Abstract
Melioidosis is a life-threatening disease caused by Burkholderia pseudomallei. The lung is the most commonly affected organ, resulting in abscess formation in patients with chronic melioidosis. Previous study has shown that B. pseudomallei was able to invade and multiply in epithelial cells. In the present study, we have demonstrated that B. pseudomallei is able to stimulate interleukin 8 (IL-8) production from the human alveolar lung epithelium cell line A549. However, the level of IL-8 production was significantly lower than when the cells were infected with other Gram-negative bacteria such as Salmonella enterica serovar Typhi (S. typhi) which were used for comparison. The degree of IκBα degradation in the B. pseudomallei-infected cells was lower than that of the S. typhi-infected cells, suggesting that B. pseudomallei is also a poorer cell activator. Inhibition of B. pseudomallei invasion by cytochalasin D did not interfere with either IL-8 production or IκBα degradation, indicating that bacterial uptake is not required for the production of this chemokine. Thus, it appears that the signalling initiated by the interaction of B. pseudomallei with the epithelial cell surface is sufficient for epithelial cell activation.
Keywords: Burkholderia pseudomallei, human lung epithelial cell, IκBα, IL-8, melioidosis
INTRODUCTION
Melioidosis is an important cause of sepsis in several tropical areas, including South-east Asia and northern Australia [1,2]. In humans, the disease is usually acquired by skin inoculation or inhalation of the dust contaminated with Burkholderia pseudomallei. The clinical features vary from an acute fulminate septicaemia to chronic debilitating localized infection [1]. Abscess formation can be found in any organ, with lung as the most commonly affected organ in those with chronic melioidosis [3,4]. The patients often present themselves with cough and fever as a result of primary lung abscess or secondary to septicaemia spread.
B. pseudomallei is a facultative intracellular Gram-negative bacillus which can survive and multiply in both phagocytic and non-phagocytic cells [5]. After internalization, the bacteria can escape from membrane-bound phagosome into the cytoplasm [5,6]. Internalized B. pseudomallei can also induce a cell-to-cell fusion, resulting in a multinucleated giant cell (MNGC) formation [6,7]. This unique phenomenon, which has never been observed in any other bacteria, thus facilitates the spreading of bacterium from one cell to another [7]. The presence of MNGC has also been observed in the tissues of patients with melioidosis [8].
The mechanism by which B. pseudomallei is able to escape host defence is not fully understood. However, we have reported previously that the macrophages infected with this bacterium failed to produce inducible nitric oxide synthase (iNOS), which is a key enzyme in antibacterial activity of the macrophages [9]. Moreover, unlike other Gram-negative bacterium, including the prototype S. typhi that has been studied more extensively in the macrophages infected with B. pseudomallei, produced significantly less cytokines such as tumour necrosis factor-alpha (TNF-α) or beta interferon (IFN-β) [9,10]. These features may facilitate the bacteria to survive inside the macrophages. In contrast to the phagocytic cells, very little information is currently available for the cytokine response in the non-phagocytic cells infected with B. pseudomallei. In the present study, we investigated the production of interleukin 8 (IL-8) from B. pseudomallei-infected human lung epithelial cells.
MATERIALS AND METHODS
Bacterial isolation
B. pseudomallei strain 844 used in this study was isolated originally from a patient admitted to Srinagarind Hospital in the melioidosis-endemic Khon Kaen province of Thailand. The bacterium was identified originally as B. pseudomallei based on its biochemical characteristics, colonial morphology on selective media, antibiotic sensitivity profiles and reaction with polyclonal antibody [11–13] and was used in our previous reports [9,10]. Salmonella enterica serovar Typhi (S. typhi) used for comparative study were maintained at Ramathibodi Hospital (Mahidol University, Bangkok, Thailand) and kept as a stock culture in our laboratory. For use in the experiments, the bacteria were cultured in Trypticase soy broth at 37°C with shaking at 120 r.p.m. The overnight cultures were washed twice in phosphate-buffered saline (PBS) and adjusted to a desired concentration by measurement of the optical density at 650 nm and the colony-forming unit (CFU) was calculated from the precalibrated standard curve.
