Abstract
Several cytokines have been implicated individually in the pathogenesis of systemic lupus erythematosus (SLE) and some, including interleukin (IL)-10, IL-12 and IL-1ra are raised during flares of disease activity. Few studies have been directed at examining the interactions between these cytokines and how their combined profile relates to disease activity. We have examined serum levels of IL-10, IL-12 and IL-1ra in a cohort of SLE patients obtained from the Queen Elizabeth Hospital, Birmingham in cross-sectional and, in a smaller group, longitudinal analyses. In the cross-sectional study, there were significant correlations between levels of the three cytokines. There were also significant correlations between levels of each cytokine and measures of disease activity. IL-10 levels correlated with ESR, anti-dsDNA antibody titres and C3D, IL-12 levels with anti-dsDNA antibody titres and IL-1ra levels with ESR, anti-dsDNA antibody titres and C3D. IL-1ra levels also correlated with CRP. Circulating IL-10 and IL-1ra levels were higher in patients with SLE than in normal controls, although in this study group they did not reach significance. Circulating IL-12 levels were, however, significantly higher in SLE compared to controls. This was true both in patients with active disease and those sampled during a quiescent phase. These data add to the evidence that cytokines such as IL-10, IL-12 and IL-1ra are important in SLE pathogenesis. In a retrospective study of serial serum samples from seven patients, we found two patients whose cytokine profile was very different from the rest of the group. In most patients normalized IL-10, IL-12 and IL-1ra levels mirrored BILAG scores closely, but in these two patients, IL-10, IL-12 and IL-1ra levels did not fluctuate with disease activity. It is possible that there is a subgroup of SLE patients whose cytokine profile could be an important indicator of their pathology. In order to confirm this and determine the frequency of such patients this study needs to be repeated with a much larger subject group. The coexistence of patient groups with different patterns of cytokine activity might explain conflicting reports of associations of levels of particular cytokines with SLE. As the observed differences could reflect different aetiologies of SLE, this information could reveal valuable endophenotypes for genetic and functional studies of SLE and might, ultimately, inform therapeutic management.
Keywords: interleukin 1 receptor antagonist (IL-1ra), interleukin-10 (IL-10), interleukin-12 (IL-12), systemic lupus erythematosus (SLE)
INTRODUCTION
Systemic lupus erythematosus (SLE) is an autoimmune disease characterized by severe dysregulation of the immune system, involving increased B cell hyperactivity, production of autoantibodies [1,2] and a wide spectrum of organ involvement. The disease has an episodic course with exacerbation and complete remissions of disease and is more common in women than men. Impaired T lymphocyte (TH cell) regulation results in high levels of autoantibodies which, coupled with failure to remove immune complexes, can cause accumulation and deposition of these complexes in the tissue, leading to vasculitis and other features of SLE, such as glomerulonephritis.
Factors such as ultraviolet (UV) light or infection exacerbate the disease, but it has a strong genetic component: 4% of cases are familial [3] and a range of concordance rates including 24% up to 65% have been reported in monozygotic twins [4]. Genetic factors, such as a high incidence of particular HLA haplotypes [5] and C4-null alleles [6], associations between alleles of genes encoding immunoglobulin constant heavy domains [7], T cell receptor chains [8] and a range of other genes [4] have been reported with presence of SLE. In addition to genetic factors affecting susceptibility to SLE, associations of interleukin (IL)-10, tumour necrosis factor (TNF)-α and the gene encoding IL-1ra alleles (IL-1RN) with clinical course of the disease have also been observed [9–12].
Genetic dissection of complex disorders is, however, extremely difficult and one of the key aspects of successful cohort analysis is effective clinical subdivision of subjects. Since a disorder such as SLE represents an end-point that could be reached in more than one way, it is important to seek key features of pathology that might be used to distinguish between patients with different aetiologies of their disease. We have investigated the profile of circulating cytokines in active and inactive SLE in an attempt to differentiate subgroups of patients with differing pathological mechanisms.
A characteristic observation in SLE is increased production of IL-10 in peripheral blood mononuclear cells (PBMC) compared to healthy controls. Increased IL-10 secretion by monocytes and lymphocytes is postulated to be responsible for increased immunoglobulin production [13]. Serum IL-10 levels are increased in lupus patients compared to controls and correlate with clinical and serological disease activity indices, especially anti-DNA antibody titres [14] and renal damage [15]. Tyrell-Price et al.[16] have suggested that the effect of IL-10 on antibody production from peripheral blood mononuclear cells depends on the disease activity (assessed by the bilag score) at the time of sampling, so decreased bilag was associated with increased antibody production. Increased production of IL-10 during pregnancy may also explain the pregnancy-associated flares and higher rate of miscarriage suffered by women with SLE [17].
