Abstract
A characteristic feature of Wegener's granulomatosis is the presence of antineutrophil cytoplasm antibodies (ANCA) to proteinase 3 (PR3). In vitro, ANCA activate neutrophils by co-ligating PR3 and FcγRIIa/IIIb receptors. ANCA are predominantly of the IgG isotype, and IgG1, IgG3 and IgG4 subclasses are particularly represented. To address the pathogenic role of individual ANCA-IgG subclass antibodies, patients' sera were screened using indirect immunofluorescence, enzyme-linked immunosorbent assay (ELISA) and subclass PR3-ELISA to identify patients with high titres of PR3-ANCA within the IgG1, IgG3 or IgG4 subclasses. Unfractionated ANCA-IgG and subclass fractions were isolated by affinity chromatography and compared for their capacities to stimulate superoxide production by primed human neutrophils. Donor neutrophils were analysed for constitutive and induced FcγRI expression by flow cytometry. The IgG1, IgG3 and IgG4 subclass fractions, isolated from three different ANCA sera, each stimulated superoxide production from neutrophils derived from multiple donors. Subsequently, IgG4 subclass fractions isolated from a further four ANCA positive sera demonstrated varying abilities to stimulate release of superoxide; unrelated to PR3-ANCA titre, neutrophil donor, or neutrophil FcγRI expression. The stimulation of superoxide release by IgG1- and IgG3-ANCA subclass fractions is consistent with the proposed mechanism of co-ligation of PR3 antigen and FcγRIIa/IIIb receptors. However, the demonstration of similar activity for the IgG4-ANCA subclass fractions isolated from some sera was unexpected. This activity was independent of neutrophil donor and expression of FcγRI, suggesting it was capable of activating neutrophils via constitutively expressed FcγRIIa/IIIb or co-ligation of other, unidentified, cell surface molecules.
Keywords: ANCA, IgG subclasses, IgG4, PR3-ANCA, Wegener's granulomatosis
INTRODUCTION
Wegener's granulomatosis (WG) is an autoimmune systemic vasculitic disease in which antineutrophil cytoplasm antibodies (ANCA) are characteristically present in the serum. The predominant specificity is for proteinase 3 (PR3), a serine protease present in cytoplasmic azurophilic granules. These antibodies can be useful diagnostic markers, because their titre correlates with disease activity, falling in remission and rising on relapse [1–3].
Indirect evidence for a pathogenic role for PR3-ANCA in vivo is provided by the benefit afforded by plasmapheresis for patients with severe acute disease [4]. Current understanding of possible pathogenic mechanism(s) promoted through PR3-ANCA in WG vasculitis is based largely on in vitro studies. PR3-ANCA have been shown to engage PR3 antigen expressed on the surface of neutrophils, through the Fab regions and to co-ligate Fcγ receptors [5–7] resulting in neutrophil activation with secretion of proinflammatory cytokines and the production of a respiratory burst [5,8–10]. There is evidence for ligation by ANCA of the low-affinity FcγRIIa and FcγRIIIb receptors, which are constitutively expressed by human neutrophils [8–10]. Involvement of the high-affinity FcγRI receptor that can be induced on culture with interferon (IFN)-γ or granulocyte macrophage colony-stimulating factor (GM-CSF) [11] has been less well studied.
The PR3 antigen is constitutively expressed, at low levels, on the surface of normal neutrophils [12], while treatment with tumour necrosis factor (TNF)-α or GM-CSF increases PR3 expression [13,14]. Exposure to TNF-α or GM-CSF also results in maturation (priming) of normal neutrophils such that on exposure to ANCA they are activated, with the generation of superoxide anions. TNF-α levels are increased in sera of patients with vasculitis, resulting in in vivo priming [15] and a threefold increase in the level of cell surface PR3 expression has been reported for patients with active WG [16].
ANCA are predominantly of the IgG isotype [17], but studies of their distribution within the IgG subclasses present a rather confused picture. Thus, claims have been made that pathogenicity is correlated with IgG1 and/or IgG4 levels of PR3-ANCA [18] and, similarly, for the related disorder microscopic polyangiitis which is associated with ANCA specific to myeloperoxidase (MPO-ANCA) [19]. Other studies have concluded that ANCA of the IgG3 subclass is more directly associated with pathogenicity in both WG and MPA [17,18,20,21]. By contrast, further studies concluded that IgG3-ANCA titres do not correlate with likelihood of relapse in patients with WG or potency of antibody activities [22,23]. These studies have attempted to correlate ANCA titre with disease activity and do not address deeper questions of the epitope specificity of antibody populations, their impact on immune complex formation and the activation of effector functions. An intriguing feature of autoimmune diseases is the restricted antigen specificity of the autoantibodies and the possible identification of a major antigenic determinant. In established disease the antibody response may be less focused due to the phenomenon of epitope spreading.
Four subclasses of human IgG are defined structurally, functionally and serologically. While a unique profile of effector functions has been ascribed to each IgG subclass (Table 1), such assignments have been shown to be simplistic; studies with defined immune complexes of each subclass have shown that multiple parameters may determine the effector ligand activated and the functional consequences, e.g. epitope density, antibody/antigen ratio [24–26]. In the context of this report it should be noted that IgG4 has been reported to bind the high-affinity receptor FcγR1 only; however, IgG4-mediated antibody-dependent cellular cytotoxicity reactions have been observed for natural killer (NK) cells isolated from the blood of donors homozygous for the FcγRIIIa-158 V allele (A. W. Morgan & J. D. Isaacs, unpublished observations). This may be added to numerous other reports of seemingly anomalous activities for IgG4 (reviewed in Aalberse & Schuurman [27]).
Table 1.
