CYTOTOXICITY IN THE COMPLEX REALM OF CD4+ T-CELLS
From the textbooks, one can readily learn that CD4+ T-cells display a variety of helper functions, necessary for the mounting of an efficient adaptive immune response. Indeed, for about two decades, the study of CD4+ T-cells has been mainly focused on their polarization into T helper 1 or T helper 2 cells and the role of these subpopulations in cellular and humoral immunity, respectively; so that CD4+ T-cells have commonly been referred to as helper T-cells. Nonetheless, the tendency may be changing: with new subsets and new functions being ascribed to CD4+ T-cells, the emphasis on Th1/Th2 studies seems on the decline. Suppressor or regulatory CD4+ T-cells raise the strongest interest nowadays: their role in regulating T-cell proliferation comes out as being particularly important and the perspective to manipulate the activity of these cells may offer new hopes of improving immune based therapies (e.g. immune responses to vaccines).
In addition, over the past two decades, many mouse and human studies have reported the acquisition of lytic capacity by CD4+ T-cells [1–9]. However, these observations were usually restricted to cell lines and CD4+ T-cell clones generated by in vitro culture, and have therefore raised much disregard, sceptics arguing that such cytotoxic CD4+ T-cells represent an in vitro artefact and have no physiological role. Interestingly, isolated but repeated reports have recently described the presence of cytotoxic CD4+ T-cells detected directly from peripheral blood, i.e. in vivo, in various human pathologies like viral infections (HIV, CMV and EBV) [10,11], rheumatoid arthritis [12], ankylosing spondylitis [13] and B-cell chronic lymphocytic leukaemia [14]. Healthy individuals seem to display few of these cells (on average no more than 2% of the whole CD4+ T-cell population), in contrast, up to 50% percent of the CD4+ T-cells in some HIV infected donors for instance can exhibit clear cytotoxic potential. Studies of clones and cell lines have suggested that CD4+ CTLs use the perforin-dependent cytotoxic mechanism, rather than the Fas-dependent pathway [8,15–17]. In keeping with these data, ex vivo (i.e. directly from blood) analysis of cytotoxic CD4+ T-cells indicate that they have lytic granules containing cytotoxic factors such as granzymes and perforin, and that their lytic activity is HLA class II restricted [10–14]. It is therefore important to appreciate that these cells are not merely an in vitro artefact.
CYTOTOXIC CD4+ T-CELLS AND DIFFERENTIATION
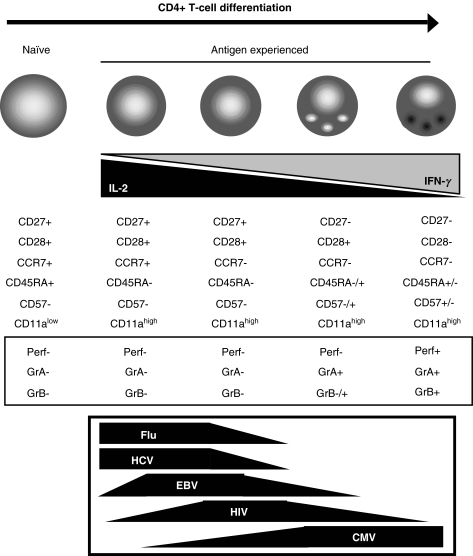
The existence of cytotoxic CD4+ T-cells in humans raises obvious questions concerning their nature and their role. There is no evidence to suggest that these CD4+ CTL are either derived from CD8+ T-cells or belong to some family of NK like cells or T regulatory cells. Instead, they seem to represent a subset of antigen experienced or memory cells, as indicated by their phenotype (CD11ahigh, CCR7–), as well as their reactivity for CMV antigens in particular, but also HIV antigens [10,11]. Interestingly, cytotoxic CD4+ T-cells are characterized by a loss of CD27 surface expression, as well as CD28, but gain of CD57, an overall phenotype that indicates an advanced stage of cellular differentiation. Increasing interest has recently been granted to the process of differentiation or post-thymic development of CD4+ T-cells, and several investigators seem to agree with a model of CD4+ T-cell differentiation characterized by a sequential down-regulation of CCR7, CD27 and then CD28, accompanied by changes of their functional characteristics [18–22] (Fig. 1). This makes an intriguing parallel with the process of CD8+ T-cell post-thymic development [20], all the more because, like in the case of CD8+ T-cells [23], virus specific CD4+ T-cells have been reported to exhibit distinct differentiation phenotypes in different infections [18,19,21,24,25]: Flu, HCV, EBV and HIV specific CD4+ T-cells are less differentiated than CMV specific CD4+ T-cells (Fig. 1). While the cytotoxic potential of CD8+ T-cells increases with differentiation, CD4+ T-cells gain such potential, with the acquisition of lytic granules with granzymes, as they lose CD27 expression, and acquisition of perforin at the CD28 negative stage, so that highly differentiated CD4+ T-cells become cytotoxic. It will be interesting to study the mechanisms of CD4+ T-cell cytolytic activity, for instance to see if this activity does not require the formation of a stable mature immunological synapse, but only of an early or lytic synapse with a low stimulation threshold, as this has recently been shown for CD8+ T-cells [26,27].