Other members of the cytokine network are also important in SLE. The role of IL-12 has also been investigated, but results are apparently contradictory, with reports of increased serum levels in SLE [18], but decreased production by monocytes isolated from people with SLE [1,19]. As with IL-10, Tyrell-Price et al.[16] suggest that the effect of IL-12 on antibody production by isolated PBMCs from patients with SLE may be affected by disease activity (bilag score) at the time of sampling. This might explain some of the contradictory data reported in the literature. In addition to the genetic evidence that IL-1ra may be important in the clinical course of the disease [9], there are several reports of higher IL-1ra levels related to active lupus [20–22] although, as with IL-12, there are also reports of lower production from isolated cells, in this case polymorphonuclear neutrophils, from SLE patients [23].
There is also evidence that serum levels of particular cytokines may be associated with increased susceptibility to particular complications of SLE [18,22,24]. We have investigated the circulating levels of IL-10, IL-12 and IL-1ra in a cohort of SLE patients at a single time-point in order to determine whether there were correlations between these cytokines or between levels of individual cytokines and disease activity or specific organ involvement. In order to examine the profile of cytokine levels in the course of the disease in individual patients, we also carried out a longitudinal study in a smaller group of patients experiencing a flare. We hoped, in this way, to determine whether cytokine profiles during flares and remissions could be used to differentiate between clinical subgroups that might have different aetiologies.
MATERIALS AND METHODS
Subjects
Peripheral blood samples were obtained at routine SLE clinic visits at the Department of Rheumatology, Queen Elizabeth Hospital and University of Birmingham. All patients attending the clinic were invited to participate. All of the patients met the American College of Rheumatology (ACR) classification criteria for SLE [25]. All subjects gave their informed consent to take part in the study and local ethical committee approval was obtained. Control serum was obtained as part of a study of factors predisposing to recurrent pregnancy loss, from 13 healthy fertile women attending Jessop Hospital for Women, Sheffield. The women had a median age of 31 years (range 24–40).
Serum was separated and frozen at −70°C until assayed for the various cytokines. Seventy-four SLE patients (72 female and two male) were assayed in the cross-sectional study. The median age was 42·6 years (range 18·5–72·1) and the median disease duration from diagnosis was 7·5 years (range 2–36 years). For the longitudinal study, we analysed between four and eight serial serum samples collected prospectively from seven female SLE patients over a period of time ranging from 3 to 12 months. These patients were selected for further analysis as they had a flare of disease activity during the 12-month study period. Details of the age, sex and therapy of each patient is contained in Table 1.
Table 1.
Details of age, sex and therapy of each of the seven patients in the longitudinal study
| Therapy | ||||
|---|---|---|---|---|
| Patient | Sex | Age (years) | At time of flare | After flare |
| 1 | F | 34 | Steroid cream | Prednisolone |
| Painkillers | ||||
| 2 | F | 26 | Prednisolone | Prednisolone (increased) |
| Azathioprine | Azathioprine (increased) | |||
| 3 | F | 35 | Prednisolone | Prednisolone (increased) |
| Azathioprine | Azathioprine | |||
| 4 | F | 32 | Hydroxychloroquine | Hydroxychloroquine |
| Prednisolone | Prednisolone | |||
| Azathioprine | ||||
| 5 | F | 35 | Hydroxychloroquine | Hydroxychloroquine |
| Prednisolone | Prednisolone (increased) | |||
| Azathioprine | Azathioprine | |||
| 6 | F | 31 | Hydroxychloroquine | Hydroxychloroquine |
| Prednisolone | ||||
| 7 | F | 53 | Prednisolone | Prednisolone (increased) |
| Hydroxychloroquine | Hydroxychloroquine | |||
| Cyclophosphamide | ||||
Disease activity
Clinical disease activity was measured using the BILAG index at each visit. The bilag index contains 86 variables that are rated by taking into account the change in activity over time. It employs complex formulae to calculate separate scores for eight organ-based systems, e.g. general, mucocutaneous, neurological, musculoskeletal, cardiorespiratory, vasculitis, renal and haematological signs, excluding immunological test results. A graded score of A indicates most active disease, B moderate disease, C mild disease, D systems affected previously and E systems never involved. Scores are complied using the bilag computer program as described previously [26]. The graded scores for each of the bilag systems were converted to a numerical score for statistical analysis using A = 9, B = 3, C = 1, D = 0 and E = 0. For the longitudinal study, a disease flare was defined as the occurrence of new symptoms and signs sufficient to score at least one new A (= 9) or three new B system scores (= 9).
Routine laboratory measures of disease activity were also recorded: these included the erythrocyte sedimentation rate (ESR), C-reactive protein levels, anti-double-stranded DNA antibody titres [measured by enzyme-linked immunosorbent assay (ELISA)], C3 and C4 levels (measured by nephelometry) and complement degradation (C3D).