Specificity of human IgG subclasses for FcγR: + refers to affinity of binding of a subclass for the stated FcγR, – denotes that a subclass is reported not to bind to the stated FcγR [44]
| IgG1 | IgG2 | IgG3 | IgG4 | |
|---|---|---|---|---|
| FcγRI (CD64) | + + + | – | + + + | + |
| FcγRII (CD32) | ||||
| FcγRIIa-H131 | + + | + + | + + + | − |
| FcγRIIa-R131 | + + | – | + + + | – |
| FcγRIIb | + + | – | + + + | + |
| FcγRIII (CD16) | ||||
| FcγRIIIa | + + | + | + + | + |
| FcγRIIIb | + + | + | + + | – |
In the present report IgG was isolated from the sera of WG patients and assayed to determine the relative titres of IgG subclass ANCA present. Serum samples from the patients with high IgG1, IgG3 or IgG4 ANCA titres were fractionated by affinity chromatography to yield polyclonal IgG1, IgG3 and IgG4 subclass fractions with ANCA activity. We report that polyclonal IgG1, IgG3 and IgG4 fractions containing PR3-ANCA exhibit differential capacity to stimulate a superoxide response in fresh, primed human neutrophils, independent of the neutrophil donor. This may be indicative of differing epitope specificities between the IgG subclass fraction and the sera from which they were isolated. The stimulatory activity of IgG4 ANCA was unexpected since IgG4 has not been reported to ligate/activate FcγRIIa or FcγRIIIb. The possible involvement of FcγRI was excluded by its absence from primed neutrophils; further, induction of FcγRI expression by overnight stimulation with IFN-γ did not result in a significant increase in superoxide production. Deglycosylation of PR3-ANCA IgG resulted in a loss of its activating ability, consistent with FcγRIIa or FcγRIIIb involvement. These findings suggest that IgG4 ANCA can activate neutrophils via constitutively expressed FcγRIIa and FcγRIIIb or co-ligation of other, unidentified, cell surface molecules. This report adds to an increasing literature in which IgG4 antibodies have been shown to exhibit unexpected activities [27].
PATIENTS AND METHODS
Sample selection
Fifty-five plasma-derived serum samples from patients with ANCA-associated vasculitis were screened to select samples with PR3-ANCA but not MPO-ANCA or antiglomerular basement membrane (GBM) antibody activity. The final panel was comprised of sera from 14 patients with PR3-ANCA who were diagnosed as suffering from active WG (six female, eight male, mean female age 66·8 years, mean male age 57·9 years). These patients had clinical evidence of WG and were new presentations. The Birmingham Vasculitis Activity Score was >10 in all patients indicating active disease, mean score was 18. All had renal biopsy confirmation of active vasculitis and accorded with the Chapel Hill Consensus Conference definition of WG [28]. All were on prednisolone and cyclophosphamide therapy for a maximum of 24–48 h prior to plasma exchange. The first litre of plasma exchange fluid was retained, aliquoted and stored at −20°C. Thawed plasma was converted to serum by the addition of 1 ml of sterile, 1 m calcium chloride per 50 ml of plasma. The plasma was incubated at 37°C for 1 h and left to clot at 4°C, overnight. The fibrin clots were pelleted by centrifugation at 3000 rpm for 10 min. Clarified serum was removed, passed through 0·2 µm filters, dispensed into 5 ml aliquots and frozen at −20°C. Studies were conducted under permission from the Local Research Ethics Committee.
Indirect immunofluorescence (IIF)
IIF was performed as described [29]. Indirect immunofluorescence results were reported as cANCA, pANCA or negative.
Anti-PR3, anti-MPO and anti-GBM antibody enzyme-linked immunosorbent assay (ELISA)
Samples were tested for PR3-ANCA, MPO-ANCA and anti-GBM using Bind-azyme kits (Binding Site, Birmingham, UK). The ELISA was performed according to the manufacturer's instructions. Briefly, standards, controls and samples, diluted 1 : 50, 1 : 100 with phosphate buffered saline (PBS)-0·05% Tween were added to wells of PR3-coated plates. Following incubation at room temperature (30 min) the plates were washed three times with 300 µl of 1× kit wash buffer and 100 µl of antihuman IgG (Fc-specific) conjugate was added and incubated for 30 min at room temperature. The plates were washed again three times with 300 µl of 1× kit wash buffer and 100 µl of 3,3′,5,5′-tetramethylbenzidine (TMB) substrate added per well. The plates were incubated at room temperature for 30 min and 100 µl of 3 m phosphoric acid stopping solution was added to each well. The optical density of the plate was read within 30 min at 450 nm.
PR3-ANCA IgG subclass ELISA
ELISA plates precoated with PR3 (Binding Site Birmingham, UK) were incubated with PR3-ANCA positive serum at dilutions of 1 : 50–1 : 10 000 in PBS-0·05% Tween 20 (PBS-T). All samples were run at a minimum of four dilutions. After a 30-min incubation at room temperature, the plates were washed three times with 300 µl of the kit wash buffer and 100 µl of antihuman IgG1, 2, 3 or 4 horseradish peroxidase-conjugated subclass specific antibody diluted 1 : 1000 with PBS-T, was added (Binding Site, Birmingham, UK). The optical density of the plate was read within 30 min at 450 nm. Anti-PR3 activity was estimated using the calibration samples supplied with the kit. Titres determined at all dilutions were normalized to a 1 : 50 dilution, and the mean was calculated.
IgG subclass purifications
All subclass IgG purifications were performed using an automated system composed of a peristaltic pump, fraction collector, UV monitor and chart recorder (Amersham Biosciences, Bucks, UK) connected to the affinity column. Affinity columns were prepared using subclass specific monoclonal antibodies for IgG1, IgG3 or IgG4 (8C/6, ZG4 and RJ4, respectively [30]) and immobilized to CNBr activated Sepharose-6B (Amersham Biosciences, Bucks, UK). Serum was loaded onto columns equilibrated in PBS and the breakthrough fraction was collected following washing with PBS. The bound IgG was eluted with 0·1 m glycine, pH 2·6 and fractions collected in tubes containing 1 m Tris base, pH 9·0 (100 µl Tris per 1 ml of eluent) to neutralize the solution. The eluted IgG subclass was dialysed extensively against changes of PBS at 4°C. The flowthrough ANCA-IgG subclass-depleted serum fraction was then loaded onto a HiTrap protein G column (Amersham Biosciences, Bucks, UK) pre-equilibrated with PBS. The column was washed with PBS and, as before, the bound IgG eluted with 0·1 m glycine, pH 2·6. The eluted IgG was extensively dialysed against changes of PBS at 4°C. The isolated ANCA-IgG subclass fractions were concentrated using Vivaspin concentrators with a 10 000 Da cut-off (Vivascience, Hanover, Germany). The fractions were 0·2 µm filtered into a sterile container and the protein concentration of both fractions determined by UV absorption at 280 nm and E1%280nm = 14·5. The samples were aliquoted and stored at 4°C until use.
Total IgG and IgG subclass concentrations
The total IgG and IgG subclass concentrations of each PR3-ANCA positive serum was determined in the routine clinical immunology laboratory employing nephelometry, as described by Grange et al. [31]. The proportion of each subclass within each sample was calculated.
