Abstract
Proteinase 3 is the major autoantigen in patients with Wegener's granulomatosis. Earlier studies have shown that circulating leucocytes from patients with Wegener's granulomatosis show elevated proteinase 3 surface expression and mRNA levels. Wegener's granulomatosis patients also have increased levels of proteinase 3 in plasma. A single nucleotide polymorphism (SNP) (− 564 A/G SNP) in the promoter region has been associated with disease. This SNP introduces a new potential Sp1 transcription factor binding site that may be responsible for the observed up-regulated expression of proteinase 3. To investigate this a 740 base pair long region of the promoter was cloned from genomic DNA. The disease-associated −564 A/G, as well as a control −621 A/G exchange, were introduced by polymerase chain reaction mutagenesis and cloned into a luciferase reporter vector. Endogenous expression levels of proteinase 3 mRNA and promoter activity of the cloned constructs were measured in three myeloid cell lines, HL-60, U937 and NB-4, and in epithelial HeLa cells. The results demonstrate a good correlation between the endogenous proteinase 3 mRNA expression and the promoter activity, as judged by luciferase activity. However, no significant differences in activity between the wild-type, polymorphic and the mutated control variant were found. In conclusion, the −564 A/G polymorphism is not responsible for the increased expression levels seen in myeloid cells from patients with Wegener's granulomatosis.
Keywords: ANCA, promoter, proteinase 3, vasculitis, Wegener's granulomatosis
INTRODUCTION
Wegener's granulomatosis and microscopic polyangiitis are systemic small vessel vasculitides of unknown aetiology. Both diseases are associated with autoantibodies against granule proteins from neutrophils and monocytes, e.g. proteinase 3 and myeloperoxidase [1]. These antibodies are called antineutrophil cytoplasmic antibodies (ANCA). Autoantibodies against proteinase 3 are found in about 85% of patients with Wegener's granulomatosis and in 45% of patients with microscopic polyangiitis [2,3]. Some correlations are found between antibody titres and clinical activity, but the mechanism by which they are formed is unclear and it is still an unresolved issue whether the ANCA are pathogenic in humans.
More recent studies have shown that proteinase 3 itself plays an important role in the disease process. The enzyme is expressed on the surface of a subset of neutrophils [4–6]. A high percentage of neutrophils expressing proteinase 3 on their surface is considered a risk factor for disease progression in Wegener's granulomatosis patients [6,7]. Primed neutrophils can be fully activated by ANCA, probably via cross-linking of the surface-bound proteinase 3 and the Fc receptor [8,9]. Moreover, the finding of increased expression of the protein in disease provides indirect evidence for a pathological role of proteinase 3. We and others have found elevated levels of circulating proteinase 3 in plasma from patients with Wegener's granulomatosis [10–12]. Furthermore, mRNA levels of proteinase 3 are also raised in monocytes of patients with Wegener's granulomatosis [13]. The molecular mechanism for increased expression of proteinase 3 in vasculitides is not clear, but possible mechanisms include increased transcription of the proteinase 3 gene.
Several transcriptional control elements have been defined in the promoter region upstream of exon 1 of the proteinase 3 gene. Within the first 200 base pairs of the proteinase 3 promoter, Sturrock et al. identified two elements, a PU.1 binding site at position −101 and a cytidine-rich site at position −190. Both elements are critical for maximal activity of the analysed promoter. The cytidine-rich site shows sequence similarities to binding sites of the transcription factor Sp1, but direct binding of Sp1 could not be demonstrated [14]. PU.1 nuclear factor did bind directly to the PU.1 site and this binding was reduced during myeloid differentiation in correlation with a reduced proteinase 3 mRNA expression [14]. Another study showed that up-regulation of proteinase 3 mRNA expression by G-CSF is associated with PU.1 binding [15], lending further support for PU.1 as an important transcription factor for proteinase 3. The activity of the promoter of proteinase 3 is also dependent on the presence of sites for C/EBP and c-Myb and on a TATA site within the first 91 base pairs of the promoter [16].
