Abstract
Acute coronary syndromes (ACS) are associated with inflammation resulting from monocyte activation. We sought for differences in the production of pro- and anti-inflammatory cytokines by monocytes from patients with ACS. C-reactive protein (CRP) and neopterin were measured in 22 patients with acute coronary syndromes, 50 patients with stable vascular disease and 22 healthy controls. Production of tumour necrosis factor (TNF)-α and interleukin (IL)-10 was determined after, respectively, 6 and 24 h of incubation of full blood with lipopolysaccharide (LPS). Levels of CRP [median, interquartile range (IQR)][1·5 mg/l (0·8–4·5) ACS patient versus 2·1 (0·9–3·6) stable disease versus 0·4 (0·3–1·2) healthy controls] (P < 0·001) and neopterin [7·4 nmol/l (6·0–8·7) ACS patient versus 7·1(6·0–8·9) stable disease versus 6·4 (5·6–7·3) healthy controls] (P = 0·07) were higher in both the patient groups. IL-10 production after LPS stimulation was greatly reduced in patients with acute coronary syndromes (16 175 pg/ml, 7559–28 470 pg/ml) as opposed to patients with stable disease (28 379 pg/ml, 12 601–73 968 pg/ml) and healthy controls (63 830 pg/ml, 22 040–168 000 pg/ml) (P = 0·003). TNF-α production was not signicantly different between the groups [7313 pg/ml (4740–12 615) ACS patient versus 11 002 (5913–14 190) stable disease versus 8229 (5225–11 364) healthy controls] (P = 0·24). Circulating monocytes in unstable coronary syndromes produce equal amounts of TNF-α but less IL-10 after stimulation with LPS in vitro as compared with healthy controls. We hypothesize that, in acute coronary syndromes, the production proinflammatory cytokines is not counterbalanced by anti-inflammatory cytokines such as IL-10.
Keywords: acute coronary syndromes, atherosclerosis, IL-10, inflammation, monocyte activation
INTRODUCTION
Atherosclerosis is an inflammatory disease of multi-factorial origin, resulting in vascular occlusion. Monocytes/macrophages are culprit cells in atherosclerotic plaque formation [1–3]. Recently, it was shown that patients with coronary artery disease have higher levels of neopterin, a marker of monocyte/macrophage activation [4–6]. This implies that monocyte/macrophage activation is an important determinant of the inflammatory response in coronary artery disease (CAD). Also, levels of interleukin (IL)-6, a proinflammatory cytokine that is produced mainly by activated monocytes/macrophages, are elevated in patients with CAD [7,8]. Finally, levels of C-reactive protein (CRP), a systemic marker of inflammation, are prognostic for the development and outcome of acute coronary syndromes [9,10]. Thus, a proinflammatory immune response because of monocyte/macrophage activation contributes to the development of acute coronary syndromes (ACS).
Less is known about the role of anti-inflammatory cytokines in human atherosclerosis. In mice, however, recent reports suggest an important role for IL-10 in preventing atherogenesis [11,12].
Because an adverse outcome in ACS is associated with elevated levels of several proinflammatory cytokines [10], and the anti-inflammatory cytokine IL-10 is supposed to be protective in atherosclerosis, we hypothesized that a disturbed cytokine balance by monocytes might be present in patients with ACS.
MATERIALS AND METHODS
Patients and controls
Three groups of subjects were studied. Group 1 consisted of 22 consecutive patients admitted to our hospital with an ACS. Seventeen patients had an acute myocardial infarction defined as: typical chest pain, ST-elevation >0·1 mV in at least two contiguous leads and creatin kinase (CK) levels of more than twice the upper limit or elevated troponin levels. Unstable angina pectoris was diagnosed in the other seven patients and was defined as: two episodes of chest pain at rest or an episode of chest pain lasting over 20 min in combination with ST-elevations >0·1 mV during pain, without elevations of CK.