Analysis of the purity of IgG subclass fractions
Sheep red blood cells (SRBC) were sensitized with purified monoclonal antibodies specific for each IgG subclass, by the chromium chloride method [32]. The appropriate IgG subclass protein agglutinates such cells. Each IgG subclass fraction was titrated in a twofold dilution series in 2% HEPES buffer in the wells of a round-bottomed 96-well microtitre plate; control wells contained 2% HEPES buffer only. Positive control plates were prepared as above using dilutions of known concentrations of purified IgG subclass protein. Sensitized SRBC (25 µl) were added and plates incubated at room temperature for 1 h. Relative concentrations of the IgG subclass proteins were determined by comparison of the haemagglutination titre end-points obtained for the IgG subclass fractions and the corresponding purified monoclonal subclass protein.
Human neutrophils
Neutrophils were isolated as described previously employing a discontinuous Percoll density gradient [33] from peripheral blood taken from healthy volunteers following informed consent and with permission from the Local Research Ethics Committee. Isolated neutrophils were diluted with Trypan blue solution and counted using a haemocytometer to determine cell viability. All preparations were >95% viable.
Neutrophils were primed for superoxide assays by exposure to cytochalasin B 1 U/ml (Sigma-Aldrich Company Ltd, Dorset, UK) for 5 min followed by 2 U/ml TNF-α (NISBC, Potters Bar, UK) for 15 min.
Culture of neutrophils with IFN-γ
Freshly isolated neutrophils were resuspended in Iscove's DMEM (Dulbecco's modified Eagle's medium) (Sigma-Aldrich Company Ltd, Dorset, UK), with added 10% autologous human serum to maintain the cells viability, in the presence or absence of 400 U/ml IFN-γ (R&D Systems Europe Ltd, Abingdon, Oxon, UK) for 18 h at 37°C. Following incubation cells were analysed by single or dual stain flow cytometry (FacScan, BD Biosciences, Oxford, UK). IFN-γ treated neutrophils were also primed as described above and used in superoxide assays; neutrophil counts were based on viable cells only.
Superoxide generation
Superoxide assays were performed as described previously using a kinetic microplate assay that measures the superoxide dismutase inhibitable reduction of ferricytochrome C [7]. The plates were scanned at 550 nm at 0, 5, 30, 60 and 100 min using a Multiscan® Bichromatic plate reader (Thermo Labsystems Oy, Helsinki, Finland), between readings the plates were incubated at 37°C. Each test was performed in triplicate.
The activity of the purified IgG subclass preparations was determined at concentrations equivalent to those that were present in the whole serum IgG. Thus, if the input total IgG was at a concentration of 200 µg/ml and the IgG4 content represented 10% of the total IgG, the purified IgG4 fractions was assayed at an initial concentration of 20 µg/ml.
Single stain flow cytometric analysis for FcγRI or PR3 expression
Aliquots of neutrophils (5 × 105) were exposed to saturating concentrations of FITC-conjugated anti-FcγRI (Medarex Inc, Princeton, NJ, USA) or unconjugated 4A5-anti-PR3 antibody (Wieslab, Lund, Sweden), the latter being followed by FITC-conjugated goat antimouse F(ab′)2 (Dako Ltd, Ely, Cambridgeshire, UK) for 30 min at 4°C. The cells were fixed in 1% paraformaldehyde solution and flow cytometric analysis performed (FacScan, BD Biosciences, Oxford, UK).
Dual stain flow cytometric analysis
Aliquots of freshly isolated neutrophils (5 × 105), cultured overnight in the presence or absence of IFN-γ (R&D Systems Europe Ltd), were stained for 45 min at 4°C with saturating concentrations of FITC conjugated anti-FcγRI (Medarex Inc.). Cells were washed with PBS, pelleted at 400 g for 5 min and resuspended in 100 µl of annexin V kit binding buffer. Cells were incubated with saturating concentration of PE conjugated annexin V (BD Biosciences, Oxford, UK) for 15 min in the dark. Following incubation, 400 µl of annexin V kit binding buffer was added and flow cytometric analysis was performed (FacScan, BD Biosciences).
Preparation of deglycosylated IgG
Purified IgG was dialysed into 40 m m potassium phosphate, 10 m m EDTA, pH 7·4. PNGase F (1 U/0·5 mg IgG) (Roche Diagnostics Ltd, East Sussex, UK) was added and the sample incubated at 37°C for 72 h. Control IgG was incubated for the same time period without the addition of enzyme. The deglycosylated and control antibody samples were purified on a 1 ml HiTrap protein G column (Amersham Biosciences, Bucks, UK) and frozen at −20°C until purification. The extent of deglycosylation was assessed by SDS-PAGE analysis of the heavy chain mass [33].
RESULTS
PR3-ANCA IgG subclass ELISA
Serum samples from 14 PR3-ANCA WG patients selected for study and six controls were analysed to determine IgG subclass PR3-ANCA titres, using a modified PR3-ELISA as described. A summary of the mean IgG subclass anti-PR3 titres for each patient is given in Table 2. The titres of subclass restricted anti-PR3 antibodies can be compared between samples for each individual IgG subclass. Comparisons between titres for each subclass within a given sample are more problematic due to possible differences between the IgG subclass specific antibodies, e.g. affinity, number of epitopes recognized, etc.
Table 2.
Summary of IgG PR3-ANCA subclass ELISA results
| P | IgG1 PR3 titre (U/ml) | IgG2 PR3 titre (U/ml) | IgG3 PR3 titre (U/ml) | IgG4 PR3 titre (U/ml) |
|---|---|---|---|---|
| 1 | 10 | 2 | 200 | 90 |
| 2 | 400 | 3 | 190 | 10 |
| 3 | 50 | 40 | 10 | 1800 |
| 4 | 10 | 4 | 40 | 10 |
| 5 | 10 | 2 | 20 | 90 |
| 6 | 20 | 3 | 180 | 10 |
| 7 | 5 | 2 | 5 | 20 |
| 8 | 50 | 6 | 2000 | 10 |
| 9 | 300 | 15 | 70 | 20 |
| 10 | 600 | 9 | 150 | 50 |
| 11 | 30 | 5 | 290 | 10 |
| 12 | 70 | 3 | 160 | 10 |
| 13 | 10 | 3 | 70 | 5 |
| 14 | 50 | 4 | 1500 | 200 |
The profile of the IgG subclass anti-PR3 titres differed for each serum sample with mainly one subclass predominating. Thus, IgG1 anti-PR3 predominated in samples P2, P9 and P10; IgG3 in P1, P4, P6, P8, P11-14; IgG4 in P3, P5 and P7. IgG2 did not predominate in any sample and significant titres were observed for P3 and P9 only.