Infection of human lung epithelial cell (A549)
Human lung epithelial cell line (A549) used in the experiments was obtained from American Type Culture Collection (ATCC, Rockville, MD, USA). The cells were cultured in Ham's F-12 (HyClone, Logan, UT) supplemented with 10% fetal bovine serum (FBS) (Gibco Laboratories, Grand Island, NY, USA) at 37°C and 5% CO2 atmosphere. If not indicated otherwise, these cells (5 × 105) were cultured in a six-well plate overnight before exposure to the bacteria at a multiplicity of infection (MOI) of 10 : 1 for 2 h. To remove extracellular bacteria, the cells were washed three times with 2 ml of PBS before replacing with the medium containing 250 µg/ml kanamycin (Gibco Laboratories). At the time indicated, the cells were lysed and subjected to immunoblotting while the supernatant was used for IL-8 analysis.
Bacterial uptake (internalization)
A standard antibiotic protection assay was performed to determine the degree of bacterial internalization by human lung epithelial cell line. After the infected cells were incubated in the medium containing 250 µg/ml of kanamycin for 1 h, the cells were washed three times with PBS and intracellular bacteria were liberated by lysing the cells with 0·1% Triton X-100 and plating the released bacteria in tryptic soy agar. The number of intracellular bacteria expressed as colony forming units (CFU), was determined by bacterial colony counting.
Reverse transcriptase-polymerase chain reaction (RT-PCR)
Total RNA was extracted from the infected cells according to the manufacturer's instructions (Eppendorf, Hamburg, Germany) before being used for cDNA synthesis by cMaster RT Enzyme (Eppendorf). The PCR reaction was conducted using cDNA as template for IL-8 and actin and amplified by Taq DNA polymerase (Invitrogen, Carlsbad, CA, USA). The primers for IL-8 were: sense 5′-ATG ACT TCC AAG CTG GCC GTG GCT-3′, antisense 5′-TCT CAG CCC TCT TCA AAA ACT TCT C-3′ and those for actin were: sense 5′-TGG CAT TGT TAC CAA CTG GGA CG-3′, antisense 5′-GCT TCT CTT TGA TGT CAC GCA CG-3′. The amplified products were electrophoresed on 1·8% agarose gel and stained with ethidium bromide before being visualized under an ultraviolet lamp.
Immunoblotting
At time intervals, the infected cells were lysed in buffer containing 20 m m Tris, 100 m m NaCl and 1% NP40. The lysates containing 30 µg of protein were electrophoresed using 10% polyacrylamide and were then electrotransferred onto nitrocellulose membrane (Schleicher & Schuell, Dassel, Germany). The membrane was blocked with 5% milk for 1 h before incubating overnight with polyclonal rabbit antibody to IκBα (Santa Cruz, Santa Cruz, CA, USA). Blots were then reacted with horseradish peroxidase-conjugated swine antirabbit IgG (Dako, Glostrup, Denmark). Protein bands were detected with an enhanced chemiluminescence kit (Roche Diagnostic, Mannheim, Germany).
Enzyme-linked immunosorbent assay (ELISA)
The concentrations of IL-8 in the supernatant of infected lung epithelial cells were measured using an ELISA kit (BD Biosciences, San Diego, CA, USA). The sensitivity of the assay system was 50 U/ml.
Statistical analysis
If not indicated otherwise, all experiments carried out in this study were conducted at least three times. The data shown are representative results. Experimental values are expressed as mean ± standard errors. The statistical significance of differences between two means was evaluated by Student's t-test, and a P-value ≤0·01 was considered significant.
