a valuable tool in determining CD4+ and CD8+ T cell responses to viral peptide epitopes in chronic infections such as cytomegalovirus [23] and human immunodeficiency virus [24]. Here this technique is applied to chronic plaque psoriasis and we demonstrate peptide-specific responses by CD8+ T cells, isolated from the blood of psoriatic individuals, to short peptides derived from both keratin 17 and streptococcal M6 protein sequences. Moreover, we show that an increased frequency of T cells responding to keratin peptides appeared to be largely restricted to the skin-homing, cutaneous lymphocyte-associated antigen (CLA)-expressing subpopulation of CD8+ T cells. In contrast, CD8+ T cells from Cw6+ non-psoriatic individuals, having a family history of psoriasis, generally only responded to M6 derived peptides, and the CLA+ subset of CD8+ T cells responded infrequently to keratin peptides compared with psoriatic individuals. Moreover, CD8+ T cells from psoriasis patients who do not carry the HLA-Cw6 allele showed significantly fewer responses to both keratin and M peptides.
MATERIALS AND METHODS
Study population
Twelve HLA-Cw*0602-positive patients (eight men, four women, mean age 45·1 years) with moderate to severe ‘eruptive’ plaque psoriasis were recruited to the study. In addition to chronic plaques, the patients had diffusely distributed papular lesions that have been referred to as eruptive lesions and such lesions are mainly confined to Cw6+ psoriatics [17]. These individuals differ from those with standard plaque psoriasis because they have guttate-like papules that are interspersed between the psoriatic plaques. As a comparison group, 11 Cw6+ healthy individuals having a family history of psoriasis were included in the study (six men, five women, mean age 46·6 years). In addition, 11 Cw6– individuals with moderate to severe plaque psoriasis were recruited (five men, six women, mean age 50·5 years). Individuals receiving immunosuppressive treatment were excluded from the study. Patients and controls had been typed previously for all known HLA-C alleles using a Dynal HLA-C low-resolution typing kit (Dynal Biotech Ltd, Merseyside, UK), a polymerase chain reaction (PCR)-based method used as instructed by the manufacturer [17]. The study was approved by the Ethical Committee of Landspitali University Hospital in Reykjavik.
Peptide antigens
To identify candidate epitopes, peptides of 9, 12 or 20 amino acids in length with sequences derived from K17 were selected based on sequence similarities with M6 protein from S. pyogenes and predicted binding of HLA-Cw6. The amino acid sequence of K17 (NCBI accession number gi:1346342) was split into a complete set of overlapping nine residue peptides that were then used as a library to compare to the sequence of M6 (gi:80042) using the FASTA algorithm [25]. The resulting sets of peptides from K17 and M6 showing sequence similarities were then scanned for sequence motifs favourable for binding to HLA-Cw6 using the BIMAS algorithm [26] and an in-house motif search using published HLA-peptide binding data [27]. The candidate peptides, and an additional four peptides from the sequences of K17 and M6 protein that either did not have shared sequences or had poor predicted binding to HLA-Cw6 for use as negative controls, were synthesized as free acids and prepared to 95% purity (Peptron, Daejeon, South Korea). Lyophilized peptides were resuspended in dimethylsulphoxide (DMSO) at stock concentrations of 4 mm. The final concentration of each peptide was 5 µm/106 cells in all experiments described.
Antigens
Streptococcal antigen was prepared from S. pyogenes serotype 12 as described previously [28] and used at a protein concentration of 2·5 µg/ml. Candida albicans cellular antigen was obtained from Greer Laboratories (Lenoir, NC, USA) and used at 40 µg/ml. Streptokinase (200 U/ml) was from Hoechst Marion Roussel AB (Stockholm, Sweden). Cell culture supernatant from a hybridoma cell line (OKT-3) was used for anti-CD3 MoAb stimulation at a dilution of 1/50, which had previously been determined as suboptimal.
Intracellular staining
PBMC were isolated from the heparinized venous blood of psoriatic or control individuals by Ficoll (Sigma-Aldrich, St Louis, MO, USA) density gradient sedimentation. Cells were then washed twice in Hanks' balanced salt solution (Gibco, Invitrogen Ltd, Paisley, UK) and resuspended in RPMI-1640 medium (Gibco) supplemented with 10% heat inactivated fetal calf serum (FCS) (Gibco), 100 U/ml penicillin (Sigma), and 100 µg/ml streptomycin (Sigma). Co-stimulatory antibodies to CD28 and CD49d (Serotec Scandinavia, Oslo, Norway) were added at a concentration of 1 µg/ml, which had been shown to be an optimal concentration. After the addition of appropriately titred antigen, culture tubes were incubated at a 5° slant at 37°C in a humidified 5% CO2 atmosphere for a total of 8 h, with the last 6 h including 10 µg/ml of the secretion inhibitor brefeldin A (Sigma). After incubation, cells were prepared for intracellular staining for IFN-γ as directed (Becton-Dickinson, San Jose, CA, USA) using the following monoclonal antibodies: anti-CD4 and CD8-PerCP-Cy5·5, CD69-PE, IFN-γ-FITC and appropriate isotype controls (Becton-Dickinson). Samples were analysed using a Becton-Dickinson FACScan flow cytometer capturing a minimum of 30 000 events gated on CD4 or CD8+ small lymphocytes. In all experiments, responses were rated positive when a population of IFN-γ+CD69+CD4/8+ events was observed to be greater than 0·05% above background isotype control staining (IgG2b+CD69+CD4/8+).