Affinity purification and characterization of ANCA positive IgG subclass fractions
IgG1, IgG3 and IgG4 subclass fractions were isolated from sera P2, P8 and P3, respectively. Each IgG subclass preparation was shown to be free of contaminating serum proteins, by SDS-PAGE, and comprised essentially of a single subclass (>95% pure as determined by the haemagglutination assay). Samples of total unfractionated IgG, IgG subclasses fractions, and IgG from healthy controls were assayed by PR3-ELISA (antihuman IgG Fc specific conjugate) at 100 µg/ml; the IgG subclass fractions were also assayed at concentrations proportional to their concentrations in the original serum sample (Table 3), i.e. for sample P2 this comprised 49% of the total IgG, therefore anti-PR3 titre was determined at 49 µg/ml.
Table 3.
Anti-PR3 ELISA results for (A) P2 IgG1 (B) P8 IgG3 and (C) P3 IgG4 fractionations. Results are shown for samples of unfractionated IgG, isolated subclasses and normal IgG for each ELISA. Each was tested at 100 µg/ml and the purified subclass antibodies were also tested at concentrations equivalent to their percentage within the original serum sample
| Sample | Anti-PR3 titre (U/ml) |
|---|---|
| (a) | |
| P2 IgG 100 µg/ml | 77 |
| P2 IgG1100 µg/ml | 52 |
| P2 IgG1 49 µg/ml | 22 |
| Normal IgG 100 µg/ml | 5·4 |
| (b) | |
| P8 IgG 100 µg/ml | 156 |
| P8 IgG3100 µg/ml | 152 |
| P8 IgG3 13 µg/ml | 115 |
| Normal IgG 100 µg/ml | 0·8 |
| (c) | |
| P3 IgG 100 µg/ml | 133 |
| P3 IgG4100 µg/ml | 120 |
| P3 IgG4 22 µg/ml | 86 |
| Normal IgG 100 µg/ml | 0·8 |
Importantly, anti-PR3 binding was detected in isolated subclass fractions for P2, P8 and P10 but not in normal IgG preparations.
Activation of primed human neutrophils with IgG1-, IgG3- and IgG4-ANCA
The ability of each ANCA-IgG positive fraction to stimulate generation of superoxide from freshly isolated primed neutrophils was determined; each sample was tested against neutrophils from a minimum of three different donors. The baseline concentration used for testing the purified subclass isolates was that which was proportional to the concentration in the original serum samples, as described above.
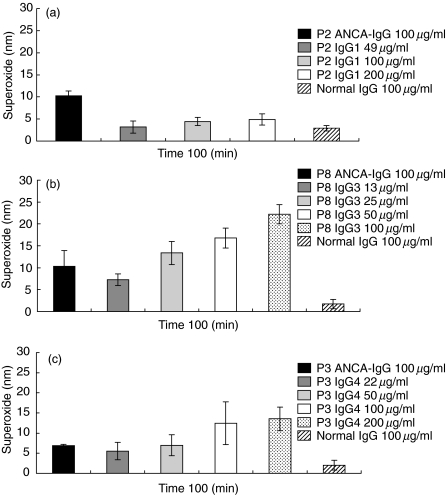
The IgG1-ANCA fraction isolated from serum P2 elicited a superoxide response in a dose-dependent manner at 100 min (Fig. 1a) and at the earlier time-points of 5, 30 and 60 min (not shown). However, it was not as vigorous as that seen within the original unfractionated ANCA-IgG. Even increasing the concentration of IgG1-ANCA to 100 and 200 µg/ml did not increase the response (4·4 ± 0·9 and 4·9 ± 1·28 nmol superoxide, respectively, at 100 min) above that seen with unfractionated P2 ANCA-IgG.
Fig. 1.
Mean superoxide production stimulated by ANCA-IgG and fractionated ANCA-IgG subclasses from patients P2, P8 and P3 in primed neutrophils. Superoxide production was measured for 100 min in response to 100 µg/ml of ANCA-IgG or normal IgG. For ANCA-IgG subclasses, IgG1-ANCA from patient P2, shown in (a), was used at 49, 100 and 200 µg/ml; IgG3-ANCA from P8, shown in (b), was used at 13, 25, 50 and 100 µg/ml and IgG4-ANCA from P3, shown in (c), was used at 22, 50, 100 and 200 µg/ml. Results are expressed as nmol/1 × 105 cells. Data shown are mean ± s.e.m. for experiments performed in triplicate on four neutrophil donors.
The IgG3-ANCA fraction isolated from serum P8 elicited rapid and vigorous responses (7·3 ± 1·3 nmol superoxide at 100 min using 13 µg/ml as present in the unfractionated P3 IgG) (Fig. 1b). Increasing the concentration of IgG3 above that present in the original sample led to a further increase in superoxide production, so that at 25, 50 and 100 µg/ml the response was 13·4 ± 2·6, 16·8 ± 2·3 and 22·2 ± 2·2 nmol, respectively, at 100 min, exceeding that obtained for unfractionated P8 IgG at 100 µg/ml (10·4 ± 3·6 nmol at 100 min).
The IgG4-ANCA fraction isolated from serum P3 also elicited a vigorous superoxide response (5·5 ± 2·2 nmol at 100 min using 22 µg/ml as present in the unfractionated P3 ANCA-IgG) (Fig. 1c). Increasing the concentration of IgG4-ANCA to 100 µg/ml and 200 µg/ml increased superoxide production to 12·4 ± 5·3 and 13·6 ± 2·9 nmol, respectively, at 100 min, more than that obtained with the original unfractionated ANCA-IgG (6·9 ± 0·28 nmol at 100 min). It was apparent from these studies that the binding activity in the PR3-ANCA ELISA did not correlate with the ability of any sample or sample fraction to activate neutrophils in the superoxide assay. It was also apparent that IgG4-ANCA was able to activate freshly isolated primed neutrophils.
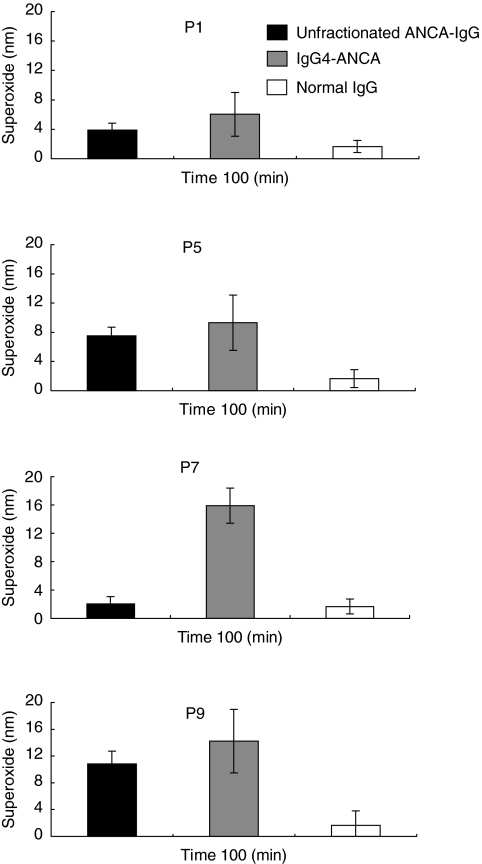
Activation of primed human neutrophils with IgG4-ANCA isolated from four individual WG patients
The generality of IgG4-ANCA activation of neutrophils was investigated following the isolation of the IgG4 subclass fraction from sera P1, P5, P7 and P9. Unfractionated ANCA-IgG and the IgG4-ANCA subclass fractions were assayed for superoxide production using four different neutrophil donors (Fig. 2). All four IgG4-ANCA fractions, tested at 200 µg/ml, produced a superoxide response. The purified P7 IgG4-ANCA was particularly activating, compared with the unfractionated ANCA-IgG from which it was purified.