RESULTS
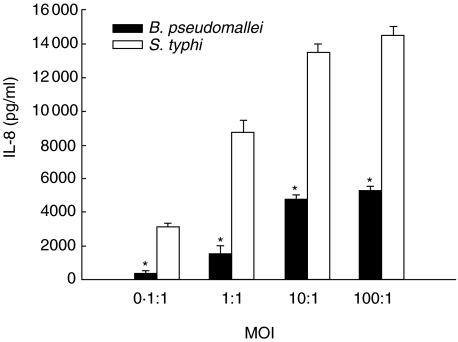
The quantity of IL-8 produced by the cells infected with either B. pseudomallei or S. typhi correlated with the MOI used (Fig. 1). However, comparing with S. typhi, the level of IL-8 induced by B. pseudomallei was significantly lower (Fig. 1). Kinetics of the IL-8 production from the cells infected with both bacteria was also similar to one another. Results presented in Fig. 2a show that the level of IL-8 increased gradually before reaching the maximum at 6 h. It should be mentioned that the viability of the cells infected with either bacterium, judged by tryphan blue staining, were more than 90% throughout the time-course of the experiments (data not shown).
Fig. 1.
IL-8 production by infected human alveolar lung epithelial cell line (A549). The cells (5 × 105 cells/well) were infected for 2 h with either B. pseudomallei or S. typhi at MOI of 0·1 : 1, 1 : 1, 10 : 1 and 100 : 1. The infected cells were washed with PBS three times before fresh culture medium containing 250 µg/ml of kanamycin was added and cultured for 8 h. The supernatants were collected and used for IL-8 analysis by ELISA. Data represent mean and standard errors of 3 separate experiments, each carried out in duplicate. *P ≤ 0·01 by Student's t-test.
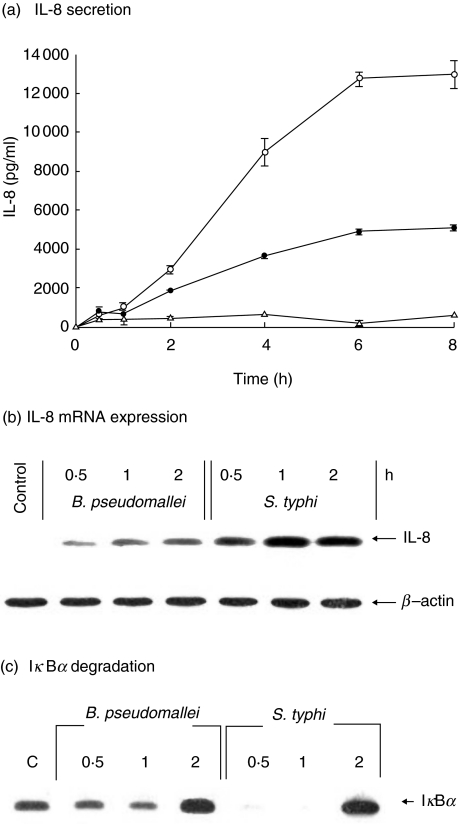
Fig. 2.
Kinetics of IL-8 production, IL-8 mRNA expression and IκBα degradation. The cells (5 × 105 cells/well) were infected with either B. pseudomallei (•) or S. typhi (○) at an MOI of 10 : 1. Uninfected cells were used as control (▵). At times indicated, the supernatants were collected and analysed for IL-8 by ELISA (a). The cells were then lysed and used for analysis of IL-8 mRNA expression by RT-PCR (b) or for IκB degradation by immunoblotting (c). Data represent mean and standard errors of three separate experiments, each carried out in duplicate.
The expression of IL-8 mRNA from the bacterial infected cells could be detected as early as 30 min of infection before reaching a maximum at 1 h (Fig. 2b). In parallel with the ELISA results above, the level of IL-8 mRNA expression in the S. typhi-infected cells was considerably higher than in the B. pseudomallei-infected cells performed at the same MOI. In accord with these observations, the level of IκBα degradation was also higher in the S. typhi-infected cells, suggesting that S. typhi was a stronger cell activator. The results presented in Fig. 2c showed that the IκBα in S. typhi-infected cells was degraded after 30 min and then reappeared after 2 h of infection.