In 1999, Gencik et al. reported an A→G SNP in the promoter region of proteinase 3 that give rise to a new potential Sp1 transcription factor binding site 564 base pairs upstream from the translation start [17]. Furthermore, the G allele frequency was significantly higher in patients with Wegener's granulomatosis compared to healthy individuals. Considering that a similar SNP is present in the myeloperoxidase gene [18] and that this SNP confers a 25-fold increase in expression [19], we speculate that the −564 A/G SNP in the proteinase 3 promoter region could be responsible for the increased expression of proteinase 3 found in patients with ANCA associated small vessel vasculitis. Therefore, we wanted to investigate the role of the Sp1 site created in the G allele in terms of increased promoter activity.
MATERIALS AND METHODS
Cloning of DNA and sequence analysis
The promoter region of the proteinase 3 gene was obtained by polymerase chain reaction (PCR) amplification of genomic DNA collected from mononuclear blood cells. In the first PCR an amplicon was obtained by running a PCR with a Pfu polymerase (Stratagene, La Jolla, CA, USA) using the following primers: 5′-CAGTGGCAC GATCTTGGCT-3′ and 5′-GCTCACTCACCGC TCAGCA-3′. The PCR product was amplified further in a second PCR using two nested primers: 5′-GGTACTCGAGCAAA TAATGAA CACTGGTCTCTCCC-3′ and 5′-ACTGAAGCTT GGTGGGGT CCAGGGTGC-3′. These primers also introduced the XhoI and HindIII restriction sites (underlined) for subsequent cloning of the second PCR product. The PCR product and the pGL3 basic vector (Promega) were cleaved, purified and ligated. The construct was sequenced and found to have an A in the −564 position. This construct was defined as proteinase 3 wild-type.
Mutagenesis
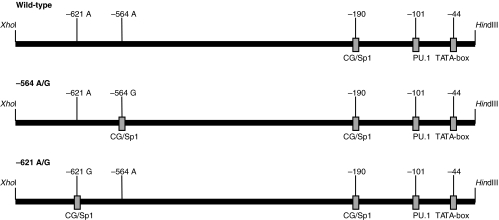
A sequence analysis revealed a second putative site, 57 base pairs further upstream of the −564 G/A SNP, in which a single mutation from A to G will introduce another Sp1 site (− 621 A/G). This site is not reported as a naturally occurring SNP in humans, but was used in the study as a control. Both the previously described SNP −564 A/G and the −621 A/G (control) site were created by mutating an A→G by site directed mutagenesis using the primers 5′-GGACCCTGGGCGAGGTCTGAG-3′ and 5′-ATGGAGTG GGCGGAGGCCAAG-3′, respectively, and the megaprimer method [20]. The PCR products were purified and ligated into the pGL3 basic vector and sequenced. The two constructs were called −564 A/G and −621 A/G, respectively, (Fig. 1).
Fig. 1.
A schematic drawing of the three different proteinase 3 promoter constructs. The wild-type vector has no additional Sp1 sites to the putative one in the CG rich element. The −564 A/G construct carries the reported SNP [17]introducing a new Sp1 site at position −564. The −621 A/G construct is mutated at position −621 from an A→G also yielding a Sp1 site. The −621 A/G SNP is not reported to occur in humans and this construct serves as a control only.
Cell culture
U937 (ATCC no CRL-1593·2), NB-4 (DSMZ no. ACC 207) and HeLa (ATCC no. CCL-2) cells were cultured in RPMI-1640 (Gibco, BRL) supplemented with 10% fetal calf serum (Gibco BRL Invitrogen AB, Sweden). HL60 clone 15 (ATCC no. CRL-1964) was cultured in identical medium supplemented with 1 mm l-glutamine and 1 mm sodium pyruvate. U937, NB-4 and HL60 clone 15 are human myeloid leukaemia cell lines expressing endogenous proteinase 3. HeLa is a human cervix carcinoma cell line that is negative for proteinase 3.