Cytokine measurements
Levels of IL-10, IL-1ra and IL-12 were assayed using commercially available ELISA kits. The samples were assayed in duplicate where possible. The IL-10 levels were measured using a high sensitivity IL-10 kit from Amersham Life Sciences, Bucks, UK. The serum was used undiluted in the assay. The detection limit of the assay was 0·1 pg/ml with interassay and intra-assay coefficients of variation (CV) of 10%. The IL-12 levels were measured using a Cytoscreen™ ultra-sensitive human IL-12 immunoassay kit from Biosource, UK. Where necessary, because of high cytokine levels, the serum was diluted 1 : 5. The detection limit of the assay was 0·8 pg/ml with an interassay and intra-assay CV of 10·2% and 4·6%, respectively. IL-1ra levels were measured using the Quantikine™ human IL-1ra ELISA kit (R&D Systems, Oxon, UK). The serum was used undiluted in the assay. The detection limit of the assay was 22 pg/ml with an interassay and intra-assay CV of 6·7% and 6·2%, respectively.
Statistical analysis
Statistical analyses were carried out using SPSS. In the cross-sectional study (Table 2), cytokine levels were not distributed normally and so the significance of differences between the levels of various cytokines in patients compared with controls was assessed using the Mann–Whitney U-test. For correlation analysis, levels of the various cytokines and measures of disease activity were log-transformed and tested using Pearson's parametric correlation analysis. The range was 0·4–32 pg/ml, 38–1044 pg/ml and 2950–79 pg/ml for IL-10, IL-12 and IL-1ra, respectively, and no zero values were obtained. For the longitudinal study, results were normalized for each patient: the mean level of each cytokine was calculated for that patient and individual data points were presented as percentages of that level.
Table 2.
Mean serum cytokine levels in healthy controls, SLE patients overall and with active and less active disease using bilag scores of 5, 6 and 9 as the cut-off points
| bilag 5 or more | bilag 6 or more | bilag 9 or more | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Serum IL-10 (pg/ml) Mean ± s.e.m. | Serum IL-12 (pg/ml) Mean ± s.e.m. | Serum IL-1ra (pg/ml) Mean ± s.e.m. | Serum IL-10 (pg/ml) Mean ± s.e.m. | Serum IL-12 (pg/ml) Mean ± s.e.m. | Serum IL-1ra (pg/ml) Mean ± s.e.m. | Serum IL-10 (pg/ml) Mean ± s.e.m. | Serum IL-12 (pg/ml) Mean ± s.e.m. | Serum IL-1ra (pg/ml) Mean ± s.e.m. | |
| Controls | 4·09 ± 2·1(n = 12) | 56·01 ± 11·57(n = 13) | 308·71 ± 74·14(n = 13) | 4·09 ± 2·1(n = 12) | 56·01 ± 11·57(n = 13) | 308·71 ± 74·14(n = 13) | 4·09 ± 2·1(n = 12) | 56·01 ± 11·57(n = 13) | 308·71 ± 74·14(n = 13) |
| SLE | 6·58 ± 0·81(n = 74) | 261·64 ± 24·78*(n = 68) | 475·90 ± 61·85(n = 71) | 6·58 ± 0·81(n = 74) | 261·64 ± 24·78*(n = 68) | 475·90 ± 61·85(n = 71) | 6·58 ± 0·81(n = 74) | 261·64 ± 24·78*(n = 68) | 475·90 ± 61·85(n = 71) |
| Active SLE | 7·55 ± 1·04(n = 52) | 271·95 ± 30·46*(n = 48) | 487·13 ± 79·66(n = 50) | 7·55 ± 1·04(n = 52) | 271·95 ± 30·46*(n = 48) | 487·13 ± 79·65(n = 50) | 7·79 ± 1·33(n = 33) | 273·72 ± 43·48*(n = 32) | 430·03 ± 78·97(n = 30) |
| Inactive SLE | 3·96 ± 0·66(n = 22) | 203·47 ± 31·18*(n = 20) | 414·82 ± 70·68(n = 21) | 4·00 ± 0·69(n = 22) | 214·55 ± 30·81*(n = 20) | 421·05 ± 73·84(n = 21) | 5·43 ± 0·90(n = 41) | 234·33 ± 24·10*(n = 36) | 494·29 ± 87·50(n = 41) |
Significant at the P = 0·001 level (two-tailed).
RESULTS
Cytokine levels in the cross-sectional study
IL-10, IL-12 and IL-1ra levels were measured in serum samples taken at a single time-point from a cohort of 74 patients with SLE (the number of samples in each group varies between analyses as there was insufficient serum to carry out all the analyses). IL-12 levels were significantly raised in patients with SLE compared to normal controls (Table 2, P < 0·01). The level of IL-12 was also significantly raised in active disease versus controls (P < 0·01) and inactive disease versus controls (P < 0·01). However, the mean levels of IL-12 were not significantly different between active and less active disease. In this study group there was a significant difference in means of IL-10 levels. They were higher in patients than controls and higher in active disease than in less active disease, but this did not reach significance (Table 2, P = 0·328 and P = 0·054, respectively). Similarly, IL-1ra levels were higher in patients than controls and higher in active disease than in quiescent disease, but this was not significant (Table 2, P = 0·116 and (P = 0·928), respectively.