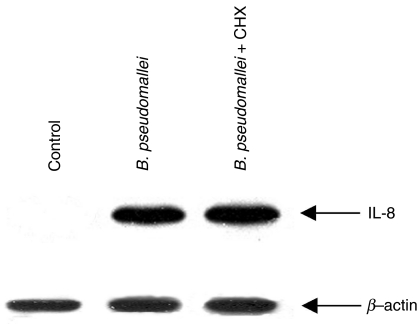
Several other cytokines have been reported to be produced by epithelial cells. Some of these are known to be able to stimulate IL-8 production from the epithelial cell [14]. In order to rule out the possibility that the IL-8 detected in B. pseudomallei-infected cells might have been stimulated by other cytokines produced by these infected cells, these cells were pretreated with cyclohexamide to inhibit protein synthesis for 2 h before being infected with B. pseudomallei. The infected cells were then lysed and analysed for IL-8 mRNA expression. The results in Fig. 3 showed that under this condition, the B. pseudomallei-infected cells were still able to up-regulate IL-8 mRNA expression, suggesting that B. pseudomallei was able to directly initiate the IL-8 production.
Fig. 3.
Effects of cyclohexamide on the expression of IL-8 mRNA in B. pseudomallei-infected cells. The cells were pretreated with cyclohexamide (5 µg/ml) for 2 h before being infected with the bacteria at MOI of 10 : 1. After 2 h of incubation, the infected cells were washed with PBS and then lysed. The expression of IL-8 mRNA was determined by RT-PCR as described in Materials and methods.
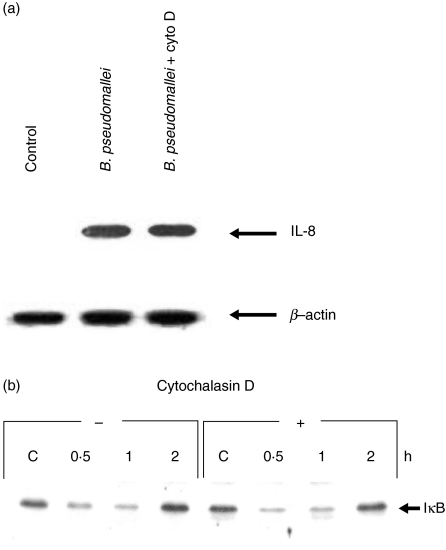
Because B. pseudomallei has been reported to be able to invade and survive inside the non-phagocytic cells [7], it should be of interest therefore to determine whether or not invasion would be required to initiate the IL-8 production. In this series of experiments, cytochalasin D was added to the test system to prevent bacterial invasion [15]. Inhibition of bacterial entry by cytochalasin D did not reduce IL-8 production significantly (data not shown). Moreover, as shown in Fig. 4a and b, inhibition of the bacterial invasion interfered with neither expression of IL-8 mRNA nor IκBα degradation. These results suggested that invasion of B. pseudomallei was not necessary for cell activation and IL-8 production. It should be noted that in the presence of cytochalasin D, the number of internalized bacteria was reduced significantly to less than 8% compared with the cells tested in the absence of this inhibitor (data not shown).
Fig. 4.
Effects of cytochalasin D pretreatment on IL-8 mRNA expression (a) and IκBα degradation (b) on the cells infected with B. pseudomallei. The cells (5 × 105 cells/well) were pretreated with cytochalasin D (2 µg/ml) for 2 h before being infected with B. pseudomallei at an MOI of 10 : 1. The cells were lysed after 2 h of infection. The expression of IL-8 mRNA was determined by RT-PCR and the degradation of IκB was detected by immunoblotting.
DISCUSSION
Burkholderia pseudomallei is a facultative intracellular bacterium which can survive and multiply in both phagocytic and non-phagocytic cells. The mechanism of this bacterium to evade host defence is not understood fully. By comparing with other Gram-negative bacteria such as S. typhi, this bacterium appeared to be a rather poor macrophage activator [9,10]. More importantly, this bacterium failed to activate iNOS expression, which is a key enzyme in macrophages defence against bacteria including B. pseudomallei[9]. The failure to stimulate iNOS production would allow the bacteria to survive and multiply inside the macrophages, allowing infection of neighbouring cells by cell-to-cell spreading, as we have reported previously [6,7].