The subpopulation of CD8+ T cells expressing CLA were examined for peptide specific responses as above, with the exception that the cells were surface immunostained for CLA (anti-CLA-FITC, Pharmingen, San Diego, CA, USA) and CD8 (anti-CD8-PerCP-Cy5·5, Becton-Dickinson) before being fixed, permeabilized and stained intracellularly for IFN-γ (anti-IFN-γ-PE). In this case, 10 000 CLA+CD8+ lymphocytes were analysed cytometrically from each culture.
Statistics
Data were tested for normality and statistical significance determined by unpaired t-test, performed with SigmaStat (SPSS, Erkrath, Germany).
RESULTS
Peptides
Candidate peptides, selected on the basis of sequence similarities and potential to bind HLA-Cw6, are listed in Table 1. These are clustered in two areas in the sequences of the K17 and M6 proteins, which contain the sequence motifs ‘ALEEANxxL’ and ‘GLRRxLD’. These motifs occur in sequences forming coils 1 A ‘ALEEANxxL’ and 1B ‘GLRRxLD’ of the rod section of keratin 17 (Fig. 1). In the M6 protein sequence, these motifs occur in a region that includes two directly repeated 27 amino acid segments and a 22 amino acid sequence highly conserved in M proteins, towards the carboxyl terminus of the protein proximal to the bacterial cell wall. These two repeated segments showed best homology to Type I keratins. The ‘ALEEANxxL’ motif was found in the sequences of a number of M proteins (including M5, M6, M12, M24) and Type I human epidermal keratins (9, 12, 13, 14, 15, 16, 17); however, it was not found in Type II keratins. The motif ‘GLRRxLD’ is present in M proteins (M5, M6, M12) and keratins 10, 12, 13, 14, 15, 17 and 19. Interestingly, this motif is present in the M5 protein three times, and twice in the sequences of M6 and M12 proteins.
Table 1.
Peptide sequences derived from keratin 17 and streptococcal M6 protein
| Group | Peptide name | Sequence |
|---|---|---|
| A | 125K17-9 | RLASYLDKV |
| 128K17-20 | SYLDKVRALEEANADLEVKI | |
| 128K17-9 | SYLDKVRA | |
| 134K17-12 | RALEEANADLEV | |
| 135K17-9 | ALEEANADL | |
| 139K17-9 | ANADLEVKI | |
| B | 217K17-9 | DVNGLRRVL |
| 220K17-9 | GLRRVLDEL | |
| 221K17-20 | LRRVLDELTLARTDLEMQIE | |
| 231K17-9 | ARTDLEMQI | |
| 238-K17-9 | QIEGLKEEL | |
| C | 261M6-9 | DIGALKQEL |
| 282M6-9 | SRKGLRRDL | |
| 324M6-9 | SRQGLRRDL | |
| 327M6-9 | GLRRDLDAS | |
| 384M6-9 | EAKALKEQL | |
| D | 338M6-20 | AKKQVEKALEEANSKLAALE |
| 338M6-12 | AKKQVEKALEEA | |
| 344M6-12 | KALEEANSKLAA | |
| 345M6-9 | ALEEANSKL | |
| 354M6-12 | AALEKLNKELEE | |
| 355M6-9 | ALEKLNKEL | |
| E | 127K17-9 | ASYLDKVRA |
| 130K17-9 | LDKVRALEE | |
| 31K17-12 | ISSVLAGASCPA | |
| 464M6-12 | ALTVMATAGVAA |
Peptide name indicates the start position in the protein sequence, protein name and sequence length. Peptides are divided into five groups corresponding to coils 1A and 1B of keratin 17 (groups A and B), repeats and conserved sequence of M6 (groups C and D) and four peptides with poor predicted binding to HLA-Cw6 (group E).
Fig. 1.
Peptides selected from keratin 17 and streptococcal M6 proteins. The shaded areas correspond to coils 1A (green) and 1B (blue) of the keratin; the two directly repeated 27 amino acid segments (pink) and a 22 amino acid conserved sequence (yellow) towards the C terminus of the M protein. Lines representing the selected peptides are colour-coded to indicate the predicted binding to HLA-Cw*0602 using the BIMAS algorithm: green, blue and red lines represent poor, intermediate and good binding predictions, respectively.