Group 2 comprised 50 consecutive patients from our out-patient clinic with stable vascular disease. This group had either CAD (n = 31), peripheral vascular disease (PVD) (n = 17) or both (n = 2). CAD was defined as a positive exercise test result, significant stenosis (>70%) at coronary angiography, previous admission for an ACS, previous percutaneous transluminal coronary angioplasty or previous coronary artery bypass grafting. Patients had to be stable for at least 6 months. Patients with PVD were recruited from the out-patient clinic for vascular surgery. PVD was defined as the presence of intermittent claudication, and was confirmed by a decreased ankle/brachial index (<0·8), Doppler ultrasonography, digital subtraction angiography or prior vascular surgery. Twenty-two healthy volunteers were recruited from the hospital staff and served as controls. People with evidence of recent infectious disease, immunological disorders, fever, use of anti-inflammatory drugs, major surgery or neoplastic disease were excluded from the study.
All subjects gave written informed consent. The study was approved by the local medical ethical committee.
Blood samples
From each patient one tube of 10 ml heparinized blood and one tube of 10 ml ethylinediaminetetraacetic acid (EDTA) blood was drawn by venapuncture (Vacutainer system, Becton Dickinson Co., Plymouth, UK). In the case of an ACS this was performed immediately at admission, before any intervention had occurred. The tubes were transported on ice. A white blood cell count was performed immediately using the Coulter STKS (Beckman Coulter Nederland BV, Mijdrecht, the Netherlands). Blood was then centrifuged at 1255 g for 10 min. The plasma supernatant was removed and stored at −70°C until further analysis. The heparin tubes were used immediately for lipopolysaccharide (LPS) stimulation tests.
In vitro whole blood LPS stimulation
Cytokine production was measured using a whole blood culture system as described elsewhere [13]. Briefly, under sterile conditions, aliquots of 1 ml of heparinized whole blood were drawn into blank 5 ml tubes (Vacutainer system, Becton Dickinson Co.). The LPS-stimulated samples were treated with 20 µl LPS (Sigma Chemical Co., St Louis, MO, USA) to a final concentration of 2·5 µg/ml blood. LPS-treated samples, together with an untreated control sample, were then incubated at 37°C in an atmosphere containing 5% CO2. After 6 h of incubation the samples for tumour necrosis factor (TNF)-α measurement were centrifuged at 1076 g for 5 min. The interleukin (IL)-10 samples were centrifuged after 24 h of incubation. The plasma supernatant was removed and stored at −20°C for further analysis.
The first 14 whole blood cultures were co-incubated with polymyxin B. Polymyxin B occupies the CD14 molecule, the major LPS-receptor that is mainly present on monocytes [14]. These samples showed no enhanced production of cytokines after stimulation (data not shown). This shows that enhanced cytokine production after LPS stimulation is achieved exclusively by the CD14 molecule and therefore by monocytes.
CRP, TN-α, IL-10 and neopterin measurement
CRP, TNF-α and IL-10 levels were determined by using a validated sandwich enzyme-linked immunosorbent assay (ELISA) [15–17]. In brief, plates (Costar, Badhoevedorp, the Netherlands) were, respectively, coated with antibodies to CRP (A-073, Dakopatts, Glostrup, Danmark), TNF-α (MoAb 610, R&D systems Inc., MN, USA) and IL-10 (MoAb 18551D, BD Pharmigen, Bedford, USA) overnight. Subsequently, for CRP measurements plates were incubated with samples in duplicate in a 1 : 125 dilution and samples were diluted with incubation buffer containing: 0·05 m Tris HCl, 0·30 m NaCl and 0·05% Tween-20. For TNF-α and IL-10 measurement plates were incubated with TNF-α and IL-10 samples in 1: 2, 8, 32 and 128 dilutions, whereas these samples were diluted with incubation buffer containing: 0·01 m phosphate-buffered saline (PBS), 0·05% Tween-20 and 0·2% gelatin. CRP, TNF-α and IL-10 standards were made, respectively, with: 4 µg/ml ORCE 02/03 (Boehringer Mannheim, Germany), 20 µg/ml r-h TNF-α (Boehringer Mannheim, Germany) and 500 ng/ml r-h IL-10 (BD Pharmingen, Bedford, USA). CRP, TNF-α and IL-10 detection was, respectively, performed with 1 : 2000 rat antihuman CRP bound to horseradish peroxidase (HRP) (P-227, Dakopatts, Glostrup, Denmark), 1 : 2000 goat antihuman TNF-α-biotin (no. 21335, Pierce, Rockford, USA) and 1 : 500 MoAb 18562D (BD Pharmingen, Bedford, USA). The TNF-α and IL-10 plates were then treated with 1 : 8000 streptavidin labelled with polyhorseradish peroxidase (CLB, Amsterdam, the Netherlands). Between each step the plates were washed five times with washing buffer containing: 0·025 m Tris HCl, 0·15 m NaCl and 0·05% Tween-20. In all three ELISAs tetramethylbenzidin (TMB) (Brunschwig Chemie, Amsterdam, the Netherlands) was used as chromogen. The chromogen reaction was stopped by adding 2 N sulphuric acid. Extinctions were measured with an Emax scanner (Molecular Devices, Sunnyvale, USA) at 450–575 nm and calculated with Softmax Pro software (Molecular Devices, Sunnyvale, USA). The detection limit of IL-10 was 20 pg/ml, that for TNF-α was 15 pg/ml and for CRP 0·1 µg/ml. Neopterin levels were measured by commercially available ELISA (Brahms Diagnostica GmbH, Germany, detection limit 2 nmol/l). All ELISAs had a coefficient of variation (CV) of less than 10%.