Fig. 2.
Mean superoxide production stimulated by unfractionated ANCA-IgG and IgG4-ANCA samples from patients P1, P5, P7 and P9 in primed neutrophils. Superoxide production was measured for 100 min in response to 100 µg/ml of unfractionated ANCA-IgG (black bars) or normal-IgG (white bars) and 200 µg/ml IgG4-ANCA (dark grey bars). Results are expressed as nmol/1 × 105 cells. Data shown are mean ± s.e.m. for experiments performed in triplicate on four neutrophil donors.s.e.m.
FcγRI expression and superoxide production by human neutrophils cultured with IFN-γ
ANCA-IgG is believed to activate neutrophils via co-ligation of FcγRIIa and/or FcγRIIIb [10]; however, IgG4 would not be expected to activate freshly isolated neutrophils through these receptors. Because IgG4 can ligate FcγRI, a possible role for FcγRI in IgG4-ANCA-induced neutrophil activation was investigated. Unfortunately, effective blocking antibodies to FcγRI are not available, so more indirect approaches had to be taken to investigate this.
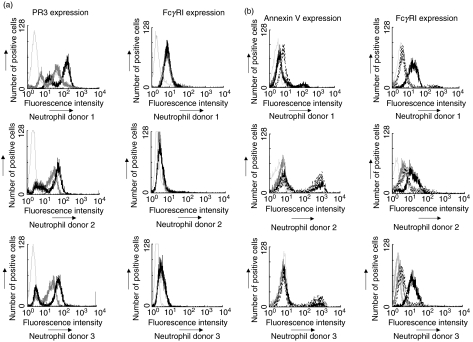
The expression of FcγRI was evaluated on freshly isolated, TNF-α-primed neutrophils and on neutrophils that had been cultured for 18 h in the presence of IFN-γ prior to priming. As established previously, freshly isolated neutrophils primed with TNF-α showed increased bimodal surface PR3 expression compared with unprimed neutrophils [34,35], indicating that PR3 expression increases but the overall proportion of low and high PR3-expressing cells remains stable, yet there was no significant effect on FcγRI expression (Fig. 3a). In contrast, FcγR1 expression was up-regulated following overnight exposure to IFN-γ. Prolonged culture of neutrophils enhances apoptosis, with characteristic up-regulation of annexin V expression. Therefore, following overnight culture in the presence or absence of IFN-γ, neutrophils were analysed by flow cytometry for the expression of annexin V and FcγRI. Freshly isolated, primed neutrophils showed low-levels of expression of annexin V and FcγRI (Fig. 3b), there is increased annexin V in donor 2 compared to other donors, which may be due to more cells being committed to apoptosis. Overall, the level of expression of annexin V was unaffected by overnight culture in the presence or absence of IFN-γ; however, expression of FcγRI was substantially increased following IFN-γ treatment.
Fig. 3.
(a) FACS analysis histograms of isolated neutrophils to detect PR3 and FcγRI expression. Dark grey histograms represent expression by unprimed, freshly isolated cells and black histograms represent expression after priming of cells for 15 min with 2 U/ml of TNF-α. Light grey bars histograms represent isotype control IgG1 binding. (b) FACS analysis histograms of isolated neutrophils, dual stained to detect annexin V and FcγRI expression. Dark grey histograms represent expression after priming of cells for 15 min with 2 U/ml of TNF-α. Black histograms represent expression after treatment with 400 U/ml of IFN-γ for 18 h while dashed line histograms represent cells cultured without IFN-γ for 18 h. Light grey histograms represent isotype control IgG1 binding.
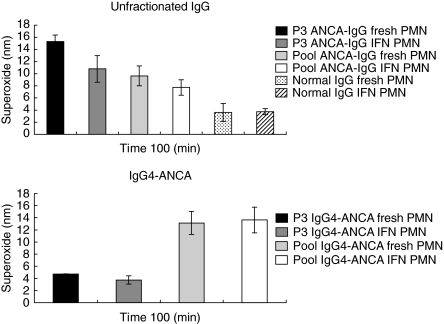
Neutrophils cultured in the presence or absence of IFN-γ were assessed for their relative abilities to produce superoxide in response to activation with unfractionated ANCA-IgG and IgG4-ANCA fractions, isolated from serum P3 and a pool of sera P1, P5, P7 and P9 (Fig. 4). For these assays the viability of the cultured neutrophils was determined by Trypan blue exclusion and the same number of viable cells were used in assays with freshly isolated and cultured neutrophils. In each case, the response obtained with fresh neutrophils was greater than for cells cultured in the presence or absence of IFN-γ. Therefore, the induction/µp-regulation of FcγRI expression following culture in the presence of IFN-γ did not enhance neutrophil activation by IgG4-ANCA.
Fig. 4.
Mean superoxide production stimulated by P3 and pooled ANCA-IgG in fresh and IFN-γ-treated neutrophils. Unfractionated ANCA-IgG and IgG4-ANCA were isolated from the serum of patient P3 and from a pool made up of serum from patients P1, P5, P7 and P9. Superoxide production was measured for 100 min in response to unfractionated 100 µg/ml ANCA or normal IgG and 200 µg/ml IgG4-ANCA. Neutrophils were cultured for 18 h in medium supplemented with 400 U/ml IFN-γ or freshly isolated from the same donors. Results are expressed as nmol/l ×105 cells superoxide measured at 100 min. Data shown are mean ± s.e.m. for experiments performed in triplicate on three neutrophil donors.