T cell responses to complex antigens
When freshly isolated PBMCs from Cw6+ psoriatic and Cw6+ control donors were stimulated for 8 h with Candida antigen, streptokinase or the streptococcal extract, both CD4+ and CD8+ T cells frequently responded to these stimuli (data not shown), with CD4+ T cells from psoriatic donors showing a significantly increased (P < 0·05) IFN-γ response to streptococcal extract compared with the healthy controls.
CD8+ T cells dominated the responses to the peptide antigens
PBMCs from Cw6+ psoriatic and Cw6+ control donors were stimulated for 8 h with peptides derived from keratin 17 and streptococcal M6 protein. The predominant responses observed were from CD8+ T cells with much weaker CD4+ T cell responses (Figs 2 and 3). For psoriatic individuals, CD8+ T cell responses were distributed throughout the range of keratin and M peptide antigens with little suggestion that any one peptide dominated the T cell response.
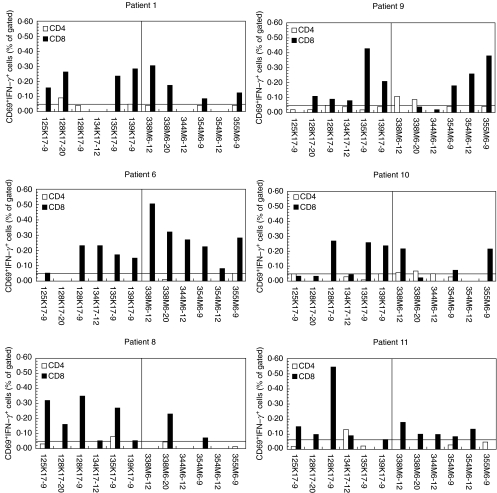
Fig. 2.
Responses of CD4+ and CD8+ T cells isolated from HLA-Cw6+ psoriatics. PBMCs were prepared from HLA-Cw*0602+ individuals with psoriasis. Following challenge with peptide antigens, PBMCs were stained for IFN-γ, CD69 and CD4 or CD8 and analysed flow cytometrically capturing a minimum of 30 000 CD4+ or CD8+ events. Six representative psoriatic patients are shown with CD4+ and CD8+ T cell responses represented with open and shaded bars, respectively. Keratin peptides are to the left and M peptides to the right of each graph. The horizontal line denotes the threshold of 0·05% over which responses were considered positive.
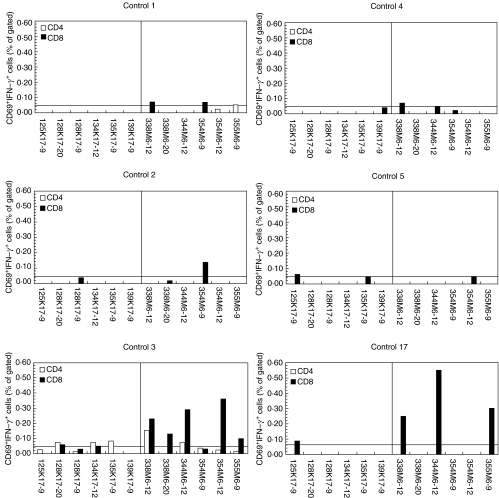
Fig. 3.
Responses of CD4+ and CD8+ T cells isolated from HLA-Cw6+ healthy controls. PBMCs were prepared from HLA-Cw*0602+ healthy controls. Following challenge with peptide antigens, PBMCs were stained for IFN-γ, CD69 and CD4 or CD8 and analysed flow cytometrically capturing a minimum of 30 000 CD4+ or CD8+ events. Six representative healthy controls are shown with CD4+ and CD8+ T cell responses represented with open and shaded bars, respectively. Keratin peptides are to the left and M peptides to the right of each graph. The horizontal line denotes the threshold of 0·05% over which responses were considered positive.
Cw6+ controls were less responsive to keratin peptides than Cw6+ psoriatics
CD8+ T cells isolated from the psoriatic donors showed responses to both keratin and M-protein-derived peptides (Fig. 2). In contrast, CD8+ T cells isolated from Cw6+ healthy controls responded to significantly fewer keratin-derived peptides compared to psoriatics (mean 1·3 versus 4·1, respectively, P = 0·001) and when responses to keratin-derived peptides were observed these were always borderline, as shown in Fig. 3. This difference was also seen in the number of M peptides to which the control and psoriatic CD8+ T cells responded (mean 2·1 versus 4·0, respectively, P = 0·037).
Interestingly, one Cw6+ patient who had finished ultraviolet B (UVB) treatment a week before the T cell analysis showed no responses to the peptide antigens while a vigorous response to streptokinase, an antigen unlikely to be encountered in skin, was demonstrated (data not shown). On a subsequent visit, 8 months later, this patient gave substantial T cell responses to four of the peptide antigens, a phenomenon that has been demonstrated previously [14,29].
The frequency of responding cells within the skin-homing CLA+CD8+ T cell population was much higher than in the CD8+ T cell population at large.