Fig. 1.
Phenotypic evolution of CD4+ T-cells along a hypothetical course of post-thymic development and distribution of virus specific cells along this process
The presence of highly differentiated CD4+ CTL seems to correlate with conditions of strong or chronic activation like in infections with CMV, EBV, or HIV, or in cases of rheumatoid arthritis. Immune activation seems therefore to be a major driving factor of CD4+ T-cell differentiation, as it is actually known to be for CD8+ T-cells [28,29]. This may provide the explanation for the common observations of CD4+ CTL in cultured cell lines and clones, likely due to the in vitro conditions of prolonged stimulation and proliferation that drive further differentiation of CD4+ T-cells and the acquisition of lytic potential. Overall, these findings portray CD4+ CTLs as highly differentiated antigen experienced CD4+ T-cells driven to this stage through activation.
THE PHYSIOLOGICAL ROLE OF CYTOTOXIC CD4+ T-CELLS
Nonetheless, the identification and characterization of CD4+ CTLs in humans have not permitted to understand their role yet. The limitation of available tools (such as class II tetramers) and identified class II restricted epitopes renders the study of CD4+ T-cells more complex than it has been for CD8+ T-cells, so that it is currently only possible to speculate as regards the physiological role of CD4+ CTLs. Obviously, it is tempting to hypothesize that they may play a role in containing viruses which infect HLA class II expressing cells (e.g. EBV in B cells or HIV in activated CD4+ T-cells), or that they would preferentially target the class II antigen presentation pathway, in infections with viruses such as HIV, EBV and CMV, which can prevent normal MHC-class I expression in order to escape immune recognition by CD8+ T-cells. However, it may also be possible that these cells represent a by-product resulting from inflammation and elevated activation, exhibiting increasing characteristics of replicative senescence and demonstrating cytotoxic potential for reasons still unclear.
The identification of similar cells in the mouse would be an important advance, enabling the elaboration of diverse strategies to uncover their role. The study of CD8+ T-cells in mouse models has provided considerable information as regards the role of CD8+ T-cell subsets in protective immunity, although a complete analogy between human and mouse remains to be reached. In the present issue of Clinical and Experimental Immunology, Lyadova et al. [30] have identified CD27- CD4+ T-cells in the mouse, which may represent highly differentiated cells and play a particular role in the immune response as postulated by the authors. These cells produce increased levels of IFNγ and their presence in the lung is related to infection with M. bovis BCG or M. tuberculosis. It is likely that the inflammatory conditions induced by these pathogens have resulted in the differentiation of CD4+ T-cells. These cells may represent an equivalent to highly differentiated CD4+ T-cells in humans. In addition of their importance in the context of mycobacteria immune control, the findings by Lyadova et al. could be the first step towards a better understanding of the role of CD4+ T-cell differentiated subsets, in particular CD4+ CTL. It will be interesting to determine if murine CD27- CD4+ T-cells exhibit lytic granules and a cytotoxic potential, for instance using new tools available in mouse, such as the marker of granules and degranulation CD107a as well as the cytotoxic factor granzyme B [31]. Depletion experiments or adoptive transfer experiments of CD27- CD4+ T-cells into infected mice (e.g. transgenic CD27 knockout mouse) may then shed light as regards the importance of these cells in controlling pathogens.
CONCLUSION
The role of CD4+ T-cells in immunity is complex as exemplified by their multiple functions. Evidence now shows that CD4+ CTL exist in vivo in humans, which challenges our ordinary view of CD4+ helper T-cells in contrast to CD8+ killer T-cells. What their role and its importance is in the immune response remain totally unclear. Nonetheless, the study of CD4+ T-cell subsets of differentiation and the identification of analogous CD4+ CTL in the mouse should provide the means to get some answers. When the existence of suppressor CD4+ T-cells was first reported, much skepticism was raised; 10 years later, these cells raise the strongest interest. Is the same fate awaiting cytotoxic CD4+ T-cells? The answer is yet to come.