Activation of primed human neutrophils with deglycosylated IgG-ANCA
Samples of IgG isolated from serum P3 and P8 and normal (control) IgG were subjected to sham and active deglycosylation. Glycosylation of IgG is necessary for activation of FcγRII and FcγRIII, but not FcγRI. Analysis by sodium dodecyl sulphate-polyacryamide gel electrophoresis (SDS-PAGE) confirmed deglycosylation of the sample exposed to PNGase F. While the P3 and P8 IgG-ANCA samples and their sham deglycosylated equivalents provoked superoxide production, the deglycosylated IgG did not (Table 4), suggesting that FcγRII and/or FcγRIII, but not FcγRI, was ligated.
Table 4.
Mean superoxide production stimulated by unfractionated ANCA IgG, deglycosylated ANCA IgG and sham deglycosylated ANCA IgG samples in fresh, primed human neutrophils. Superoxide production was measured in response to 100 µg/ml of all IgG samples. Data are shown at 100 min ± standard error. Results are expressed as nmol/1 × 105 cells. Experiments performed in triplicate on three neutrophil donors
| Sample | Superoxide production at 100 min (nm) |
|---|---|
| P8 IgG | 6·3 (± 1·3) |
| P8 IgG sham deglycosylation | 6·5 (± 1·4) |
| P8 IgG deglycosylated | 0·7 (± 0·9) |
| P3 IgG | 2·3 (± 1·0) |
| P3 IgG sham deglycosylation | 2·6 (± 0·9) |
| P3 IgG deglycosylated | 0·7 (± 0·7) |
| Normal IgG | 0·7 (± 0·4) |
| Normal IgG sham deglycosylation | 1·2 (± 0·2) |
| Normal IgG deglycosylated | 0·1 (± 0·2) |
DISCUSSION
We are engaged in a detailed study of parameters contributing to ANCA activation of human neutrophils. A number of sera from patients with active WG were screened and a panel of 14 were selected that contained PR3-ANCA but not MPO-ANCA. In the present report we demonstrate that IgG4 PR3-ANCA has the ability to activate cytokine-primed neutrophils. This finding is difficult to explain in terms of the consensus view that PR3-ANCA activates neutrophils following binding to surface PR3 antigen and co-ligation of FcγRIIa and/or FcγRIIIb as IgG4 is reported to bind FcγRI and not FcγRIIa/FcγRIIIb. However, close scrutiny of the literature provides evidence of numerous well-defined systems in which, unexpectedly, it has been concluded that IgG4 may act though FcγRII and/or FcγRIII receptors; these interactions may be independent of, or aided by, the presence of complement [24,27].
Initially we identified three sera (P2, P8 and P3) with high titre PR3-ANCA of IgG1, IgG3 or IgG4 subclass, respectively. Polyclonal IgG subclass fractions were isolated from patient sera by affinity purification. All IgG fractions were shown to retain PR3-ANCA activity, as determined by ELISA, and the IgG subclass fraction shown to be >95% pure, with respect to subclass composition. The IgG1-ANCA, IgG3-ANCA and IgG4-ANCA were each shown to activate primed neutrophils with the production of superoxide anion. The activity of the IgG1 and IgG3 ANCA fractions is consistent with proposed mechanisms of neutrophil activation by PR3-ANCA, namely PR3 engagement and co-ligation of FcγRIIa and/or FcγRIIIb. However, the activity of the IgG4 fraction was unexpected. To evaluate the generality of these findings, four sera that gave positive IgG4-ANCA titres in ELISA were subject to IgG4 subclass affinity purification. The individual IgG4-subclass fractions activated primed neutrophils. Interestingly the activating potential of isolated IgG4 sample P7 was greater than that of the original unfractionated ANCA-IgG, showing that the PR3-ANCA IgG subclass titre is not a guide to functionality, indeed this was also so of the unfractionated PR3-ANCA IgG samples. This may relate to affinity of PR3-ANCA or competition for epitopes by polyclonal PR3-ANCA and purifying the IgG subclasses may have reduced this, helping to establish which subclasses are contributing to ANCA activity. In vivo, antibody/antigen ratios and complement activating ability may also affect the functional outcome [24].
IgG4 is known to bind and activate FcγRI and there are reports that neutrophils may express FcγRI constitutively, at very low levels, and that FcγRI is induced/µp-regulated following culture of neutrophils with G-CSF or IFN-γ [36]. To explore the possibility that IgG4-ANCA were activating primed neutrophils by ligation of FcγRI, the expression of FcγRI on freshly isolated primed neutrophils and on neutrophils following overnight culture in the presence of IFN-γ was evaluated by flow cytometry. Overnight culture of neutrophils is known to promote apoptosis, which could affect neutrophil viability and their ability to be activated, so expression of annexin V, a marker of apoptotic cells, was also tested. In addition, equal numbers of live neutrophils were employed for the activation studies. PR3 expression was up-regulated following priming with TNFα for 15 min as expected, but FcγRI expression was at background levels. FcγRI expression was unaffected by priming or overnight culture in the absence of IFN-γ but was induced significantly on culture in the presence of IFN-γ. Despite these increases in FcγRI expression, the levels of superoxide production from viable neutrophils cultured overnight with IFN-γ and primed with TNF-α were less (with P3) or unchanged (with pooled IgG4 samples) than for fresh primed neutrophils. These data do not support a role for FcγRI in IgG4 PR3-ANCA activation of primed neutrophils. Further, deglycosylation of PR3-ANCA IgG abolished the ability to induce a respiratory burst suggesting that IgG4-ANCA can ligate FcγRIIa/FcγRIIIb but not FcγRI. These results demonstrate clearly that polyclonal IgG4-ANCA isolated from the sera of some WG patients can activate neutrophils to produce a superoxide response, reproducibly and independent of neutrophil donor.
If IgG4-ANCA in common with IgG1-ANCA and IgG3-ANCA can ligate FcγRIIIb on neutrophils, then yet other properties may determine the biological outcome. For example, the extent of fucosylation [37] and/or galactosylation and sialylation [38] of the IgG antibody, the glycosylation status of the FcγRIII [39] or FcγRIII polymorphisms that may influence FcγRIII engagement and activation [38,40]. Numerous other parameters could be added including the allotype of the antibody, the epitope target and the antigen on which it is expressed. How such variables interact with IgG4 has yet to be determined. ANCA may also interact with monocytes [41] and these cells express FcγRIIIa. There is some evidence that IgG4 is bound by only the FcγRIIIa-158 V isoform [42,43]. This difference in binding affinity also appears to translate to alterations in IgG4-mediated effector functions, with IgG4-mediated antibody-dependent cellular cytotoxicity reactions occurring only in those individuals that carry the FcγRIIIa-158 V allele (A. W. Morgan & J. D. Isaacs, unpublished observations).