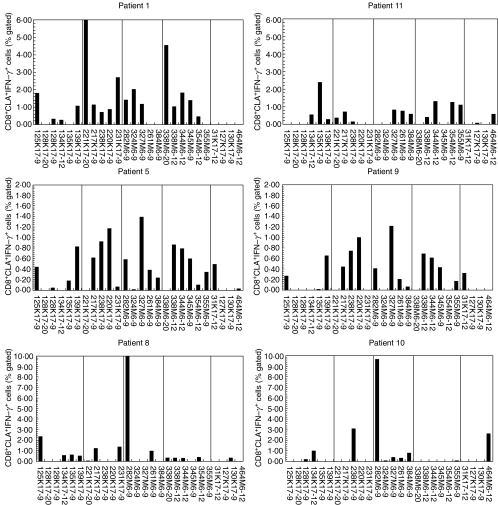
As psoriasis is considered to be a T cell-mediated skin disease, we next focused our analysis on the skin-homing, CLA+ subpopulation of CD8+ T cells. Furthermore, the number of keratin and M protein peptides used as test antigens was increased (all peptides in groups A to E, Table 1). The responses of the CLA+CD8+ T cells to the peptide antigens were far stronger than those of the CD8+ T cell population at large, as shown in Fig. 4.
Fig. 4.
Responses of CLA+CD8+ T cells isolated from HLA-Cw6+ psoriatics. PBMCs were prepared from HLA-Cw*0602+ individuals with psoriasis. Following challenge with peptide antigens, PBMCs were stained for CLA, IFN-γ, and CD8 and analysed flow cytometrically capturing a minimum of 10 000 CLA+CD8+ events. Six representative psoriatic patients are shown. The vertical lines divide the peptides into five groups, A to E, as in detailed Table 1.
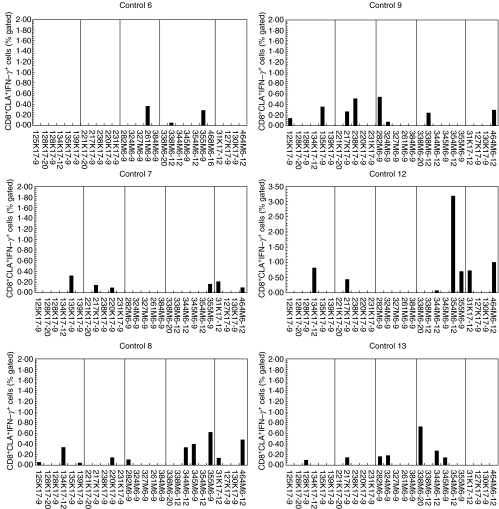
CD8+CLA+ T cells from Cw6+ controls responded more weakly and not as frequently to keratin peptides compared with Cw6+ psoriatics
In concordance with results from the CD8+ T cell population, CLA+CD8+ T cells from the Cw6+ controls (Fig. 5) showed much weaker and significantly less frequent responses to the keratin peptides compared to Cw6+ psoriatics (P < 0·001). Although Cw6+ controls responded to a number of keratin and M-protein-derived peptides, their responses were generally much weaker and to fewer keratin peptides (mean 6·3 versus 2·5, respectively, P < 0·001) and M peptides (mean 6·6 versus 3·9, respectively, P = 0·004) than was observed in the psoriatic patients. Of note are the poor and infrequent responses seen when the negative control peptides were used (Figs 4 and 5, far right-hand pane in all graphs). These peptides, listed in Table 1, group E, were designed to bind poorly to HLA-Cw6 and gave very few responses from both patients and controls.
Fig. 5.
Responses of CLA+CD8+ T cells isolated from HLA-Cw6+ healthy controls. PBMCs were prepared from HLA-Cw*0602+ healthy controls. Following challenge with peptide antigens, PBMCs were stained for CLA, IFN-γ, and CD8 and analysed flow cytometrically capturing a minimum of 10 000 CLA+CD8+ events. Six representative healthy controls are shown. The vertical lines divide the peptides into five groups, A to E, as in detailed Table 1.
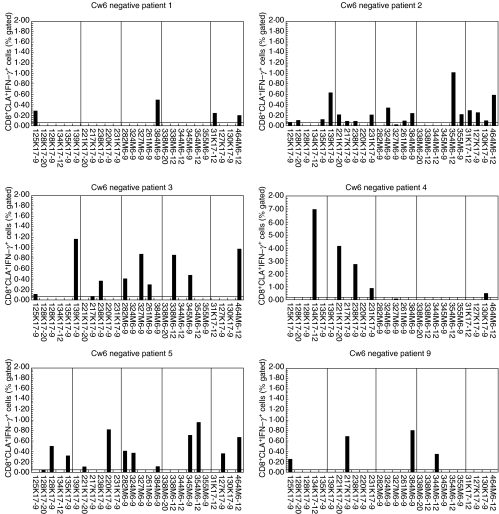
Cw6– psoriatic individuals responded less frequently to keratin and M peptides compared with Cw6+ psoriatics
The HLA-Cw6– psoriasis patients showed moderate CD8+CLA+ T cell responses to the keratin and M peptides (Fig. 6); however, these patients responded to significantly fewer peptides than did the Cw6+ psoriatics (Fig. 7), and this applied for both keratin peptides (mean 6·3 versus 3·9, P = 0·024) and M peptides (mean 6·6 versus 3·4, P < 0·001). There was not a significant difference in the number of peptides to which the Cw6– psoriatics and Cw6+ controls responded, the frequency of responding cells was, however, much greater for the Cw6– psoriatics than Cw6+ controls (Figs 5 and 6).