Correlations of cytokine levels and measurements of disease activity
To determine whether there was a relationship between levels of the various cytokines, we carried out pairwise analysis using Pearson's correlation on log-transformed data. Significant correlations were found between IL-10 and IL-1ra and IL-10 and IL-12 (Table 3). Scatter plots of these correlations appeared linear and did not reveal any extreme outliers. We also found correlations between log-transformed measurements of disease activity and log-transformed values of the various cytokines. These included IL-10 with ESR, anti-dsDNA antibody titres and C3D, IL-12 with anti-dsDNA antibody titres and IL-1ra with ESR, anti-dsDNA antibody titres, C3D and CRP (Table 4). There were no significant correlations between log-transformed levels of cytokines and bilag scores when judged globally. There was no significant difference in levels of any of the cytokines investigated between patients with and without particular ACR classification criteria [25].
Table 3.
Correlation analysis on log-transformed cytokine levels in SLE patients using bilag scores of 5, 6 and 9 as the cut-off points
| bilag of 5 or more | bilag of 6 or more | bilag of 9 or more | ||||||
|---|---|---|---|---|---|---|---|---|
| All SLE | Active SLE | Inactive SLE | Active SLE | Inactive SLE | Active SLE | Inactive SLE | ||
| IL-10 + | Pearson's correlation significance | 0·293* | 0·331* | −0·081 | 0·329* | −0·086 | 0·390* | 0·045 |
| IL-12 | (two-tailed) | 0·016 | 0·023 | 0·742 | 0·024 | 0·726 | 0·033 | 0·795 |
| N = | 67 | 47 | 19 | 47 | 19 | 30 | 36 | |
| IL-1 + | Pearson's correlation significance | 0·308* | 0·425** | 0·052 | 0·427** | 0·052 | 0·527** | 0·181 |
| IL-1ra | (two-tailed) | 0·009 | 0·008 | 0·838 | 0·007 | 0·837 | 0·010 | 0·31 |
| N = | 71 | 38 | 18 | 38 | 18 | 23 | 33 | |
| IL-12 + | Pearson's correlation significance | 0·211 | 0·288 | −0·009 | 0·288 | −0·014 | 0·506* | −0·073 |
| (two-tailed) | 0·092 | 0·094 | 0·975 | 0·093 | 0·959 | 0·016 | 0·706 | |
| IL-1ra | N = | 65 | 35 | 16 | 35 | 16 | 22 | 29 |
Correlation is significant at the P = 0·05 level (two-tailed),
correlation is significant at the P = 0·01 level (two-tailed).
Table 4.
Correlation between log transformed cytokine levels and measurements of disease activity or inflammation
| bilag | ESR | dsDNA | C3D | CRP | ||
|---|---|---|---|---|---|---|
| IL-10 | Pearson's correlation | 0·212 | 0·384** | 0·601*** | 0·353** | 0·193 |
| P = (two-tailed) | 0·076 | 0·001 | 0·0001 | 0·003 | 0·114 | |
| N = | 71 | 69 | 36 | 70 | 68 | |
| IL-12 | Pearson's correlation | 0·016 | 0·228 | 0·357* | 0·158 | 0·119 |
| P = (two-tailed) | 0·898 | 0·072 | 0·045 | 0·211 | 0·356 | |
| N = | 65 | 63 | 32 | 64 | 62 | |
| IL-1ra | Pearson's correlation | −0·067 | 0·361** | 0·438** | 0·326** | 0·440*** |
| P = (two-tailed) | 0·580 | 0·002 | 0·008 | 0·006 | 0·0001 | |
| N = | 71 | 69 | 36 | 70 | 68 |
Correlation is significant at the P = 0·05 level (two-tailed);
correlation is significant at the P = 0·01 level (two-tailed);
correlation is significant at the P = 0·001 level (two-tailed).
When patients with inactive and active disease were analysed separately, correlations between IL-10 and IL-1ra (P = 0·05) and IL-12 and IL-1ra (P = 0·05) were revealed (Table 3) in active disease. No significant correlations between levels of the three cytokines were observed in samples taken when the disease was less active (Table 3). When patients with active and quiescent disease were analysed separately, all the correlations in active disease shown in Table 4 remained significant with the exception of IL-12 levels and anti-dsDNA antibodies, which were significant in neither group. Correlations between IL-10 and ESR and C3D and between IL-1ra and anti-dsDNA antibodies were also different and were not significant in patients with less active disease.