Total RNA extraction
Exponentially growing U937, NB-4, HeLa and HL60 cells were harvested and total RNA was isolated using the Trizol LS reagent kit (Invitrogen, Life Technologies, Sweden) according to the manufacturer's instructions. RNA-concentration was determined from A260 using the Nanodrop® ND-1000 Spectrophotometer (Nanodrop Technologies, Delaware, USA).
Real-time reverse transcription-polymerase chain reaction (real time RT-PCR)
Fifty ng of RNA converted to cDNA, using oligo d(T)16 primers and the TaqMan® Reverse Transcription kit (Applied Biosystems, Foster City, CA, USA), was used per 25 µl reaction in which proteinase 3 cDNA was amplified by quantitative real-time PCR (TaqMan®) using an ABI PRISM 7000 Sequence Detector (Applied Biosystems) according to the manufacturer's instructions. Primers and probe were purchased from Applied Biosystems (Assays-on-Demand no. HS-00160521_m1). RT-real time PCR of β2-microglobulin (Applied Biosystems no. 4326319E) was used as internal control for equal loading. Data were collected and analysed with Sequence Detector version 1·1 software (Applied Biosystems). Relative quantitative data were calculated based on the ΔΔCT method: normalization: (ΔCT = CT (sample) − CT (β2-microglobulin); ΔΔCT = ΔCT (sample) − ΔCT (calibrator); relative quantification = 2–ΔΔCT[21].
Transient transfections and luciferase assay
Conditions for electroporation were optimized for each cell line to obtain maximal luciferase activity, as described previously [22]. U937, NB-4 and HeLa cells were transiently transfected with 15 µg of pGL3-reporter plasmid and 0·1 µg Renilla vector, pRL-SV40 (Promega) as internal control and HL60 cells with 35 µg reporter plasmid and 0·1 µg Renilla vector. Electroporation was performed in a 0·4 cm cuvette (Bio-Rad Hercules, CA, USA) with cells suspended in 500 µl culture medium using Genepulser II equipment (Bio-Rad). Cell number and electrical settings during electroporation were as follows: U937: 8 × 106/ml, 280 V, 960 µF; NB-4: 8 × 106/ml, 280 V, 960 µF; HL60: 5 × 106/ml 340 V, 960 µF. HeLa cells were plated at a density of 2 × 106/well in a six-well culture plate and transfected the next day at 70% confluence using the Lipofectin® reagent (Invitrogen, Life Technologies). After transfection, U937 cells were incubated for 16 h, NB-4 and HL60 cells for 10 h and Hela-cells for 20 h, after which the amount of luciferase was determined using a Dual-Luciferase reporter kit (Promega SDS Biosciences, Sweden) according to the manufacturer's instructions and a TD-20/20 luminometer (Turner Design Sunnyvale, CA, USA). Firefly luciferase values were corrected for the transfection efficiency by normalization to the Renilla luciferase values.
RESULTS
Proteinase 3 mRNA expression in myeloid cell lines
For our studies we chose the monocytic cell line U937 and the promyelocytic cell lines NB-4 and HL60, all expressing endogenous proteinase 3 mRNA [23–25]. As a negative control we used the epithelial HeLa cell line without proteinase 3 expression [14]. In order to determine the relative amount of endogenous proteinase 3 mRNA in the various tested cell lines we performed real-time RT-PCR analyses. In all the myeloid cell lines, U937, NB-4 and HL60 proteinase 3 mRNA expression was verified. The weakest expression level of proteinase 3 mRNA was present in U937 cells, while NB-4 and HL60 showed intermediary and high levels, respectively (Table 1). In HeLa cells the proteinase 3 mRNA expression was undetectable, as expected.
Table 1.