Fig. 6.
Responses of HLA-Cw6– psoriatic individuals to keratin and M peptides. PBMCs were prepared from psoriatic individuals who do not carry the HLA-Cw*0602 allele. Following challenge with peptide antigens, PBMCs were stained for CLA, IFN-γ, and CD8 and analysed flow cytometrically capturing a minimum of 10 000 CLA+CD8+ events. Six representative individuals are shown. The vertical lines divide the peptides into five groups, A to E, as in detailed Table 1.
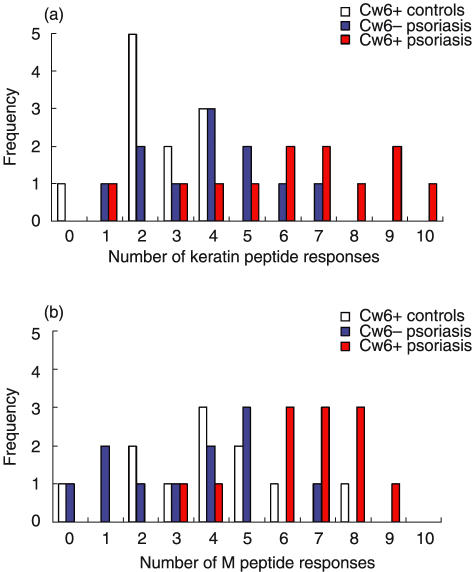
Fig. 7.
Responses of CD8+CLA+ T cells from 12 HLA-Cw6+ psoriatics, 11 Cw6– psoriatics and 11 Cw6+ healthy controls to (a) keratin peptides and (b) M peptides. All peptide responses above the threshold of 0·05% of the CD8+CLA+ T cell population are summarized here. (a) HLA-Cw6+ psoriatic patients responded to significantly more keratin peptides than did Cw6– psoriatics (P = 0·024) or Cw6+ healthy controls (P < 0·001) (b) Similarly, Cw6+ psoriatics showed more frequent responses to M peptides than did Cw6– psoriatics (P < 0·001) or controls (P = 0·004).
DISCUSSION
We have demonstrated previously that PBMCs isolated from psoriatic individuals exhibit an increased frequency of IFN-γ-producing T cells specific for keratin-derived peptides that share sequences with M proteins [14]. By now selecting peptides that are predicted to bind HLA-Cw6, we show that Cw6+ psoriatic individuals respond to both keratin- and M-protein-derived peptides, whereas Cw6+ healthy controls, with a family history of psoriasis, have less frequent and borderline responses, especially to the keratin peptides. Furthermore, we show that Cw6+ psoriatics respond significantly more frequently than Cw6– psoriatics.
We demonstrate that CD8+ T cells dominated the responses to the peptide antigens, and only a few and weak CD4+ T cell responses were recorded. This was unlikely to be due to the 2-h incubation period being too brief to allow antigen uptake and presentation via class II MHC, as CD4+ T cell responses to complex antigens such as Candida antigen, streptococcal extract and streptokinase were observed frequently. More probably, the CD4+ T cells showed poor responses because the peptide antigens were selected for binding to HLA-Cw6 both in terms of size and anchor residues, and the class II HLA that are in linkage disequilibrium with Cw6, e.g. HLA-DR7 [30], are unlikely to efficiently present these peptides.
Psoriasis is considered to be a T cell-mediated inflammatory condition of the skin and the frequency of skin-homing, CLA+CD8+ T cells in the blood of psoriasis patients correlates closely with the severity of their disease [31]. In addition, an increase in the number of CD8+ T cells especially within the epidermal compartment of the skin has been demonstrated during disease exacerbations [32]. Thus, we chose to focus our analysis on the CLA+CD8+ T cell population, which showed about 10 times higher frequency of responding cells than was observed in the CD8+ T cell population at large. This heavy skew towards those cells that have the potential to home to the skin suggests strongly that the T cells have been primed to these peptides within environments that induce skin-homing characteristics, such as skin-draining lymph nodes and possibly the pharyngeal tonsils. Furthermore, the observations that the responses to keratin peptides were restricted largely to the skin-homing population of CD8+ T cells and that the majority of T cells in the epidermal compartment are CD8+[3,4,32] are consistent with these cells having an effector role in psoriasis [2].