Cytokine production in whole blood after LPS stimulation was calculated by subtracting the cytokine concentration in the unstimulated sample from that in the LPS-incubated sample. Cytokine production per 106 monocytes was calculated by dividing the total cytokine production by the number of monocytes in the peripheral blood smear.
Statistical analysis
For comparison of the baseline characteristics between groups, continuous variables were analysed by Kruskall–Wallis or Wilcoxon's two-sample test. In case of categorical variables, a χ2 test or a Fisher's exact test was used when appropriate. Descriptive statistics are presented as median values and interquartile range or as percentages. Univariate anova was performed to identify factors influencing the inflammatory parameters. Variables having a univariate P-value <0·15 were included in a multivariate analysis. Backward selection was used to construct a multivariate model. Correlations were calculated using Spearman's rho.
A two-sided P-value <0·05 was considered statistically significant. All statistical analyses were carried out using sas version 6·12 (Cary, NC, USA).
RESULTS
Patient characteristics are presented in Table 1. The patients with ACS were predominantly male, compared to patients with stable vascular disease and healthy controls (95 versus 66 versus 68% male, P = 0·03). However, multivariate analysis showed that gender was not a major determinant influencing outcome. Risk factors for cardiovascular disease were not different between the patient groups.
Table 1.
Baseline characteristics
| Acute coronary syndromes (n = 22) | Stable atherosclerosis (n = 50) | Healthy controls (n = 22) | P* | |
|---|---|---|---|---|
| Age, years | 52 (41–65) | 54 (46–60) | 48 (44–53) | 0·09 |
| Sex [M/F (%M)] | 21/1 (95) | 33/17 (66) | 15/7 (68) | 0·03 |
| Systolic blood pressure (mmHg) | 135 (115–153) | 140 (130–158) | 0·24 | |
| Total cholesterol (mmol/l) | 5·8 (5·3–6·9) | 5·6 (4·7–6·2) | 0·11 | |
| Medication | ||||
| Aspirin, n (%) | 14 (64) | 33 (66) | 1·00 | |
| Nitrates, n (%) | 10 (45) | 8 (16) | 0·02 | |
| Statins, n (%) | 4 (18) | 36 (72) | <0·001 | |
| Beta-blockers, n (%) | 6 (27) | 33 (66) | 0·04 | |
| ACE-inhibitors, n (%) | 2 (9) | 16 (32) | 0·04 | |
| Calcium antagonists, n (%) | 4 (18) | 9 (18) | 1·00 | |
| Diuretics, n (%) | 1 (5) | 6 (12) | 0·43 | |
| Risk factors | ||||
| Smoking, n (%) | 14 (64) | 38 (76) | 0·39 | |
| Family history, n (%) | 7 (32) | 25 (50) | 0·20 | |
The P-values for three groups were calculated with the Kruskall–Wallis or χ2 test, where appropriate. The P-values for two groups were calculated using either Wilcoxon's two-sample test or Fisher's exact test.
With respect to cardiovascular medication, patients with stable vascular disease used more lipid-lowering medication, beta-blockers, ACE-inhibitors and nitrates, whereas the use of aspirin, diuretics and calcium antagonists did not differ between both groups.