Expression levels of proteinase-3 mRNA in different cell lines. Relative expression of mRNA was determined by TaqMan real-time RT-PCR. Shown are mean values of two independent experiments performed in duplicate and normalized to those of U937 cells
| Cell line | Relative expression |
|---|---|
| U937 | 1·0 |
| NB-4 | 13·0 |
| HL60 | 480·5 |
| HeLa | Undetectable |
Promoter activity in myeloid cell lines
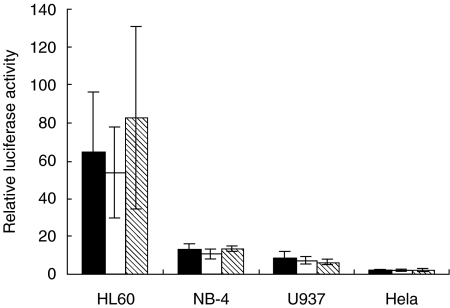
In order to reveal possible differences in transcriptional activity between the three polymorphic variants of the proteinase 3 promoter (wild-type, −564 A/G and −621 A/G), we transfected promoter-reporter plasmids to the cell lines. As shown in Fig. 2, three variants of the proteinase 3 promoter showed the highest luciferase activity in HL60 cells, compared to the activity in the two other myeloid cell lines NB-4 and U937. When transfected to non-myeloid HeLa-cells no significant promoter activity was seen. Thus, the promoter activity shows good correlation to the levels of expression of endogenous proteinase 3 (Table 1) and demonstrates that the promoter confers a myeloid-specific expression. Within each cell line, however, none of the three different promoter fragments showed any significant difference in activity (Fig. 2).
Fig. 2.
Proteinase 3 promoter activities in different cell lines. Variant wild-type (filled bars), variant −564 A/G (open bars), and variant −621 A/G (marked bars) of the proteinase 3-promoter were transiently transfected into HL60, NB-4, U937 and HeLa cells, respectively. Luciferase activity was determined as described in Materials and methods. Values shown are normalized to those obtained by transfection with pGL3 basic vector. Shown are mean ± s.e.m. values from three independent experiments, each one performed in duplicate.
In conclusion, the high expression level of proteinase 3 mRNA in HL60 cells is in good correlation with the high promoter activity in this cell line. However, the three different promoter variants showed no significant mutual differences in activity in any of the cell lines analysed, arguing strongly against the polymorphic variants conferring different expression levels of proteinase 3 mRNA.
DISCUSSION
Understanding the transcriptional, and perhaps the post-transcriptional, regulation of proteinase 3 expression is fundamental to understand the quantitative differences in the expression of proteinase 3 in patients with Wegener's granulomatosis and healthy people [6,12,26]. Certain identified polymorphisms in the proteinase 3 gene and promoter [17] were found to be over-represented in patients with Wegener's granulomatosis, but whether or not the polymorphisms are indeed causing quantitative differences in proteinase 3 expression has so far not been revealed. Our hypothesis in this study was that the previously identified proteinase 3 promoter polymorphism, an A→G SNP at position −564, results in increased proteinase 3 promoter activity contributing to increased proteinase 3 expression.
To test this hypothesis, we transiently transfected promyelocytic HL60 and NB-4 cells and monocytic U937 cells, and HeLa with three variants of the proteinase 3 promoter (−564 A/G; −621 A/G and wild-type) (Fig. 1). While HL60 and NB-4 are of promyelocytic phenotype, U937 cells show some monocytic characteristics. The highest promoter activity was found in HL60 cells, which also was the cell line showing highest levels of endogenous proteinase 3 mRNA, indicating that analysed promoter activity correlates to endogenous gene expression. However, within each cell line, our data revealed no significant differences in promoter activity among the three different promoter fragments, thus arguing strongly against our hypothesis.
Another common target antigen of ANCA in small-vessel vasculitis is myeloperoxidase which is, like proteinase 3, expressed at the promyelocytic stage during neutrophil maturation and shows a similar expression pattern with proteinase 3 during normal myeloid differentiation [27]. One study [18] showed an association between a common and functional myeloperoxidase promoter polymorphism (−463 G/A) and an increased incidence of ANCA-associated small-vessel vasculitis. This association could not, however, be confirmed by Fiebeler et al. [28]. Another study revealed that this common polymorphism (−463 G/A) in the myeloperoxidase promoter created a functional Sp1 binding site resulting in a 25-fold increase of transcriptional activity [29].