Because lung is the most commonly affected organ found in patients with melioidosis, in the present study we turned our attention to the interaction of this bacterium with human alveolar lung epithelium cells. Epithelial cells have the ability to produce an array of chemokines, including IL-8, which is a potent chemoattractant for polymorphonuclear cells and can direct recruitment of these cells into the infected sites [16]. In this study we have demonstrated that although B. pseudomallei could induce the human lung epithelium to produce IL-8 (Fig. 1), the level of cytokine and gene expression exhibited by B. pseudomallei-infected cells was significantly lower than those exposed to other Gram-negative bacteria such as S. typhi (Figs 1 and 2). One possible mechanism that depresses the cytokine gene expression in the infected cells involves nuclear translocation of transcription factors. NF-κB is an important regulator of IL-8 gene expression [17]. It is well documented that degradation of IκBα regulates NF-κB activation by forming a complex with NF-κB, preventing its translocation to the nucleus [18]. In response to stimuli, IκBα is degraded by proteosome, thereby freeing NFκB which then enters the nucleus. In normal circumstances, the IκBα is rapidly resynthesized [18]. The data presented in Fig. 4, showing that the IκBα from the B. pseudomallei-infected cells was degraded to a lesser extent than those infected with S. typhi, suggested that B. pseudomallei was not able to stimulate NF-κB translocation as extensively as S. typhi (Fig. 2b). This could be one possible mechanism that causes the low IL-8 production in this model.
Several factors have been reported to play critical roles in the activation of epithelial cells. Infection of HeLa cells with invasive Shigella flexneri induced NF-κB translocation leading to the up-regulation of IL-8 mRNA [19]. In contrast, non-invasive S. flexneri was unable to activate NF-κB and the expression of IL-8 gene was impaired. Similarly, intracellular growth of Mycobacterium tuberculosis was necessary to elicit IL-8 production by alveolar epithelial cell [20]. However, the inhibition of B. pseudomallei invasion by pretreatment of the cells with cytochalasin D did not interfere with IL-8 production and gene expression (Fig. 4). Moreover, the level of IκBα degradation was similar to the cells which were not treated with this inhibitor (Fig. 4b). Altogether these results suggested that the activation of lung epithelial cells by B. pseudomallei did not require bacterial entry into the cells. Recently, we demonstrated that B. pseudomallei was able to adhere to the surface of human lung epithelial cells [21]. It is therefore logical to conclude from the data presented in the present study that adherence to the cell surface alone by B . pseudomallei is sufficient to initiate a signal for IL-8 production. Several components on the surface of Gram-negative bacteria, e.g. lipopolysaccharide (LPS) have the ability to stimulate epithelial cells which lead to NF-κB activation and IL-8 production [22]. One possible mechanism for the IL-8 production in our model is the interaction of LPS of B. pseudomallei to the surface of the human lung epithelial cells. Another bacterial product, the flagellin, could also elicit IL-8 production in lung epithelial cells via TLR5 [23]. The role of B. pseudomallei flagellin in lung epithelial cell activation remains to be investigated.
Melioidosis presents itself as a febrile illness, ranging from acute septicaemia to a chronic localized infection. This disease is characterized by abscess formation in various organs. Airway inflammation, characterized by influx and activation of neutrophils, plays a major role in the pathology of human melioidosis. In this study we demonstrated that B. pseudomallei is able to stimulate IL-8 release from lung epithelial cells. The production of this chemokine may contribute to an influx of neutrophils that may lead ultimately to the lung damage observed in the patients with melioidosis. Accumulation of neutrophils may not only cause pathology of lung tissue, but it may also enhance host defence against B. pseudomallei. Even though the neutrophils were reported to have little activity against B. pseudomallei, increased neutrophil production by granulocyte colony-stimulating factor (G-CSF) has been shown recently to decrease the mortality rate significantly in patients with melioidosis [24,25]. Production of IL-8 from lung epithelial cells infected with B. pseudomallei may be sufficient to induce influx of neutrophils to the sites of infection and provide a sufficient degree of protective inflammatory response against B. pseudomallei infection.