Levels of CRP, neopterin and white blood cell counts
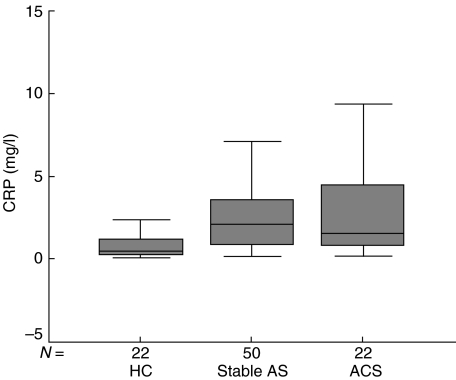
As expected, CRP levels were higher in patients suffering from vascular disease compared with healthy controls (P < 0·001). However, no difference was found between CRP values of both patient groups (P = 0·90) (Table 2, Fig. 3).
Table 2.
Biological and experimental parameters
| Acute coronary syndromes (n = 22) | Stable atherosclerosis (n = 50) | Healthy controls (n = 22) | P* | |
|---|---|---|---|---|
| CRP mg/L | 1·51 (0·84–4·50) | 2·09 (0·91–3·57) | 0·43 (0·30–1·20) | <0·001 |
| IL-10 after LPS challenge (pg/ml) | 16 175 (7559–28 470) | 28 379 (12 601–73 968) | 63 830 (22 040–168 000) | 0·003 |
| IL-10 (pg per 106 monocytes) | 30 339 (15 150–44 049) | 45 730 (22 876–189 000)† | 110 000 (38 259–357 000) | 0·002 |
| TNF-α after LPS challenge (pg/ml) | 7 313 (4740–12 615) | 11 002 (5913–14,190) | 8 229 (5227–11 364) | 0·29 |
| TNF-α (pg per 106 monocytes) | 15 234 (9263–20 356) | 17 950 (11 585–25 670)† | 18 980 (11 616–25 827) | 0·35 |
| White blood cell count (×106 cells)* | 9·2 (7·5–12·4) | 7·1 (6·3–8·4) | 5·7 (5·2–6·7) | <0·001 |
| Neopterin (nmol/l) | 7·4 (6·0–8·7) | 7·1 (6·0–8·9) | 6·4 (5·6–7·3) | 0·07 |
The P-values for three groups were calculated with the Kruskall–Wallis or χ2 test, where appropriate.
n = 49.
Fig. 3.
Levels of CRP. HC: healthy controls; stable AS: stable atherosclerotic disease; ACS: acute coronary syndromes.
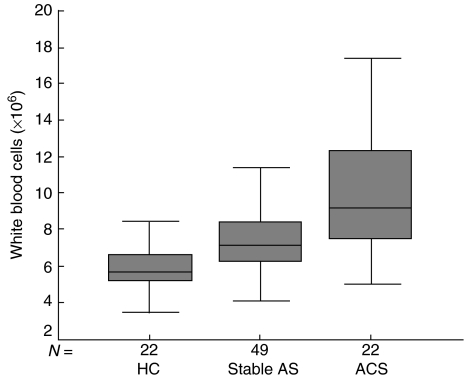
CRP levels correlated with neopterin levels (r = 0·401, P < 0·001) and white blood cell count (r = 0·272, P = 0·004). White blood cell counts were highest in patients with ACS compared to patients with stable atherosclerotic disease and healthy control groups, respectively (P < 0·001) (Table 2, Fig. 4).
Fig. 4.
White blood cell counts. HC: healthy controls; stable AS: stable atherosclerotic disease; ACS: acute coronary syndromes.
LPS-induced IL-10 production
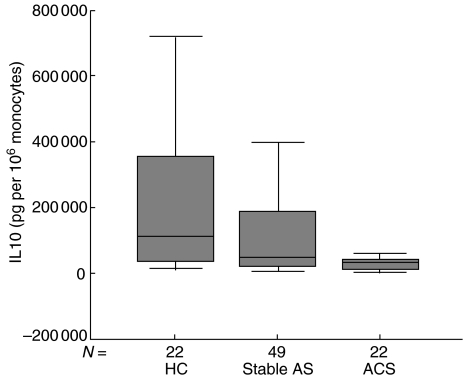
IL-10 levels are provided in Table 2 and displayed graphically in Fig. 1. IL-10 production per 106 monocytes (P = 0·002) as well as total IL-10 production (P = 0·003) after LPS stimulation was markedly and significantly lower in the ACS group compared to patients with stable atherosclerosis and healthy controls. In the unstimulated samples, an insignificant spontaneous production of cytokines was found (data not shown). IL-10 production in stable atherosclerosis patients was not significantly lower compared to healthy controls (P = 0·163).