Longitudinal study
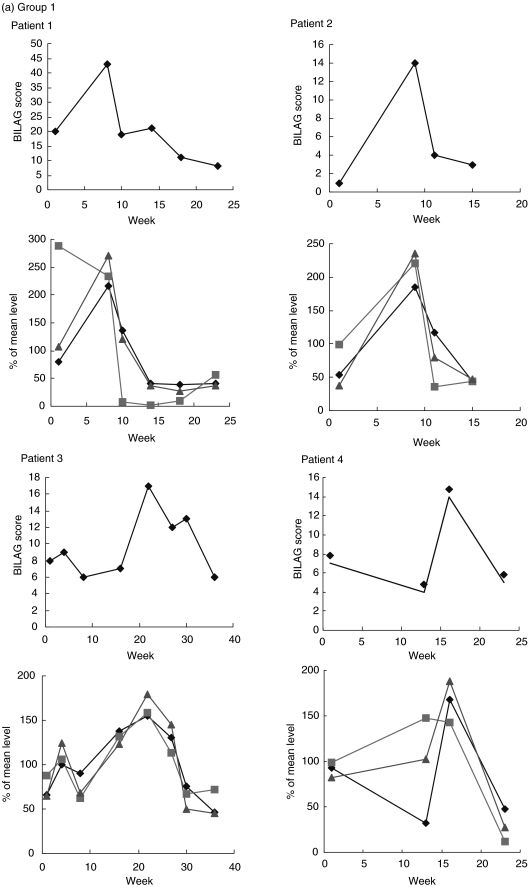
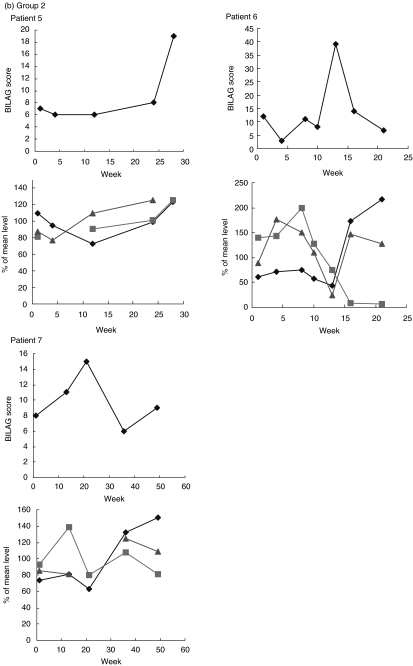
IL-10, IL-12 and IL-1ra levels were measured in sequential serum samples taken from seven patients over a period of time that included a flare of disease activity. The profile of relative IL-10 and IL-1ra levels were similar, but IL-12 levels seemed more variable, rising before the flare in patients 1, 4 and 7. Based on the cytokine profiles observed, it may be possible to divide the patients into groups according to IL-10 and IL-1ra profile. In patients 1, 2, 3 and 4, circulating IL-10 and IL-1ra levels closely mirrored bilag scores of disease activity (Fig. 1a). In patient 5, levels of IL-10 and IL-1ra did not appear to reflect disease activity quite as markedly, but in patients 6 and 7 the data were strikingly different, with levels of IL-10 and IL-1ra rising before the flare and lowest when bilag score was highest (Fig. 1b).
Fig. 1.
Top graphs for each patient are times of disease flare indicated by the bilag score, lower graphs are levels of IL-10, IL-12 and IL-1ra (all cytokines are expressed as deviations from the mean level for that patient). (a) Group 1: patients whose IL-10 and IL-ra levels mirror disease activity score. (b) Group 2: patients whose IL-10 and IL-ra levels do not closely mirror disease activity score. ⋄, IL-10; ░, IL-12;  , IL-ra.
, IL-ra.
DISCUSSION
We have investigated cytokine levels in SLE cross-sectionally and also, in a smaller group of patients, longitudinally. The results of the longitudinal study indicate that patients differ based on their normalized cytokine profile. In five of seven patients normalized IL-10, IL-12 and IL-1ra levels fluctuated with disease activity (assessed by bilag score), whereas in the two others the levels of cytokines did not fluctuate with disease activity. Although the number of patients investigated in this way was very small, the data on the different cytokines was consistent. Normalization of the data facilitates meaningful comparison of individuals with very different serum cytokine levels and compensates for the marked interindividual differences in basal levels of circulating cytokines that are observed in all populations studied.
The study of Tyrell-Price et al. [16] demonstrated marked interindividual variation in autoantibody production by PBMCs from SLE patients after treatment with IL-10 and IL-12. This study also included only a small number of subjects. It is possible that a larger study, combining profiling of circulating IL-10, IL-1ra and IL-12 levels with stimulation of isolated PBMCs for the same patients might clarify whether patients whose PBMCs respond differently to cytokine treatment represent members of the different categories described above.
IL-10 is increased in SLE [14]. In our study, differences in mean serum IL-10 levels between patients and controls and between patients sampled during active and less active periods of disease were not significant. There were, however, highly significant correlations between serum IL-10 levels and three of the four clinical markers investigated, confirming an important role for IL-10 in lupus. This is underlined further by genetic studies of IL-10 promoter polymorphisms that report association with susceptibility to or the risk of particular complications of SLE [10,27–29].