Cw6+ healthy volunteers were recruited as controls because the majority of the peptide antigens used were selected based on their likelihood to bind HLA-Cw6, and selection of these Cw6+ non-psoriatic individuals who have at least one close relative with psoriasis should highlight the aberrant T cell responses of the psoriatic individuals. It was not unexpected, however, that the PBMCs from the HLA-Cw6– psoriatics responded to the keratin and M peptides. Although these peptides were designed primarily for favourable binding to Cw6, they are not exclusively Cw6-restricted and most probably have the ability to bind other HLA class I molecules. Thus, the Cw6– psoriatics in this study responded to both the keratin and M peptides, but only very occasionally as strongly as, and less frequently than did the Cw6+ psoriatics.
It is interesting that the two peptide motifs examined here, ALEEANxxL and GLRRxLD, exist in Type I keratins other than K16 and K17. Keratins 12, 13, and 15 all contain at least one occurrence of both of these motifs and are not expressed in normal skin (K13 and K15 are expressed in non-keratinizing epithelia and K12 expression is restricted to cornea [33]). These motifs in K12, K13 and K15 could also give rise to antigen-mimetic peptides; yet, the tissues expressing these keratins do not exhibit psoriatic lesions. The key feature of K16 and K17 may be their largely novel and massive expression during keratinocyte hyperproliferation, whereby large amounts of these epitopes are expressed on the surface of keratinocytes that are then recognized by skin-homing CD8+ T cells. It is unknown how psoriasis is initiated, but we have suggested [2] that dendritic cells carrying streptococcal antigens could migrate from the tonsils to the skin, and they could prime autoreactive CD8+ T cells and induce the effector responses against epitopes presented by keratinocytes. Interestingly, dendritic cells containing bacterial peptidoglycan have been detected in nonlymphoid organs [34] and it has been demonstrated that uninvolved skin of psoriatics is more susceptible to stimulation with bacterial superantigen [35]. Moreover, cross-presentation by dendritic cells requires a large amount of antigens [36]; thus, the combination of the amount of, and the location of, cross-reactive epitopes may be critical for their recognition.
There was a marked variation in the pattern of responses to the peptide antigens in the psoriatic individuals (Figs 2 and 4), which suggests that different autoantigen(s) may dominate the psoriatic response in different individuals. Both keratin 17 and M6 protein can provide many possible T cell epitopes of which one or a very few epitopes may dominate the CD8+ T cell responses. Indeed, using a similar methodology and HIV-1 seropositive HLA-A2 individuals, it was demonstrated that different individuals show immunodominant responses to different CD8+ T cell epitopes from the HIV Gag protein [24]. Subsequent to antigen recognition via molecular mimicry, further epitopes may be recognized from the keratin due to epitope spreading; hence, T cells isolated from individuals with chronic psoriasis are unlikely to respond to a single dominant peptide. The observation that Cw6– psoriatics responded to fewer peptide antigens than did the Cw6+ psoriatics suggests that epitope spreading may be less evident in the Cw6– psoriatic disease. Additionally, some variability was seen in the response of the same donor on different visits (data not shown), which may reflect fluctuations in the course of psoriasis, in that the disease may periodically flare up and improve spontaneously which may alter the number of, and specificity of, the T cells sampled from peripheral blood.
In conclusion, we show that individuals with psoriasis have circulating CLA+CD8+ T cells that produce IFN-γ in response to keratin peptides. Our findings are consistent with the notion that CD8+ T cells with specificity for epitopes shared by M protein and human keratin may play an important effector role in psoriasis by targeting keratin peptides presented by HLA class I molecules on keratinocytes.
Acknowledgments
This work was supported by the EU Training and Mobility of Researchers programme as part of the ‘European Union Network for Investigation of the Pathogenesis of Psoriasis vulgaris’ research network. The authors thank Gunnhildur Ingolfsdottir and Inga Skaftadottir for their help and advice with flow cytometry.