Fig. 1.
Levels of IL-10. HC: healthy controls; stable AS: stable atherosclerotic disease; ACS: acute coronary syndromes.
In the overall population, IL-10 production after monocyte activation was correlated negatively with neopterin levels (r = −0·192, P = 0·042) and white blood cell count (r = −0·276, P = 0·003).
LPS-induced TNF-α production
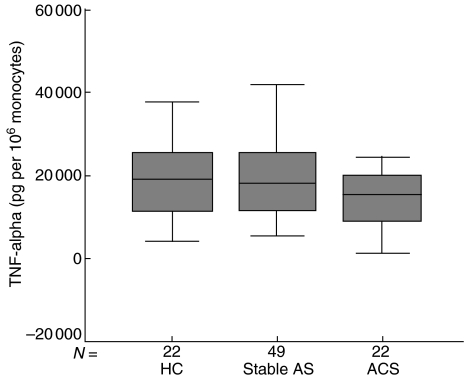
LPS-induced TNF-α per 10-6 monocytes as well as total TNF-α levels for the ACS, stable vascular disease and healthy control groups were not significantly different between the groups (P = 0·29) (Table 2, Fig. 2).
Fig. 2.
Levels of TNF-α per 106 monocytes. HC: healthy controls; stable AS: stable atherosclerotic disease; ACS: acute coronary syndromes.
Linear correlations for the whole population were found between TNF-α and monocyte count (r = 0·347, P < 0·001).
DISCUSSION
The pathogenesis of plaque rupture is characterized by an inflammatory process in which activated monocytes play an important role [2]. Monocyte activation in unstable coronary syndromes is thought to be a result of enhanced cytokine production by T lymphocytes [18,19]. Pro- and anti-inflammatory cytokines can either activate or deactivate this inflammatory reaction.
We demonstrate for the first time that in patients with acute coronary syndromes, activated monocytes produce lower amounts of IL-10 after LPS stimulation, but that the LPS-induced production of TNF-α, a proinflammatory cytokine, by monocytes did not differ between stable and unstable atherosclerotic disease. Patients with stable and unstable atherosclerotic disease displayed a comparable inflammatory state, reflected by elevated levels of CRP and white blood cell counts. Furthermore, levels of neopterin tended to be higher in these patients as well, indicating monocyte/macrophage activation, as has been reported previously [4–6].
Our findings suggest that a lack of anti-inflammatory counterbalance might be the culprit for enhanced inflammation as found in patients with unstable atherosclerotic disease.
Interleukin-10 is a major immunomodulatory cytokine that is produced by monocytes/macrophages, T helper Type II cells, regulatory T cells and B cells. It inhibits a broad array of inflammatory processes such as T helper Type I cell (proinflammatory) cytokine production, antigen presentation and antigen-specific T cell proliferation [20,21]. It is demonstrated that IL-10 decreases inflammatory activity in vivo, as shown in T helper Type I mediated diseases such as psoriasis [22].
In recent years much attention has been given to the role of IL-10 in the pathogenesis of atherosclerosis and the acute coronary syndromes. A protective role for IL-10 in atherosclerosis has been emphasized by a number of studies. Uyemura et al. demonstrated that IL-10 was virtually absent in atherosclerotic plaques and that it had deactivating properties on the T helper 1 immune response [20]. Recently, a protective role for IL-10 in atherosclerosis was suggested by demonstrating that IL-10 transgenic mice had reduced lesion size after an atherogenic diet [11], and Mallat et al. demonstrated abundant atherosclerosis in IL-10–/– mice, a process that could be prevented partly by the transfer of murine IL-10 [12].