Circulating IL-1ra levels have also been reported to be raised in women with SLE [20] compared to control subjects. Other groups have confirmed this finding, but have reported lack of correlation between serum IL-1ra levels and SLEDAI scores during flares in disease [22]. The two studies may have included varying proportions of patients with active and inactive disease at the time of sampling and different numbers of patients from the two cytokine profile groups that we describe. In our cross-sectional study group, IL-1ra was higher in SLE patients than in control samples, but this was not significant, due perhaps to the limited number of control samples. Interestingly, Heward et al.[30] did not find an association between IL-1RN genotype and SLE, but this polymorphism may not affect IL-1ra levels. We did observe highly significant correlation of IL-1ra levels with all clinical parameters assessed. Interestingly, IL-1ra levels showed a highly significant correlation with CRP, a general marker of inflammation, not generally raised in lupus patients. In lupus patients, who as a group have an increased risk of atherosclerosis, CRP is a good predictor of cardiovascular risk [31]. Approximately 70% of the patients in our study had raised CRP levels, but unfortunately we do not have data on atherosclerosis in this patient group. It has also been suggested recently that IL-1ra is an important factor in atherosclerosis in general [32–34] and in atherosclerosis related to infective agents [35]. Polymorphisms in the IL-1ra gene are associated with atherosclerosis [36] and IL-1ra-deficient mice develop severe arteritis [37]. IL-1ra levels may, therefore, may be worth investigating as an indicator of long-term cardiovascular risk in SLE. Genetic association studies indicate that polymorphisms in the IL-1 gene cluster may, while not necessarily promoting susceptibility to SLE, have important implications for clinical outcome [9].
There are also puzzling differences between reports of IL-12 activity in lupus. Liu et al.[1] found lower IL-12 production by PBMCs from SLE patients compared with PBMCs from controls, with levels lowest in samples from patients with high anti-dsDNA antibody titres. Tokano et al.[18] found raised serum IL-12 levels in SLE patients compared to controls, but reported that patients with high levels of IL-12 had less frequent organ involvement in SLE. In particular, patients with pulmonary involvement had a high level of IL-12, although the number of cases was very small. In our study, mean IL-12 level was significantly higher in patients with SLE than in controls and also significantly different in controls compared to active and in controls compared to quiescent disease, but was not significantly different in active compared to quiescent disease. Among the clinical parameters that we investigated, IL-12 levels correlated only with anti-dsDNA antibody titres. Analysis of our cohort of SLE patients did not reveal any association of the cytokines investigated with particular ARA clinical criteria. Tokano et al. [18] reported that a rise in circulating IL-12 level precedes a flare of disease activity and this can be observed in patients 1 and 4 as well as patients 6 and 7.
The lack of significant differences in circulating IL-10, IL-12 or IL-1ra between active and less active disease could be explained by differing cytokine profiles surrounding a flare in disease activity in different patients. From this and other studies, it is clear that cytokine levels are directly or indirectly involved in the pathological processes underlying SLE, but it is important to recognize that a clinical diagnosis of SLE might be reached by a range of aetiological routes and that different factors and pathologies may be operating in different subclasses of patients. Further, in patients with SLE, different cytokine profiles might be associated with flares initiated by different factors (UV, infection, hormones or stress, for example) or different organ systems. Our observation must be replicated in a prospective study including a much larger group of patients to clarify the full implications of differences in cytokine profiles as related to indices of disease activity. In this future study, it might also be useful to analyse patients with a single A-score separately to prevent confusion of those with widespread mild disease as opposed to severe disease affecting only a single organ/system.
A major difficulty in the analysis of complex diseases lies in the definition of such subclasses of disease, by identification of specific endophenotypes. If our observations are confirmed by a larger study, we believe that longitudinal cytokine profiling is a possible approach to identifying subgroups of patients with differing pathologies. This could have important implications for subdivision of patients included in genetic studies as well as for assessment of susceptibility to certain complications, which could, ultimately, be useful in monitoring and management of their disease.
If the phenomena described here are confirmed in a much larger study, they may go some way towards explaining conflicting reports of cytokines associated with SLE. Since different patients tested might belong to different cytokine profile groups, it is difficult to assess the significance of cytokine levels in patients sampled at a single time-point. This may explain the differences between various published studies, particularly those including modest numbers of patients.
Acknowledgments
We would like to thank the Arthritis Research Council (ARC) for their financial support in order to carry out this work. Lupus UK supports the Birmingham lupus cohort collection.