REFERENCES
- 1.Krensky AM, Reiss CS, Mier JW, et al. Long-term human cytolytic T-cell lines allospecific for HLA-DR6 antigen are OKT4+ Proc Natl Acad Sci USA. 1982;79:2365–9. doi: 10.1073/pnas.79.7.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fleischer B. Acquisition of specific cytotoxic activity by human T4+ T lymphocytes in culture. Nature. 1984;308:365–7. doi: 10.1038/308365a0. [DOI] [PubMed] [Google Scholar]
- 3.Lukacher AE, Morrison LA, Braciale VL, et al. Expression of specific cytolytic activity by H-2I region-restricted, influenza virus-specific T lymphocyte clones. J Exp Med. 1985;162:171–87. doi: 10.1084/jem.162.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCarthy SA, Singer A. Recognition of MHC class I allodeterminants regulates the generation of MHC class II-specific CTL. J Immunol. 1986;137:3087–92. [PubMed] [Google Scholar]
- 5.Man S, Lechler RI, Batchelor JR, et al. Individual variation in the frequency of HLA class II-specific cytotoxic T lymphocyte precursors. Eur J Immunol. 1990;20:847–54. doi: 10.1002/eji.1830200420. [DOI] [PubMed] [Google Scholar]
- 6.Huang Z, Vafai A, Lee J, et al. Specific lysis of targets expressing varicella-zoster virus gpI or gpIV by CD4+ human T-cell clones. J Virol. 1992;66:2664–9. doi: 10.1128/jvi.66.5.2664-2669.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Littaua RA, Oldstone MB, Takeda A, et al. A CD4+ cytotoxic T-lymphocyte clone to a conserved epitope on human immunodeficiency virus type 1 p24: cytotoxic activity and secretion of interleukin-2 and interleukin-6. J Virol. 1992;66:608–11. doi: 10.1128/jvi.66.1.608-611.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Norris PJ, Sumaroka M, Brander C, et al. Multiple effector functions mediated by human immunodeficiency virus- specific cd4 (+) t-cell clones. J Virol. 2001;75:9771–9. doi: 10.1128/JVI.75.20.9771-9779.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mahon BP, Katrak K, Nomoto A, et al. Poliovirus-specific CD4+ Th1 clones with both cytotoxic and helper activity mediate protective humoral immunity against a lethal poliovirus infection in transgenic mice expressing the human poliovirus receptor. J Exp Med. 1995;181:1285–92. doi: 10.1084/jem.181.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Appay V, Zaunders JJ, Papagno L, et al. Characterization of CD4(+) CTLs ex vivo. J Immunol. 2002;168:5954–8. doi: 10.4049/jimmunol.168.11.5954. [DOI] [PubMed] [Google Scholar]
- 11.Zaunders JJ, Dyer WB, Wang B, et al. Identification of circulating antigen-specific CD4+ T lymphocytes with a CCR5+, cytotoxic phenotype in an HIV-1 long-term nonprogressor and in CMV infection. Blood. 2004;103:2238–47. doi: 10.1182/blood-2003-08-2765. [DOI] [PubMed] [Google Scholar]
- 12.Namekawa T, Wagner UG, Goronzy JJ, et al. Functional subsets of CD4 T cells in rheumatoid synovitis. Arthritis Rheum. 1998;41:2108–16. doi: 10.1002/1529-0131(199812)41:12<2108::AID-ART5>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 13.Duftner C, Goldberger C, Falkenbach A, et al. Prevalence, clinical relevance and characterization of circulating cytotoxic CD4+CD28- T cells in ankylosing spondylitis. Arthritis Res Ther. 2003;5:R292–300. doi: 10.1186/ar793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Porakishvili N, Kardava L, Jewell AP, et al. Cytotoxic CD4+ T cells in patients with B cell chronic lymphocytic leukemia kill via a perforin-mediated pathway. Haematologica. 2004;89:435–43. [PubMed] [Google Scholar]
- 15.Williams NS, Engelhard VH. Identification of a population of CD4+ CTL that utilizes a perforin- rather than a Fas ligand-dependent cytotoxic mechanism. J Immunol. 1996;156:153–9. [PubMed] [Google Scholar]
- 16.Echchakir H, Bagot M, Dorothee G, et al. Cutaneous T cell lymphoma reactive CD4+ cytotoxic T lymphocyte clones display a Th1 cytokine profile and use a fas-independent pathway for specific tumor cell lysis. J Invest Dermatol. 2000;115:74–80. doi: 10.1046/j.1523-1747.2000.00995.x. [DOI] [PubMed] [Google Scholar]