In a clinical study, Smith et al. noticed that levels of IL-10 were decreased in patients with acute coronary syndromes [23]. In line with their findings, we demonstrate that, in particular, monocytes of these patients have a deficient production of this apparently important cytokine.
The study as presented here is cross-sectional and samples are small, therefore we cannot provide insight into whether the lack of IL-10 production by monocytes is transient or persistent in our patients. It may therefore have been that the monocytes of the patients with ACS were ‘exhausted’ in their IL-10 production; this could explain the negative overall correlation that was found between IL-10 levels and levels of neopterin and white blood cell counts. However, one would have expected a lower TNF-α production as well. Because patients with acute coronary syndromes are supposed to have sympathetic arousal, it can be speculated that the lower IL-10 production is the result of stimulation of inflammatory cells by catecholamines. However, it was reported previously that catecholamines enhance IL-10 production in patients with an acute myocardial infarction [24]. The fact that patients with stable atherosclerotic disease have a decreased IL-10 production compared to healthy controls, but higher than in patients with ACS, suggests that it is a chronic feature of atherosclerotic cardiovascular disease that may be enhanced by an acute exacerbation. It can therefore be speculated that restoration of the inflammatory cytokine balance, e.g. by supplying recombinant IL-10, may prove beneficial in patients with ACS. However, this issue remains speculative, but provides a challenge for future research.
Acknowledgments
The authors wish to acknowledge Dr J. J. van den Dungen for referring patients for participation, Mrs W. Oost-Kort for her valuable technical assistance in laboratory measurements and Dr P. C. Limburg for his valuable advice. Dr Paul L. van Haelst is supported by the Netherlands Heart Foundation, Grant D99-020. This project has been supported by the Foundation ‘De Drie Lichten’ in the Netherlands.
REFERENCES
- 1.Ross R. Atherosclerosis − an inflammatory disease. N Engl J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 2.Libby P. Molecular bases of the acute coronary syndromes. Circulation. 1995;91:2844–50. doi: 10.1161/01.cir.91.11.2844. [DOI] [PubMed] [Google Scholar]
- 3.Moreno PR, Falk E, Palacios IF, Newell JB, Fuster V, Fallon JT. Macrophage infiltration in acute coronary syndromes. Implications for plaque rupture. Circulation. 1994;90:775–8. doi: 10.1161/01.cir.90.2.775. [DOI] [PubMed] [Google Scholar]
- 4.Schumacher M, Halwachs G, Tatzber F, et al. Increased neopterin in patients with chronic and acute coronary syndromes. J Am Coll Cardiol. 1997;30:703–7. doi: 10.1016/s0735-1097(97)00172-1. [DOI] [PubMed] [Google Scholar]
- 5.Gupta S, Fredericks S, Schwartzman RA, Holt DW, Kaski JC. Serum neopterin in acute coronary syndromes. Lancet. 1997;349:1252–3. doi: 10.1016/s0140-6736(05)62447-6. [DOI] [PubMed] [Google Scholar]
- 6.Garcia-Moll X, Coccolo F, Cole D, Kaski JC. Serum neopterin and complex stenosis morphology in patients with unstable angina. J Am Coll Cardiol. 2000;35:956–62. doi: 10.1016/s0735-1097(99)00640-3. [DOI] [PubMed] [Google Scholar]
- 7.Rifai N, Joubran RYuH, Asmi M, Jouma M. Inflammatory markers in men with angiographically documented coronary heart disease. Clin Chem. 1999;45:1967–73. [PubMed] [Google Scholar]
- 8.Biasucci LM, Liuzzo G, Grillo RL, et al. Elevated levels of C-reactive protein at discharge in patients with unstable angina predict recurrent instability. Circulation. 1999;99:855–60. doi: 10.1161/01.cir.99.7.855. [DOI] [PubMed] [Google Scholar]
- 9.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336:973–9. doi: 10.1056/NEJM199704033361401. [DOI] [PubMed] [Google Scholar]