REFERENCES
- 1.Christophers E. Psoriasis − epidemiology and clinical spectrum. Clin Exp Dermatol. 2001;26:314–20. doi: 10.1046/j.1365-2230.2001.00832.x. [DOI] [PubMed] [Google Scholar]
- 2.Gudjonsson JE, Johnston A, Sigmundsdottir H, Valdimarsson H. Immunopathogenic mechanisms in psoriasis. Clin Exp Immunol. 2004;135:1–8. doi: 10.1111/j.1365-2249.2004.02310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker BS, Swain AF, Griffiths CE, Leonard JN, Fry L, Valdimarsson H. Epidermal T lymphocytes and dendritic cells in chronic plaque psoriasis: the effects of PUVA treatment. Clin Exp Immunol. 1985;61:526–34. [PMC free article] [PubMed] [Google Scholar]
- 4.Bos JD, Hagenaars C, Das PK, Krieg SR, Voorn WJ, Kapsenberg ML. Predominance of ‘memory’ T cells (CD4+, CDw29+) over ‘naive’ T cells (CD4+, CD45R+) in both normal and diseased human skin. Arch Dermatol Res. 1989;281:24–30. doi: 10.1007/BF00424268. [DOI] [PubMed] [Google Scholar]
- 5.Veal CD, Capon F, Allen MH, et al. Family-based analysis using a dense single-nucleotide polymorphism-based map defines genetic variation at PSORS1, the major psoriasis-susceptibility locus. Am J Hum Genet. 2002;71:554–64. doi: 10.1086/342289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Telfer NR, Chalmers RJ, Whale K, Colman G. The role of streptococcal infection in the initiation of guttate psoriasis. Arch Dermatol. 1992;128:39–42. [PubMed] [Google Scholar]
- 7.Gudjonsson JE, Thorarinsson AM, Sigurgeirsson B, Kristinsson KG, Valdimarsson H. Streptococcal throat infections and exacerbation of chronic plaque psoriasis: a prospective study. Br J Dermatol. 2003;149:530–4. doi: 10.1046/j.1365-2133.2003.05552.x. [DOI] [PubMed] [Google Scholar]
- 8.Fischetti VA. Surface proteins on Gram-positive bacteria. In: Fischetti VA, Novick RP, Ferretti JJ, Portnoy DA, Rood JI, editors. Gram-positive pathogens. Washington DC: ASM Press; 2000. pp. 11–24. [Google Scholar]
- 9.Bisno AL, Brito MO, Collins CM. Molecular basis of group A streptococcal virulence. Lancet Infect Dis. 2003;3:191–200. doi: 10.1016/s1473-3099(03)00576-0. [DOI] [PubMed] [Google Scholar]
- 10.Sigmundsdottir H, Sigurgeirsson B, Troye-Blomberg M, Good MF, Valdimarsson H, Jonsdottir I. Circulating T cells of patients with active psoriasis respond to streptococcal M-peptides sharing sequences with human epidermal keratins. Scand J Immunol. 1997;45:688–97. doi: 10.1046/j.1365-3083.1997.d01-438.x. [DOI] [PubMed] [Google Scholar]
- 11.McFadden J, Valdimarsson H, Fry L. Cross-reactivity between streptococcal M surface antigen and human skin. Br J Dermatol. 1991;125:443–7. doi: 10.1111/j.1365-2133.1991.tb14769.x. [DOI] [PubMed] [Google Scholar]
- 12.Leigh IM, Navsaria H, Purkis PE, McKay IA, Bowden PE, Riddle PN. Keratins (K16 and K17) as markers of keratinocyte hyperproliferation in psoriasis in vivo and in vitro. Br J Dermatol. 1995;133:501–11. doi: 10.1111/j.1365-2133.1995.tb02696.x. [DOI] [PubMed] [Google Scholar]
- 13.Valdimarsson H, Baker BS, Jonsdottir I, Powles A, Fry L. Psoriasis: a T-cell-mediated autoimmune disease induced by streptococcal superantigens? Immunol Today. 1995;16:145–9. doi: 10.1016/0167-5699(95)80132-4. [DOI] [PubMed] [Google Scholar]
- 14.Gudmundsdottir AS, Sigmundsdottir H, Sigurgeirsson B, Good MF, Valdimarsson H, Jonsdottir I. Is an epitope on keratin 17 a major target for autoreactive T lymphocytes in psoriasis? Clin Exp Immunol. 1999;117:580–6. doi: 10.1046/j.1365-2249.1999.01013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cunningham MW, Antone SM, Smart M, Liu R, Kosanke S. Molecular analysis of human cardiac myosin-cross-reactive B- and T-cell epitopes of the group A streptococcal M5 protein. Infect Immun. 1997;65:3913–23. doi: 10.1128/iai.65.9.3913-3923.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pruksakorn S, Currie B, Brandt E, et al. Identification of T cell autoepitopes that cross-react with the C-terminal segment of the M protein of group A streptococci. Int Immunol. 1994;6:1235–44. doi: 10.1093/intimm/6.8.1235. [DOI] [PubMed] [Google Scholar]
- 17.Gudjonsson JE, Karason A, Antonsdottir AA, et al. HLA-Cw6-positive and HLA-Cw6-negative patients with psoriasis vulgaris have distinct clinical features. J Invest Dermatol. 2002;118:362–5. doi: 10.1046/j.0022-202x.2001.01656.x. [DOI] [PubMed] [Google Scholar]
- 18.Flohr T, Buwitt U, Bonnekoh B, Decker T, Bottger EC. Interferon-gamma regulates expression of a novel keratin class I gene. Eur J Immunol. 1992;22:975–9. doi: 10.1002/eji.1830220415. [DOI] [PubMed] [Google Scholar]