Given that the −564 A/G SNP of the proteinase 3 promoter also creates a novel potential Sp1 binding site, we expected an increased transcription from this SNP variant. This was not found to be the case, explained possibly by the long distance between the new/additional potential binding site for Sp1 and the TATA-box. A study on the effect on transcription of two GC sites (i.e. an additional Sp1 transcription binding site) at various distances from the TATA box in the E1B promoter showed the importance of the extremely close spacing between the GC site and the TATA box [30]. The previously reported GC-rich potential Sp1 site in the wild-type proteinase 3 promoter is localized at −190 base pairs up-stream from the proteinase 3 gene and is separated from the TATA box by only 146 base pairs (Fig. 1) [14,31], consistent with the importance for maximal activity of the promoter. However, the additional potential Sp1 binding site at position –564, created by the −564 A/G SNP, is 520 base pairs separated from the TATA box, which might be too far to positively affect the transcriptional activity of the promoter. The reason why the additional Sp1 site at position –463 in the myeloperoxidase promoter gives an increased promoter activity, and not the additional Sp1 site at position –564 in the proteinase 3 promoter, might be the close distance of the Sp1 site to the alternative transcriptional start site P2 in the myeloperoxidase promoter [32]. In summary, our results argue strongly against the hypothesis that the −564 A/G SNP in the promoter region of proteinase 3 is increasing the promoter activity and thus being the mechanism explaining the elevated levels of proteinase 3 found in patients with Wegener's granulomatosis.
Acknowledgments
The authors thank Lena Gunnarsson and Caroline Lejon-Nilsson for their skilful laboratory assistance. This work was supported by Swedish Scientific Research Council ( nos K2004–71XD-15152, K2004–71 PD-15151, K2002–74X-09487 and 11546), the Swedish Society for Medical Research (SSMF), the Swedish Kidney Foundation (Rnj), the Kock Foundation, the Österlund Foundation and the Crafoord Foundation.
REFERENCES
- 1.Hellmich B, Csernok E, Gross WL. 20 years with ANCA (antineutrophil cytoplasmic autoantibodies): from seromarker to a major pathogenic player in vasculitis. J Leukoc Biol. 2003;74:1–2. doi: 10.1189/jlb.0403134. [DOI] [PubMed] [Google Scholar]
- 2.Tervaert JW, van der Woude FJ, Fauci AS, et al. Association between active Wegener's granulomatosis and anticytoplasmic antibodies. Arch Intern Med. 1989;149:2461–5. doi: 10.1001/archinte.149.11.2461. [DOI] [PubMed] [Google Scholar]
- 3.Kallenberg CG, Brouwer E, Weening JJ, et al. Anti-neutrophil cytoplasmic antibodies: current diagnostic and pathophysiological potential. Kidney Int. 1994;46:1–15. doi: 10.1038/ki.1994.239. [DOI] [PubMed] [Google Scholar]
- 4.Witko-Sarsat V, Cramer EM, Hieblot C, et al. Presence of proteinase 3 in secretory vesicles: evidence of a novel, highly mobilizable intracellular pool distinct from azurophil granules. Blood. 1999;94:2487–96. [PubMed] [Google Scholar]