- 10.Biasucci LM, Liuzzo G, Fantuzzi G, et al. Increasing levels of interleukin (IL)-1Ra and IL-6 during the first 2 days of hospitalization in unstable angina are associated with increased risk of in-hospital coronary events. Circulation. 1999;99:2079–84. doi: 10.1161/01.cir.99.16.2079. [DOI] [PubMed] [Google Scholar]
- 11.Pinderski Oslund LJ, Hedrick CC, Olvera T, et al. Interleukin-10 blocks atherosclerotic events in vitro and in vivo. Arterioscler Thromb Vasc Biol. 1999;19:2847–53. doi: 10.1161/01.atv.19.12.2847. [DOI] [PubMed] [Google Scholar]
- 12.Mallat Z, Besnard S, Duriez M, et al. Protective role of interleukin-10 in atherosclerosis. Circ Res. 1999;85:17–24. doi: 10.1161/01.res.85.8.e17. [DOI] [PubMed] [Google Scholar]
- 13.de Bont N, Netea MG, Rovers C, et al. LPS-induced cytokine production and expression of LPS-receptors by peripheral blood mononuclear cells of patients with familial hypercholesterolemia and the effect of HMG-CoA reductase inhibitors. Atherosclerosis. 1998;139:147–52. doi: 10.1016/s0021-9150(98)00074-4. [DOI] [PubMed] [Google Scholar]
- 14.Coyne CP, Fenwick BW. Inhibition of lipopolysaccharide-induced macrophage tumor necrosis factor-alpha synthesis by polymyxin B sulfate. Am J Vet Res. 1993;54:305–14. [PubMed] [Google Scholar]
- 15.Hazenberg BPC, Limburg PC, Bijzet J, van Rijswijk MH. SAA versus CRP serum levels in different inflammatory conditions, studied by ELISA using polyclonal anti-AA and monoclonal anti-SAA antibodies. In: Isobe T, Araki S, Uchino F, Kito S, Tsubura E, editors. Amyloid and amyloidosis. New York: Plenum Press; 1988. pp. 85–94. [Google Scholar]
- 16.Yap SH, Moshage HJ, Hazenberg BP, et al. Tumor necrosis factor (TNF) inhibits interleukin (IL)-1 and/or IL-6 stimulated synthesis of C-reactive protein (CRP) and serum amyloid A (SAA) in primary cultures of human hepatocytes. Biochim Biophys Acta. 1991;1091:405–8. doi: 10.1016/0167-4889(91)90207-e. [DOI] [PubMed] [Google Scholar]
- 17.Fijen JW, Zijlstra JG, De Boer P, et al. Suppression of the clinical and cytokine response to endotoxin by RWJ-67657, a p38 mitogen-activated protein-kinase inhibitor, in healthy human volunteers. Clin Exp Immunol. 2001;124:16–20. doi: 10.1046/j.1365-2249.2001.01485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hansson GK. Cell-mediated immunity in atherosclerosis. Curr Opin Lipidol. 1997;8:301–11. doi: 10.1097/00041433-199710000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Weyand CM, Goronzy JJ, Liuzzo G, Kopecky SL, Holmes DR, Jr, Frye RL. T-cell immunity in acute coronary syndromes. Mayo Clin Proc. 2001;76:1011–20. doi: 10.4065/76.10.1011. [DOI] [PubMed] [Google Scholar]
- 20.Uyemura K, Demer LL, Castle SC, et al. Cross-regulatory roles of Interlekin (IL)-12 and IL-10 in atherosclerosis. J Clin Invest. 1996;97:2130–8. doi: 10.1172/JCI118650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moore KW, de Waal MR, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 22.Asadullah K, Friedrich M, Hanneken S, et al. Effects of systemic interleukin-10 therapy on psoriatic skin lesions: histologic, immunohistologic, and molecular biology findings. J Invest Dermatol. 2001;116:721–7. doi: 10.1046/j.0022-202x.2001.01317.x. [DOI] [PubMed] [Google Scholar]
- 23.Smith DA, Irving SD, Sheldon J, Cole D, Kaski JC. Serum levels of the antiinflammatory cytokine interleukin-10 are decreased in patients with unstable angina. Circulation. 2001;104:746–9. doi: 10.1161/hc3201.094973. [DOI] [PubMed] [Google Scholar]
- 24.Riese U, Brenner S, Docke WD, et al. Catecholamines induce IL-10 release in patients suffering from acute myocardial infarction by transactivating its promoter in monocytic but not in T-cells. Mol Cell Biochem. 2000;212:45–50. [PubMed] [Google Scholar]