In conclusion, we predict that the mere presence of PR3-ANCA in the serum of a patient with Wegener's granulomatosis will not predict detailed pathogenic potential of the autoantibody, as multiple factors, including IgG subclass and epitope specificity, will determine this.
Acknowledgments
M. Holland was supported by the Medical Research Council.
REFERENCES
- 1.Savage COS. Nephrology forum. ANCA-related renal disease. Kidney Int. 2001;60:1614–27. doi: 10.1046/j.1523-1755.2001.00962.x. [DOI] [PubMed] [Google Scholar]
- 2.Boomsma MM, Stegeman CA, van der Leij M, et al. Prediction of relapses in Wegener's granulomatosis by measurement of antineutrophil cytoplasmic antibody levels: a prospective study. Arthritis Rheum. 2000;43:2025–33. doi: 10.1002/1529-0131(200009)43:9<2025::AID-ANR13>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 3.Jayne DR, Gaskin G, Pusey CD, Lockwood CM. ANCA and predicting relapse in systemic vasculitis. Q J Med. 1995;88:127–33. [PubMed] [Google Scholar]
- 4.Gaskin G, Pusey CD. Plasmapheresis in antineutrophil cytoplasmic antibody-associated systemic vasculitis. Ther Apher. 2001;5:176–81. [PubMed] [Google Scholar]
- 5.Kettritz R, Jennette JC, Falk RJ. Crosslinking of ANCA antigens stimulates superoxide release by human neutrophils. J Am Soc Nephrol. 1997;8:386–94. doi: 10.1681/ASN.V83386. [DOI] [PubMed] [Google Scholar]
- 6.Keogan MT, Esnault VLM, Green AJ, Lockwood CM, Brown DL. Activation of normal neutrophils by anti-neutrophil cytoplasm antibodies. Clin Exp Immunol. 1992;90:228–34. doi: 10.1111/j.1365-2249.1992.tb07934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Radford DJ, Lord JM, Savage COS. The activation of the neutrophil respiratory burst by anti-neutrophil cytoplasm antibody (ANCA) from patients with systemic vasculitis requires tyrosine kinases and protein kinase C activation. Clin Exp Immunol. 1999;118:171–9. doi: 10.1046/j.1365-2249.1999.01043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Porges AJ, Redecha PB, Kimberly WT, Csernok E, Gross W, Kimberly RP. Anti-neutrophil cytoplasmic antibodies engage and activate human neutrophils via FcγRIIa. J Immunol. 1994;153:1271–80. [PubMed] [Google Scholar]
- 9.Mulder AHL, Heeringa C, Brouwer E, Limburg PC, Kallenberg CGM. Activation of granulocytes by anti-neutrophil cytoplasmic antibodies (ANCA): a FcγRII-dependent process. Clin Exp Immunol. 1994;98:270–8. doi: 10.1111/j.1365-2249.1994.tb06137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ben-Smith A, Dove SK, Martin A, Wakelam MJO, Savage COS. Autoantibodies from patients with systemic vasculitis activate primed neutrophils via Fcγ receptor dependent pathways. Blood. 2001;98:1448–55. doi: 10.1182/blood.v98.5.1448. [DOI] [PubMed] [Google Scholar]
- 11.Buckle AM, Jayaram Y, Hogg N. Colony-stimulating factors and interferon-gamma differentially affect cell surface molecules shared by monocytes and neutrophils. Clin Exp Immunol. 1990;81:339–45. doi: 10.1111/j.1365-2249.1990.tb03342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campbell EJ, Campbell MA, Owen CA. Bioactive proteinase 3 on the cell surface of human neutrophils: quantification, catalytic activity and susceptibility to inhibition. J Immunol. 2000;165:3366–74. doi: 10.4049/jimmunol.165.6.3366. [DOI] [PubMed] [Google Scholar]
- 13.Csernok E, Ernst M, Schmitt W, Bainton DF, Gross WL. Activated neutrophils express proteinase 3 on their plasma membrane in vitro and in vivo. Clin Exp Immunol. 1994;95:244–50. doi: 10.1111/j.1365-2249.1994.tb06518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hellmich B, Csernok E, Trabandt T, Gross WL, Ernst M. Granulocyte-macrophage colony stimulating factor (GM-CSF) but not granulocyte colony stimulating factor (G-CSF) induced plasma membrane expression of proteinase 3 (PR3) on neutrophils in vitro. Clin Exp Immunol. 2000;120:392–8. doi: 10.1046/j.1365-2249.2000.01205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deguchi Y, Shibata N, Kishimoto S. Enhanced expression of the tumour necrosis factor/cachectin gene in peripheral blood mononuclear cells from patients with systemic vasculitis. Clin Exp Immunol. 1990;81:311–4. doi: 10.1111/j.1365-2249.1990.tb03336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harper L, Cockwell P, Adu D, Savage COS. Neutrophil priming and apoptosis in ANCA-associated vasculitis. Kidney Int. 2001;59:1729–38. doi: 10.1046/j.1523-1755.2001.0590051729.x. [DOI] [PubMed] [Google Scholar]
- 17.Jayne DRW, Weetman AP, Lockwood CM. IgG subclass distribution of autoantibodies to neutrophil cytoplasmic antigens in systemic vasculitis. Clin Exp Immunol. 1991;84:476–81. [PMC free article] [PubMed] [Google Scholar]
- 18.Brouwer E, Cohen Tervaert JW, Horst G, et al. Predominance of IgG1 and IgG4 subclasses of anti-neutrophil cytoplasmic autoantibodies (ANCA) in patients with Wegener's granulomatosis and clinically related disorders. Clin Exp Immunol. 1991;83:379–86. doi: 10.1111/j.1365-2249.1991.tb05647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Locke IC, Leaker B, Cambridge G. A comparison of the characteristics of circulating anti-myeloperoxidase autoantiboides in vasculitis with those in non-vasculitic conditions. Clin Exp Immunol. 1999;115:369–76. doi: 10.1046/j.1365-2249.1999.00809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Esnault VLM, Jayne DRW, Weetman AP, Lockwood CM. IgG subclass distribution and relative functional affinity of anti-myeloperoxidase antibodies in systemic vasculitis at presentation and during follow-up. Immunology. 1991;74:714–8. [PMC free article] [PubMed] [Google Scholar]