- 5.Csernok E, Ernst M, Schmitt W, et al. Activated neutrophils express proteinase 3 on their plasma membrane in vitro and in vivo. Clin Exp Immunol. 1994;95:244–50. doi: 10.1111/j.1365-2249.1994.tb06518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Witko-Sarsat V, Lesavre P, Lopez S, et al. A large subset of neutrophils expressing membrane proteinase 3 is a risk factor for vasculitis and rheumatoid arthritis. J Am Soc Nephrol. 1999;10:1224–33. doi: 10.1681/ASN.V1061224. [DOI] [PubMed] [Google Scholar]
- 7.Rarok AA, Stegeman CA, Limburg PC, et al. Neutrophil membrane expression of proteinase 3 (PR3) is related to relapse in PR3-ANCA-associated vasculitis. J Am Soc Nephrol. 2002;13:2232–8. doi: 10.1097/01.asn.0000028642.26222.00. [DOI] [PubMed] [Google Scholar]
- 8.Ben-Smith A, Dove SK, Martin A, et al. Antineutrophil cytoplasm autoantibodies from patients with systemic vasculitis activate neutrophils through distinct signaling cascades: comparison with conventional Fcgamma receptor ligation. Blood. 2001;98:1448–55. doi: 10.1182/blood.v98.5.1448. [DOI] [PubMed] [Google Scholar]
- 9.Yang JJ, Tuttle RH, Hogan SL, et al. Target antigens for anti-neutrophil cytoplasmic autoantibodies (ANCA) are on the surface of primed and apoptotic but not unstimulated neutrophils. Clin Exp Immunol. 2000;121:165–72. doi: 10.1046/j.1365-2249.2000.01228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baslund B, Petersen J, Permin H, et al. Measurements of proteinase 3 and its complexes with alpha 1-proteinase inhibitor and anti-neutrophil cytoplasm antibodies (ANCA) in plasma. J Immunol Meth. 1994;175:215–25. doi: 10.1016/0022-1759(94)90364-6. [DOI] [PubMed] [Google Scholar]
- 11.Henshaw TJ, Malone CC, Gabay JE, et al. Elevations of neutrophil proteinase 3 in serum of patients with Wegener's granulomatosis and polyarteritis nodosa. Arthritis Rheum. 1994;37:104–12. doi: 10.1002/art.1780370116. [DOI] [PubMed] [Google Scholar]
- 12.Ohlsson S, Wieslander J, Segelmark M. Increased circulating levels of proteinase 3 in patients with anti-neutrophilic cytoplasmic autoantibodies-associated systemic vasculitis in remission. Clin Exp Immunol. 2003;131:528–35. doi: 10.1046/j.1365-2249.2003.02083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohlsson S, Hellmark T, Pieters K, et al. Increased monocyte transcription of the proteinase 3 gene, cause or consequence of ANCA associated vasculitis? J Am Soc Nephrol. 2003;14:634A. [Google Scholar]
- 14.Sturrock A, Franklin KF, Hoidal JR. Human proteinase-3 expression is regulated by PU.1 in conjunction with a cytidine-rich element. J Biol Chem. 1996;271:32392–402. doi: 10.1074/jbc.271.50.32392. [DOI] [PubMed] [Google Scholar]
- 15.Lutz PG, Moog-Lutz C, Coumau-Gatbois E, et al. Myeloblastin is a granulocyte colony-stimulating factor-responsive gene conferring factor-independent growth to hematopoietic cells. Proc Natl Acad Sci USA. 2000;97:1601–6. doi: 10.1073/pnas.97.4.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lutz PG, Houzel-Charavel A, Moog-Lutz C, et al. Myeloblastin is an Myb target gene: mechanisms of regulation in myeloid leukemia cells growth-arrested by retinoic acid. Blood. 2001;97:2449–56. doi: 10.1182/blood.v97.8.2449. [DOI] [PubMed] [Google Scholar]
- 17.Gencik M, Meller S, Borgmann S, et al. Proteinase 3 gene polymorphisms and Wegener's granulomatosis. Kidney Int. 2000;58:2473–7. doi: 10.1046/j.1523-1755.2000.00430.x. [DOI] [PubMed] [Google Scholar]
- 18.Reynolds WF, Stegeman CA, Cohen Tervaert JW. –463 G/A myeloperoxidase promoter polymorphism is associated with clinical manifestations and the course of disease in MPO–ANCA-associated vasculitis. Clin Immunol. 2002;103:154–60. doi: 10.1006/clim.2002.5206. [DOI] [PubMed] [Google Scholar]