Acknowledgments
This work was supported by research grants from the Thailand Research Fund.
REFERENCES
- 1.White N. Melioidosis. Lancet. 2003;361:1715–22. doi: 10.1016/s0140-6736(03)13374-0. [DOI] [PubMed] [Google Scholar]
- 2.Dharakul T, Songsivilai S. The many facets of melioidosis. Trends Microbiol. 1999;7:138–46. doi: 10.1016/s0966-842x(99)01477-8. [DOI] [PubMed] [Google Scholar]
- 3.Dhiensiri T, Puapairoj S, Susaengrat W. Pulmonary melioidosis: clinical–radiologic correlation in 183 cases in northeastern Thailand. Radiology. 1988;166:711–5. doi: 10.1148/radiology.166.3.3340766. [DOI] [PubMed] [Google Scholar]
- 4.Currie BJ, Fisher DA, Howard DM, et al. Endemic melioidosis in tropical northern Australia: a 10 years prospective study and review of the literature. Clin Infect Dis. 2000;31:981–6. doi: 10.1086/318116. [DOI] [PubMed] [Google Scholar]
- 5.Jone A, Beveridge TJ, Woods DE. Intracellular survival of Burkholderia pseudomallei. Infect Immun. 1996;64:782–90. doi: 10.1128/iai.64.3.782-790.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harley VS, Dance DAB, Drasar BJ, Tovey G. Effects of Burkholderia pseudomallei and other Burkholderia species on eukaryotic cells in tissue culture. Microbios. 1998;96:71–93. [PubMed] [Google Scholar]
- 7.Kespichayawattana W, Rattanachetkul S, Wanun T, Utaisincharoen P, Sirisinha S. Burkholderia pseudomallei induces cell fusion and actin-associated membrane protusion: a possible mechanism for cell-to-cell spreading. Infect Immun. 2000;68:5377–84. doi: 10.1128/iai.68.9.5377-5384.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong KT, Puthucheary SD, Vadievelu J. The histopathology of human melioidosis. Histopathology. 1995;26:51–5. doi: 10.1111/j.1365-2559.1995.tb00620.x. [DOI] [PubMed] [Google Scholar]
- 9.Utaisincharoen P, Tangthawornchaikul N, Kespichayawattana W, Chaisuriya P, Sirisinha S. Burkholderia pseudomallei interferes with inducible nitric oxide synthase (iNOS) production: a possible mechanism of evading macrophage killing. Microbiol Immunol. 2001;45:307–13. doi: 10.1111/j.1348-0421.2001.tb02623.x. [DOI] [PubMed] [Google Scholar]
- 10.Utaisincharoen P, Anuntagool N, Limposuwan K, Chaisuriya P, Sirisinha S. Involvement of IFN-β in enhancing inducible nitric oxide synthase (iNOS) production and antimicrobial activity of B. pseudomallei-infected macrophages. Infect Immun. 2003;71:3053–7. doi: 10.1128/IAI.71.6.3053-3057.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anuntagool N, Intachote P, Wuthiekanun N, White NJ, Sirisinha S. Lipopolysaccharide from nonvirulent Ara+Burkholderia pseudomallei isolates is immunologically indistinguishable from lipopolysaccharide from virulent Ara– clinical isolate. Clin Diagn Lab Immunol. 1998;5:225–9. doi: 10.1128/cdli.5.2.225-229.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawahara K, Dejsirilert S, Danbara H, Ezaki T. Extraction and characterization of lipopolysaccharide from Pseudomonas pseudomallei. FEMS Microbiol Lett. 1992;96:129–34. doi: 10.1016/0378-1097(92)90392-2. [DOI] [PubMed] [Google Scholar]
- 13.Wuthiekanun V, Smith MD, Dance DAB, Walsh AL, Pitt TL, White NJ. Biochemical characteristics of clinical and environmental isolates of Burkholderia pseudomallei. J Med Microbiol. 1996;45:408–12. doi: 10.1099/00222615-45-6-408. [DOI] [PubMed] [Google Scholar]