REFERENCES
- 1.Liu TF, Jones BM. Impaired production of IL-12 in systemic lupus erythematosus I. Excessive production of IL-10 suppresses production of IL-12 by monocytes. Cytokine. 1998;10:140–7. doi: 10.1006/cyto.1997.0268. [DOI] [PubMed] [Google Scholar]
- 2.Mehrian R, Quismorio FP, Jr, Strassmann G, et al. Synergistic effect between IL-10 and bcl-2 genotypes in determining susceptibility to systemic lupus erythematosus. Arthritis Rheum. 1998;41:596–602. doi: 10.1002/1529-0131(199804)41:4<596::AID-ART6>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 3.Fielder AH, Walport MJ, Batchelor JR, et al. Family study of the major histocompatibility complex in patients with systemic lupus erythematosus: importance of null alleles of C4A and C4B in determining disease susceptibility. Br Med J (Clin Res Ed) 1983;286:425–8. doi: 10.1136/bmj.286.6363.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Criswell LA, Amos CI. Update on genetic risk factors for systemic lupus erythematosus and rheumatoid arthritis. Curr Opin Rheumatol. 2000;12:85–90. doi: 10.1097/00002281-200003000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Grandos J, Vargas-Alarcon G, Andrade F, Melin-Aldana H, Alcocer-Varela J, Alarcon-Segovia D. The role of HLA-DR alleles and complotypes through the ethnic barrier in systemic lupus erythematosus in Mexicans. Lupus. 1996;5:184–9. doi: 10.1177/096120339600500304. [DOI] [PubMed] [Google Scholar]
- 6.Huang DF, Siminovitch KA, Liu XY, et al. Population and fanily studies of three disease related polymorphic genes in systemic lupus erythematosus. J Clin Invest. 1995;95:1766–72. doi: 10.1172/JCI117854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumar A, Martinez-Tarquino C, Maria-Forte A, et al. Immunoglobulin heavy chain constant-region gene polymorphsims in systemic lupus erythematosus. Arthritis Rheum. 1991;34:1553–6. doi: 10.1002/art.1780341212. [DOI] [PubMed] [Google Scholar]
- 8.Tebib JG, Alcocer-Varela J, Alarcon-Segovia D, Schur PH. Associations between a T cell receptor restriction fragment length polymorphism and systemic lupus erythematosus. J Clin Invest. 1990;86:1961–7. doi: 10.1172/JCI114930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blakemore AI, Tarlow JK, Cork MJ, Gordon C, Emery P, Duff GW. Interleukin-1 receptor antagonist gene polymorphism as a disease severity factor in systemic lupus erythematosus. Arthritis Rheum. 1994;37:1380–5. doi: 10.1002/art.1780370917. [DOI] [PubMed] [Google Scholar]
- 10.Rood MJ, Keijsers V, van der Linden MW, et al. Neuropsychiatric systemic lupus erythematosus is associated with imbalance in interleukin 10 promoter haplotypes. Ann Rheum Dis. 1999;58:85–9. doi: 10.1136/ard.58.2.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hajeer AH, Worthington J, Davies EJ, Hillarby MC, Poulton K, Ollier WE. TNF microsatellite a2, b3 and d2 alleles are associated with systemic lupus erythematosus. Tissue Antigens. 1997;49:222–7. doi: 10.1111/j.1399-0039.1997.tb02742.x. [DOI] [PubMed] [Google Scholar]
- 12.Tjernstrom F, Hellmer G, Nived O, Truedsson L, Sturfelt G. Synergetic effect between interleukin-1 receptor antagonist allele (IL1RN*2) and MHC class II (DR17,DQ2) in determining susceptibility to systemic lupus erythematosus. Lupus. 1999;8:103–8. doi: 10.1191/096120399678847560. [DOI] [PubMed] [Google Scholar]
- 13.Llorente L, Zou W, Levy Y, et al. Role of IL-10 in the B lymphocyte hyperactivity and autoantiody production of human SLE. J Exp Med. 1995;181:839–44. doi: 10.1084/jem.181.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Houssiau FA, Lefebvre C, Vanden Berghe M, Lambert M, Devogelaer JP, Renauld JC. Serum IL-10 titres in systemic lupus erythematosus reflect disease activity. Lupus. 1995;4:393–5. doi: 10.1177/096120339500400510. [DOI] [PubMed] [Google Scholar]
- 15.Kotzin BL. Systemic lupus erythematosus. Cell. 1996;85:303–6. doi: 10.1016/s0092-8674(00)81108-3. [DOI] [PubMed] [Google Scholar]
- 16.Tyrell-Price J, Lydyard PM, Isenberg DA. The effect of interleukin-10 and interleukin-12 on the in vitro production of anti-double stranded DNA antibodies from patients with systemic lupus erythematosus. Clin Exp Immunol. 2001;124:118–25. doi: 10.1046/j.1365-2249.2001.01466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilder RL. Hormones, pregnancy, and autoimmune diseases. Ann NY Acad Sci. 1998;840:45–50. doi: 10.1111/j.1749-6632.1998.tb09547.x. [DOI] [PubMed] [Google Scholar]