- 17.Yasukawa M, Ohminami H, Arai J, et al. Granule exocytosis, and not the fas/fas ligand system, is the main pathway of cytotoxicity mediated by alloantigen-specific CD4(+) as well as CD8(+) cytotoxic T lymphocytes in humans. Blood. 2000;95:2352–5. [PubMed] [Google Scholar]
- 18.Amyes E, Hatton C, Montamat-Sicotte D, et al. Characterization of the CD4+ T cell response to Epstein-Barr virus during primary and persistent infection. J Exp Med. 2003;198:903–11. doi: 10.1084/jem.20022058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yue FY, Kovacs CM, Dimayuga RC, et al. HIV-1-specific memory CD4(+) T cells are phenotypically less mature than cytomegalovirus-specific memory CD4 (+) T cells. J Immunol. 2004;172:2476–86. doi: 10.4049/jimmunol.172.4.2476. [DOI] [PubMed] [Google Scholar]
- 20.Appay V, Rowland-Jones SL. Lessons from the study of T-cell differentiation in persistent human virus infection. Semin Immunol. 2004;16:205–12. doi: 10.1016/j.smim.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 21.Harari A, Petitpierre S, Vallelian F, et al. Skewed representation of functionally distinct populations of virus-specific CD4 T cells in HIV-1-infected subjects with progressive disease: changes after antiretroviral therapy. Blood. 2004;103:966–72. doi: 10.1182/blood-2003-04-1203. [DOI] [PubMed] [Google Scholar]
- 22.Gamadia LE, Rentenaar RJ, van Lier RA, et al. Properties of CD4 (+) T cells in human cytomegalovirus infection. Hum Immunol. 2004;65:486–92. doi: 10.1016/j.humimm.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 23.Appay V, Dunbar PR, Callan M, et al. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat Med. 2002;8:379–85. doi: 10.1038/nm0402-379. [DOI] [PubMed] [Google Scholar]
- 24.Day CL, Seth NP, Lucas M, et al. Ex vivo analysis of human memory CD4 T cells specific for hepatitis C virus using MHC class II tetramers. J Clin Invest. 2003;112:831–42. doi: 10.1172/JCI18509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lucas M, Day CL, Wyer JR, et al. Ex vivo phenotype and frequency of influenza virus-specific CD4 memory T cells. J Virol. 2004;78:7284–7. doi: 10.1128/JVI.78.13.7284-7287.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Faroudi M, Utzny C, Salio M, et al. Lytic versus stimulatory synapse in cytotoxic T lymphocyte/target cell interaction: manifestation of a dual activation threshold. Proc Natl Acad Sci USA. 2003;100:14145–50. doi: 10.1073/pnas.2334336100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Purbhoo MA, Irvine DJ, Huppa JB, et al. T cell killing does not require the formation of a stable mature immunological synapse. Nat Immunol. 2004;5:524–30. doi: 10.1038/ni1058. [DOI] [PubMed] [Google Scholar]
- 28.Papagno L, Spina CA, Marchant A, et al. Immune Activation and CD8(+) T-Cell Differentiation towards Senescence in HIV-1 Infection. Plos Biol. 2004;2:E20. doi: 10.1371/journal.pbio.0020020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gamadia LE, van Leeuwen EM, Remmerswaal EB, et al. The size and phenotype of virus-specific T cell populations is determined by repetitive antigenic stimulation and environmental cytokines. J Immunol. 2004;172:6107–14. doi: 10.4049/jimmunol.172.10.6107. [DOI] [PubMed] [Google Scholar]
- 30.Lyadova IV, Oberdorf S, Kapina MA, et al. CD4 T cells producing IFN–gamma in the lungs of mice challenged with mycobacteria express CD27–negative phenotype. Clin Exp Immunol. 2004;138:21–9. doi: 10.1111/j.1365-2249.2004.02573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wolint P, Betts MR, Koup RA, et al. Immediate Cytotoxicity But Not Degranulation Distinguishes Effector and Memory Subsets of CD8+ T Cells. J Exp Med. 2004;199:925–36. doi: 10.1084/jem.20031799. [DOI] [PMC free article] [PubMed] [Google Scholar]