- 21.Mulder AHL, Stegeman CA, Kallenberg CGM. Activation of granulocytes by anti-neutrophil cytoplasmic antibodies (ANCA) in Wegener's granulomatosis: a predominant role for the IgG3 subclass of ANCA. Clin Exp Immunol. 1995;101:227–32. doi: 10.1111/j.1365-2249.1995.tb08343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nowack R, Grab I, Flores-Suarez L-F, Schnulle P, Yard B, Van der Woude FJ. ANCA titres, even of IgG subclasses, and soluble CD14 fail to predict relapses in patients with ANCA-associated vasculitis. Nephrol Dial Transplant. 2001;16:1631–7. doi: 10.1093/ndt/16.8.1631. [DOI] [PubMed] [Google Scholar]
- 23.Harper L, Radford D, Plant T, Drayson M, Adu D, Savage COS. IgG from MPO-ANCA positive patients stimulates greater activation of primed neutrophils than IgG from PR3-ANCA positive patients. Arthritis Rheum. 2001;44:921–30. doi: 10.1002/1529-0131(200104)44:4<921::AID-ANR149>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 24.Valim YM, Lachmann PJ. The effect of antibody isotype and antigenic epitope density on the complement-fixing ability of immune complexes: a systematic study using chimeric anti-NIP antibodies with human Fc regions. Clin Exp Immunol. 1991;84:1–8. doi: 10.1111/j.1365-2249.1991.tb08115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Voice KJ, Lachmann PJ. Neutrophil Fc gamma and complement receptors involved in binding soluble IgG immune complexes and in specific granule release induced by soluble IgG imuune complexes. Eur J Immunol. 1997;27:2514–23. doi: 10.1002/eji.1830271008. [DOI] [PubMed] [Google Scholar]
- 26.Greenwood J, Clark M, Waldmann H. Structural motifs involved in human IgG antibody effector functions. Eur J Immunol. 1993;23:1098–104. doi: 10.1002/eji.1830230518. [DOI] [PubMed] [Google Scholar]
- 27.Aalberse RC, Schuurman J. IgG4 breaking the rules. Immunology. 2001;105:9–19. doi: 10.1046/j.0019-2805.2001.01341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jennette JC, Falk RJ, Andrassy K, et al. Nomenclature of systemic vasculitides: the proposal of an International Consensus Conference. Arthritis Rheum. 1994;37:187–92. doi: 10.1002/art.1780370206. [DOI] [PubMed] [Google Scholar]
- 29.Savige J, Gillis D, Davies D, et al. International Consensus Statement on testing and reporting of antineutrophil cytolasmic antibodies (ANCA) Am J Clin Pathol. 1999;111:507–13. doi: 10.1093/ajcp/111.4.507. [DOI] [PubMed] [Google Scholar]
- 30.Jefferis R, Reimer CB, Skvaril F, et al. Evaluation of monoclonal antibodies having specificity for human IgG subclasses: results of IUIS/WHO collaborative study. Immunol Lett. 1985;10:223–52. doi: 10.1016/0165-2478(85)90082-3. [DOI] [PubMed] [Google Scholar]
- 31.Grange J, Roch AM, Quash GA. Nephelometric assay of antigens and antibodies with latex particles. J Immunol Meth. 1977;18:365–75. doi: 10.1016/0022-1759(77)90190-9. [DOI] [PubMed] [Google Scholar]
- 32.Ling NR, Bishop S, Jefferis R. Use of antibody-coated red cells for the sensitive detection of antigen and in rosette tests for cells bearing surface immunoglobulins. J Immunol Meth. 1977;15:279–89. doi: 10.1016/0022-1759(77)90065-5. [DOI] [PubMed] [Google Scholar]
- 33.Holland M, Takada K, Okomoto T, et al. Hypogalatosylation of serum IgG in patients with ANCA-associated systemic vasculitis. Clin Exp Immunol. 2002;129:183–90. doi: 10.1046/j.1365-2249.2002.01864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Halbwachs-Mecarelli L, Bessou G, Lesavre P, Lopez S, Witko-Sarsat V. Bimodal distribution of Pr3 surface expression reflects a constitutive heterogeneity in the PMN pool. FEBS Lett. 1995;374:29–33. doi: 10.1016/0014-5793(95)01073-n. [DOI] [PubMed] [Google Scholar]
- 35.Van Rossum AP, Limburg PC, Kallenberg CGM. Membrane proteinase 3 expression on resting neutrophils as a pathogenic factor in PR3-ANCA-associated vasculitis. Clin Exp Immunol. 2003;21(Suppl. 32):S64–S68. [PubMed] [Google Scholar]
- 36.Buckle AM, Hogg N. The effect of IFN-γ and colony stimulating factor on the expression of neutorphil cell membrane receptors. J Immunol. 1989;143:2295–301. [PubMed] [Google Scholar]
- 37.Shinkawa T, Nakamura K, Yamane N, et al. The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. J Biol Chem. 2003;278:3466–73. doi: 10.1074/jbc.M210665200. [DOI] [PubMed] [Google Scholar]
- 38.Kumpel BM, De Haas M, Koene HR, Van De Winkel JG, Goodrick MJ. Clearance of red cells by monoclonal IgG3 anti-D in vivo is affected by the VF polymorphism of Fcgamma RIIIa (CD16) Clin Exp Immunol. 2003;132:81–6. doi: 10.1046/j.1365-2249.2003.02119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Galon J, Robertson MW, Galinha A, et al. Affinity of the interaction between Fc gamma receptor type III (Fc gamma RIII) and monomeric human IgG subclasses. Role of Fc gamma RIII glycosylation. Eur J Immunol. 1997;27:1928–32. doi: 10.1002/eji.1830270816. [DOI] [PubMed] [Google Scholar]
- 40.Cartron G, Dacheux L, Salles G, et al. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood. 2002;99:754–8. doi: 10.1182/blood.v99.3.754. [DOI] [PubMed] [Google Scholar]
- 41.Ralston DR, Marsh CB, Lowe MP, Wewers MD. Antineutrophil cytoplasmic antibodies induce monocyte IL-8 release. J Clin Invest. 1997;100:1416–24. doi: 10.1172/JCI119662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu J, Edberg JC, Redecha PB, et al. A novel polymorphism of FcgRIIIa (CD16) alters receptor function and predisposes to autoimmune disease. J Clin Invest. 1997;100:1059–70. doi: 10.1172/JCI119616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koene HR, Kleijer M, Algra J, von Roos D, dem Borne AEGK, de Haas M. FcγRIIIa-158V/F polymorphism influences the binding of IgG by natural killer cell FcγRIIIa, independently of the FcγRIIIa-48L/R/H phenotype. Blood. 1997;90:1109–14. [PubMed] [Google Scholar]
- 44.Heijnen IA, van de Winkel JG. Human IgG Fc receptors. Int Rev Immunol. 1997;16:29–55. doi: 10.3109/08830189709045702. [DOI] [PubMed] [Google Scholar]