- 19.Troyanovsky SM, Leube RE, Franke WW. Characterization of the human gene encoding cytokeratin 17 and its expression pattern. Eur J Cell Biol. 1992;59:127–37. [PubMed] [Google Scholar]
- 20.Fierlbeck G, Rassner G, Muller C. Psoriasis induced at the injection site of recombinant interferon gamma. Results of immunohistologic investigations. Arch Dermatol. 1990;126:351–5. [PubMed] [Google Scholar]
- 21.Uyemura K, Yamamura M, Fivenson DF, Modlin RL, Nickoloff BJ. The cytokine network in lesional and lesion-free psoriatic skin is characterized by a T-helper type 1 cell-mediated response. J Invest Dermatol. 1993;101:701–5. doi: 10.1111/1523-1747.ep12371679. [DOI] [PubMed] [Google Scholar]
- 22.Prinz JC, Gross B, Vollmer S, et al. T cell clones from psoriasis skin lesions can promote keratinocyte proliferation in vitro via secreted products. Eur J Immunol. 1994;24:593–8. doi: 10.1002/eji.1830240315. [DOI] [PubMed] [Google Scholar]
- 23.Suni MA, Picker LJ, Maino VC. Detection of antigen-specific T cell cytokine expression in whole blood by flow cytometry. J Immunol Meth. 1998;212:89–98. doi: 10.1016/s0022-1759(98)00004-0. [DOI] [PubMed] [Google Scholar]
- 24.Betts MR, Casazza JP, Patterson BA, et al. Putative Immunodominant human immunodeficiency virus-specific CD8+ T-cell responses cannot be predicted by major histocompatibility complex Class I haplotype. J Virol. 2000;74:9144–51. doi: 10.1128/jvi.74.19.9144-9151.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pearson WR, Lipman DJ. Improved tools for biological sequence comparison. Proc Natl Acad Sci USA. 1988;85:2444–8. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parker KC, Bednarek MA, Coligan JE. Scheme for ranking potential HLA-A2 binding peptides based on independent binding of individual peptide side-chains. J Immunol. 1994;152:163–75. [PubMed] [Google Scholar]
- 27.Falk K, Rotzschke O, Grahovac B, et al. Allele-specific peptide ligand motifs HLA-C molecules. Proc Natl Acad Sci USA. 1993;90:12005–9. doi: 10.1073/pnas.90.24.12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pruksakorn S, Galbraith A, Houghten RA, Good MF. Conserved T and B cell epitopes on the M protein of group A streptococci. Induction of bactericidal antibodies. J Immunol. 1992;149:2729–35. [PubMed] [Google Scholar]
- 29.Sigmundsdottir H, Gudjonsson JE, Valdimarsson H. The effects of ultraviolet B treatment on the expression of adhesion molecules by circulating T lymphocytes in psoriasis. Br J Dermatol. 2003;148:996–1000. doi: 10.1046/j.1365-2133.2003.05318.x. [DOI] [PubMed] [Google Scholar]
- 30.Chicz RM, Urban RG, Gorga JC, Vignali DA, Lane WS, Strominger JL. Specificity and promiscuity among naturally processed peptides bound to HLA-DR alleles. J Exp Med. 1993;178:27–47. doi: 10.1084/jem.178.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sigmundsdottir H, Gudjonsson JE, Jonsdottir I, Ludviksson BR, Valdimarsson H. The frequency of CLA+ CD8+ T cells in the blood of psoriasis patients correlates closely with the severity of their disease. Clin Exp Immunol. 2001;126:365–9. doi: 10.1046/j.1365-2249.2001.01688.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sigmundsdottir H, Johnston A, Gudjonsson JE, Bjarnason B, Valdimarsson H. Methotrexate markedly reduces the expression of vascular E-selectin, cutaneous lymphocyte-associated antigen and the numbers of mononuclear leucocytes in psoriatic skin. Exp Dermatol. 2004;13:426–34. doi: 10.1111/j.0906-6705.2004.00177.x. [DOI] [PubMed] [Google Scholar]
- 33.Moll R, Franke WW, Schiller DL, Geiger B, Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982;31:11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- 34.Schrijver IA, van Meurs M, Melief M-J, et al. Bacterial peptidoglycan and immune reactivity in the central nervous system in multiple sclerosis. Brain. 2001;124:1544–54. doi: 10.1093/brain/124.8.1544. [DOI] [PubMed] [Google Scholar]
- 35.Travers JB, Hamid QA, Norris DA, et al. Epidermal HLA-DR and the enhancement of cutaneous reactivity to superantigenic toxins in psoriasis. J Clin Invest. 1999;104:1181–9. doi: 10.1172/JCI6835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kurts C, Miller JFAP, Subramaniam RM, Carbone FR, Heath WR. Major histocompatibility complex Class I-restricted cross-presentation is biased towards high dose antigens and those released during cellular destruction. J Exp Med. 1998;188:409–14. doi: 10.1084/jem.188.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]