- 19.Morishita K, Tsuchiya M, Asano S, et al. Chromosomal gene structure of human myeloperoxidase and regulation of its expression by granulocyte colony-stimulating factor. J Biol Chem. 1987;262:15208–13. [PubMed] [Google Scholar]
- 20.Barik S. Site-directed mutagenesis by double polymerase chain reaction: megaprimer method. In: White BA, editor. Methods in molecular biology. Vol. 15. Totowa, NJ: Humana Press Inc.; 1993. pp. 277–86. [DOI] [PubMed] [Google Scholar]
- 21.Ginzinger DG. Gene quantification using real-time quantitative PCR: an emerging technology hits the mainstream. Exp Hematol. 2002;30:503–12. doi: 10.1016/s0301-472x(02)00806-8. [DOI] [PubMed] [Google Scholar]
- 22.Lennartsson A, Pieters K, Ullmark T, et al. AM-1, PU. 1, and Sp3 regulate expression of human bactericidal/permeability-increasing protein. Biochem Biophys Res Commun. 2003;311:853–63. doi: 10.1016/j.bbrc.2003.10.067. [DOI] [PubMed] [Google Scholar]
- 23.Rao NV, Rao GV, Marshall BC, et al. Biosynthesis and processing of proteinase 3 in U937 cells. J Biol Chem. 1996;271:2972–8. doi: 10.1074/jbc.271.6.2972. [DOI] [PubMed] [Google Scholar]
- 24.Labbaye C, Zhang J, Casanova JL, et al. Regulation of myeloblastin messenger RNA expression in myeloid leukemia cells treated with all-trans retinoic acid. Blood. 1993;81:475–81. [PubMed] [Google Scholar]
- 25.Bories D, Raynal MC, Solomon DH, et al. Down-regulation of a serine protease, myeloblastin, causes growth arrest and differentiation of promyelocytic leukemia cells. Cell. 1989;59:959–68. doi: 10.1016/0092-8674(89)90752-6. [DOI] [PubMed] [Google Scholar]
- 26.Halbwachs-Mecarelli L, Bessou G, Lesavre P, et al. Bimodal distribution of proteinase 3 (PR3) surface expression reflects a constitutive heterogeneity in the polymorphonuclear neutrophil pool. FEBS Lett. 1995;374:29–33. doi: 10.1016/0014-5793(95)01073-n. [DOI] [PubMed] [Google Scholar]
- 27.Chen T, Meier R, Ziemiecki A, et al. Myeloblastin/proteinase 3 belongs to the set of negatively regulated primary response genes expressed during in vitro myeloid differentiation. Biochem Biophys Res Commun. 1994;200:1130–5. doi: 10.1006/bbrc.1994.1568. [DOI] [PubMed] [Google Scholar]
- 28.Fiebeler A, Borgmann S, Woywodt A, et al. No association of G-463A myeloperoxidase gene polymorphism with MPO–ANCA-associated vasculitis. Nephrol Dial Transplant. 2004;19:969–71. doi: 10.1093/ndt/gfh025. [DOI] [PubMed] [Google Scholar]
- 29.Piedrafita FJ, Molander RB, Vansant G, et al. An Alu element in the myeloperoxidase promoter contains a composite SP1-thyroid hormone-retinoic acid response element. J Biol Chem. 1996;271:14412–20. doi: 10.1074/jbc.271.24.14412. [DOI] [PubMed] [Google Scholar]
- 30.Segal R, Berk AJ. Promoter activity and distance constraints of one versus two Sp1 binding sites. J Biol Chem. 1991;266:20406–11. [PubMed] [Google Scholar]
- 31.van der Geld YM, Limburg PC, Kallenberg CG. Proteinase 3, Wegener's autoantigen: from gene to antigen. J Leukoc Biol. 2001;69:177–90. [PubMed] [Google Scholar]
- 32.Lin KM, Austin GE. Functional activity of three distinct myeloperoxidase (MPO) promoters in human myeloid cells. Leukemia. 2002;16:1143–53. doi: 10.1038/sj.leu.2402514. [DOI] [PubMed] [Google Scholar]