- 14.Spriggs DR, Imamura K, Rodriguez C, Saribas E, Kufe DW. Tumor necrosis factor expression in human epithelial tumor cell lines. J Clin Invest. 1988;81:455–60. doi: 10.1172/JCI113341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martinez JJ, Mulvey MA, Schilling JD, Pinkner S, Hultgren SJ. Type 1 pilus-mediated bacterial invasion of bladder epithelial cells. EMBO J. 2000;19:2803–12. doi: 10.1093/emboj/19.12.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eckmann L, Kagnoff MF, Fierer J. Epithelial cells secret the chemokine interleukin-8 in response to bacterial entry. Infect Immun. 1993;61:4569–74. doi: 10.1128/iai.61.11.4569-4574.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mukaida N, Mahe Y, Matsushima K. Cooperative interaction of nuclear factor κB and cis-regulatory enhancer binding protein-like factor binding elements in activation the interleukin-8 gene by pro-inflammatory cytokines. J Biol Chem. 1993;256:21128–33. [PubMed] [Google Scholar]
- 18.Vema I, Stevenson M, Van Schwaz JKEM, Antwap D, Miyamoto S. Ral/NF-κB/IκB family: intimate tales of association and dissociation. Genes Dev. 1995;9:2723–35. doi: 10.1101/gad.9.22.2723. [DOI] [PubMed] [Google Scholar]
- 19.Philpott DJ, Yamaoka S, Israel A, Sansonetti PJ. Invasive Shigella flexneri activates NF-κB through a lipopolysaccharide-dependent innate intracellular response and leads to IL-8 expression in epithelial cells. J Immunol. 2000;165:903–14. doi: 10.4049/jimmunol.165.2.903. [DOI] [PubMed] [Google Scholar]
- 20.Lin Y, Zhang M, Barnes P. Chemokine production by a human alveolar epithelial cell line in response to Mycobacteria tuberculosis. Infect Immun. 1998;66:1121–6. doi: 10.1128/iai.66.3.1121-1126.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kespichayawattana W, Intachote P, Utaisincharoen P, Sirisinha S. Virulent Burkholderia pseudomallei is more efficient than avirulent Burkholderia thailandensis in invasion of and adherence to culture human epithelial cells. Microb Pathog. 2004;36:287–92. doi: 10.1016/j.micpath.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 22.Utaisincharoen P, Tangthawornchaikul N, Kespichayawattana W, Anuntagool N, Chaisuriya P, Sirisinha S. Kinetic studies of the production of nitric oxide and tumor necrosis factor-alpha in macrophages stimulated with Burkholderia pseudomallei endotoxin. Clin Exp Immunol. 2000;122:324–9. doi: 10.1046/j.1365-2249.2000.01386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liaudet L, Szabo C, Evgenov OV, et al. Flagellin from Gram-negative bacteria is a potent mediator of acute pulmonary inflammation in sepsis. Shock. 2003;19:131–7. doi: 10.1097/00024382-200302000-00008. [DOI] [PubMed] [Google Scholar]
- 24.Egan AM, Gordon DL. Burkholderia pseudomallei activates complement and is ingested but not killed by polymorphonuclear leukocytes. Infect Immun. 1996;64:4952–9. doi: 10.1128/iai.64.12.4952-4959.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng AC, Stephens DP, Anstey NM, Currie BJ. Adjunctive granulocyte colony-stimulating factor for treatment of septic shock due to melioidosis. Clin Infect Dis. 2004;38:32–7. doi: 10.1086/380456. [DOI] [PubMed] [Google Scholar]