- 18.Tokano Y, Morimoto S, Kaneko H, et al. Levels of IL-12 in the sera of patients with systemic lupus erythematosus (SLE) − relation to Th1 and Th2 derived cytokines. Clin Exp Immunol. 1999;116:169–73. doi: 10.1046/j.1365-2249.1999.00862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horwitz DA, Gray JD, Behrendsen SC, et al. Decreased production of IL-12 and other Th1 type cytokines in patients with recent onset systemiclupus erythematosus. Arthritis Rheum. 1998;41:838–44. doi: 10.1002/1529-0131(199805)41:5<838::AID-ART10>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 20.Suzuki H, Takemura H, Kashiwagi H. IL-1ra in patients with active systemic lupus erythematosus. Enhanced production by monocytes and correlation with disease activity. Arthritis Rheum. 1995;38:1055–9. doi: 10.1002/art.1780380806. [DOI] [PubMed] [Google Scholar]
- 21.Chang DM. Interleukin-1 and interleukin-1 receptor antagonist in systemic lupus erythematosus. Immunol Invest. 1997;26:649–59. doi: 10.3109/08820139709088547. [DOI] [PubMed] [Google Scholar]
- 22.Sturfelt G, Roux-Lombard P, Wollheim FA, Dayer JM. Low levels of IL-1ra coincide with kidney involvement in systemic lupus erythematosus. Br J Rheumatol. 1997;36:1283–9. doi: 10.1093/rheumatology/36.12.1283. [DOI] [PubMed] [Google Scholar]
- 23.Hsieh SC, Tsai CY, Sun KH, et al. Defective spontaneous and bacterial lipopolysaccharide-stimulated production of interleukin-1 receptor antagonist by polymorphonuclear neutrophils of patients with active systemic lupus erythematosus. Br J Rheumatol. 1995;34:107–12. doi: 10.1093/rheumatology/34.2.107. [DOI] [PubMed] [Google Scholar]
- 24.Mok CC, Lanchbury JS, Chan DW, Lau CS. IL-10 promoter polymorphisms in Southern Chinese patients with systemic lupus erythematosus. Arthritis Rheum. 1998;41:1090–5. doi: 10.1002/1529-0131(199806)41:6<1090::AID-ART16>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 25.Tan EM, Cohen AS, Fries JF, et al. The revised criteria for the 1982 classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–7. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 26.Hay EM, Bacon PA, Gordon C, et al. The bilag index: a reliable and valid instrument for measuring clinical disease activity in systemic lupus erythematosus. Q J Med. 1993;86:447–58. [PubMed] [Google Scholar]
- 27.Turner DM, Williams DM, Sankaran D, Lazarus M, Sinnott PJ, Hutchinson IV. An investigation of polymorphism in the interleukin-10 gene promoter. Eur J Immmunogenet. 1997;24:1–8. doi: 10.1111/j.1365-2370.1997.tb00001.x. [DOI] [PubMed] [Google Scholar]
- 28.Lazarus M, Hajeer AH, Turner D, et al. Genetic variation in the interleukin 10 gene promoter and systemic lupus erythematosus. J Rheumatol. 1997;24:2314–7. [PubMed] [Google Scholar]
- 29.Gibson AW, Edberg JC, Wu J, Westendorp RG, Huizinga TW, Kimberly RP. Novel single nucleotide polymorphisms in the distal IL-10 promoter affect IL-10 production and enhance the risk of systemic lupus erythematosus. J Immunol. 2001;166:3915–22. doi: 10.4049/jimmunol.166.6.3915. [DOI] [PubMed] [Google Scholar]
- 30.Heward J, Allahabadia A, Gordon C, et al. The interleukin-1 receptor antagonist gene shows no allelic association with three autoimmune diseases. Thyroid. 1999;9:627–8. doi: 10.1089/thy.1999.9.627. [DOI] [PubMed] [Google Scholar]
- 31.Manzi S, Selzer F, Sutton-Tyrrell K, et al. Prevalence and risk factors of carotid plaque in women with systemic lupus erythematosus. Arthritis Rheum. 1999;42:51–60. doi: 10.1002/1529-0131(199901)42:1<51::AID-ANR7>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 32.Elhage R, Maret A, Pieraggi MT, Thiers JC, Arnal JF, Bayard F. Differential effects of interleukin-1 receptor antagonist and tumour necrosis factor binding protein on fatty-streak formation in apolipoprotein E-deficient mice. Circulation. 1998;97:242–4. doi: 10.1161/01.cir.97.3.242. [DOI] [PubMed] [Google Scholar]
- 33.Fiotti N, Giansante C, Ponte E, et al. Atherosclerosis and inflammation. Patterns of cytokine regulation in patients with peripheral arterial disease. Atherosclerosis. 1999;145:51–60. doi: 10.1016/s0021-9150(99)00013-1. [DOI] [PubMed] [Google Scholar]
- 34.Dewberry R, Holden H, Crossman D, Francis S. Interleukin-1 receptor antagonist expression in human endothelial cells and atherosclerosis. Arterioscler Thromb Vasc Biol. 2000;20:2394–400. doi: 10.1161/01.atv.20.11.2394. [DOI] [PubMed] [Google Scholar]
- 35.Auer J, Berent R, Weber T, Eber B. Interleukin-1 receptor antagonist gene polymorphism, infectious burden, and coronary artery disease. Clin Infect Dis. 2002;34:1536–7. doi: 10.1086/340531. [DOI] [PubMed] [Google Scholar]
- 36.Francis SE, Camp NJ, Dewberry RM, et al. Interleukin-1 receptor antagonist gene polymorphism and coronary artery disease. Circulation. 1999;99:861–6. doi: 10.1161/01.cir.99.7.861. [DOI] [PubMed] [Google Scholar]
- 37.Nicklin MJ, Hughes DE, Barton JL, Ure JM, Duff GW. Arterial inflammation in mice lacking the interleukin 1 receptor antagonist gene. J Exp Med. 2000;191:303–12. doi: 10.1084/jem.191.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]