Abstract
Systemic sclerosis (SSc) is characterized by multi-organ fibrosis with an autoimmune background. Although autoantibodies are detected frequently in SSc patients, the role of autoantibody in the development of fibrosis remains unknown. Connective tissue homeostasis is a balance between the synthesis and degradation of the extracellular matrix (ECM); ECM degradation is regulated mainly by matrix metalloproteinases (MMPs). Anti-MMP-1 antibody is suggested to inhibit MMP-1 and be involved in the development of the fibrosis in SSc. However, the accumulation of various ECM components in the tissue of SSc cannot be explained by the anti-MMP-1 antibody alone. In this study, we examined the presence or levels of antibody to MMP-3, a protein which degrades various ECM components relevant to SSc fibrosis. Enzyme-linked immunosorbent assay (ELISA) using human recombinant MMP-3 revealed that IgG anti-MMP-3 autoantibody levels were elevated significantly in the sera from SSc patients, but not in patients with active systemic lupus erythematosus or dermatomyositis. IgG and IgM anti-MMP-3 antibody levels were significantly higher in diffuse cutaneous SSc, a severe form, than those in limited cutaneous SSc. Consistently, IgG anti-MMP-3 antibody levels correlated significantly with fibrosis of the skin, lung and renal blood vessels. The presence of IgG anti-MMP-3 autoantibody in sera from SSc patients was confirmed by immunoblotting analysis. Remarkably, MMP-3 activity was inhibited by IgG anti-MMP-3 antibody. These results suggest that anti-MMP-3 antibody is a serological marker that reflects the severity of SSc and also suggest that it may contribute to the development of fibrosis by inhibiting MMP-3 activity and reducing the ECM turnover.
Keywords: autoantibody, extracellular matrix, matrix metalloproteinase-3, systemic sclerosis
INTRODUCTION
Systemic sclerosis (SSc) is a multi-system disorder of connective tissue characterized by excessive fibrosis in the skin and various internal organs such as the lungs, kidneys, oesophagus and heart. Excessive accumulation of extracellular matrix (ECM) components, especially types I and III collagen, is a main pathogenic event in SSc [1–3]. Increased expression of types V, VI and VII collagen, fibronectin, glycosaminoglycans and decorin in tissue and fibroblasts cultured from patients with SSc has also been reported [4–6].
Matrix metalloproteinases (MMPs), zinc-dependent endopeptidases, are the major enzymes responsible for degradation of ECM components. MMP-1 degrades extracellular fibres comprised of types I, II, III, VII, VIII, X and XI collagen, fibronectin, laminin, tenascin and vitronectine, while MMP-3 (stromelysin-1) has a broader range of substrates, including types III, IV, V, VII, IX and X collagen, decorin, elastin, fibrillin, fibronectin, laminin, tenascin, vitronectine, osteonectin, pro-MMP-1, pro-MMP-8, pro-MMP-9 and pro-MMP-13 [7]. MMP-3 is expressed by fibroblasts, chondrocytes, osteoblasts, endothelial cells, smooth muscle cells and macrophages. MMPs are synthesized and secreted from cells to the extracellular space as proenzymes and are converted to active forms by limited digestion by proteases including plasmin [8]. The activity of MMP is inhibited specifically by tissue inhibitors of metalloproteinases (TIMPs), which bind to activated MMPs with 1 : 1 molar stoichiometry [9]. Reduced collagen degradation due to MMP insufficiency might play an important role in SSc [10].
Systemic autoimmunity is one of the central features of SSc because antinuclear antibodies are detected in more than 90% of patients with SSc [11]. Although the pathogenic role of autoantibodies in SSc remains unknown, a relationship between autoantibodies and ongoing tissue damage has been suggested: antitopoisomerase I antibody levels correlate closely with disease activity and severity in SSc [12]. Furthermore, the elimination of autoantibody production results in diminished skin fibrosis in a tight-skin mouse, a genetic model for human SSc [13]. We have reported that anti-MMP-1 autoantibody that is able to inhibit the enzymatic activity of MMP-1 is detected in patients with SSc [10]. However, ECM components relevant to the fibrosis associated with SSc, including type V collagen, decorin, osteonectin, elastin and fibrillin, are degraded not by MMP-1, but by MMP-3. Therefore, we hypothesized that autoantibody against MMP-3 is also produced in SSc. To test this possibility, the presence or levels of anti-MMP-3 antibodies, their clinical correlation and their functional significance were investigated in this study.
MATERIALS AND METHODS
Serum samples
Serum samples were obtained from 58 Japanese patients with SSc (54 females and four males). All patients fulfilled the criteria proposed by the American College of Rheumatology [14]. These patients were grouped according to the classification system proposed by LeRoy et al. [15]: 30 patients (all females) had limited cutaneous SSc (lSSc) and 28 patients (24 females and four males) had diffuse cutaneous SSc (dSSc). The age of patients with SSc [mean ± standard deviation (s.d.)] was 51 ± 15. Patients with dSSc were 47 ± 18 years old while those with lSSc were 53 ± 12 years old. Anti-topoisomerase I antibody was positive for 24 (dSSc, 23 and lSSc, one), anticentromere antibody for 24 (all lSSc), anti-U1RNP antibody for four (dSSc, three and lSSc, one), and anti-Th/To antibody for two (all dSSc). Five patients (dSSc, two and lSSc, three) had antinuclear antibodies by indirect immunofluorescence using HEp-2 cells as substrate, but their specificities were not identified by autoantibody-specific enzyme-linked immunosorbent assay (ELISA) or immunoprecipitation. The disease duration of patients with lSSc and dSSc was 9·7 ± 10·0 and 5·2 ± 6·8 years, respectively. None of the SSc patients were treated with steroids, d-penicillamine or immunosuppressive therapy. Twenty-two patients with systemic lupus erythematosus (SLE), who fulfilled American College of Rheumatology criteria [16], were also examined as disease control. These patients had active SLE as determined by the SLE disease activity index [17]. In addition, 14 patients with dermatomyositis (DM) that fulfilled Bohan & Peter criteria [18,19] were included. Twenty-four age- and sex-matched Japanese healthy individuals were used as normal controls. Fresh venous blood samples were centrifuged shortly after clot formation. All samples were stored at −70°C prior to use. The protocol was approved by Kanazawa University Graduate School of Medical Science, and informed consent was obtained from all patients.
Clinical assessment
Complete medical histories, physical examinations and laboratory tests were conducted for all patients. Skin score was measured by scoring technique of the modified Rodnan total skin thickness score (modified Rodnan TSS) [20]. The anatomical areas were rated as 0 (normal skin thickness), 1 + (mild but definite thickening), 2 + (moderate skin thickening) and 3 + (severe skin thickening) and the modified Rodnan TSS was derived by summation of the score from all 17 areas (range 0–51). Organ involvement was defined as described previously [21]: lung = bibasilar fibrosis on chest radiography and high resolution computed tomography; oesophagus = hypomotility shown by barium radiography; joint = inflammatory polyarthralgias or arthritis; heart = peri-carditis, congestive heart failure or arrhythmias requiring treatment; kidney = malignant hypertension and rapidly progressive renal failure without any other explanation; and muscle = proximal muscle weakness and elevated serum creatinine kinase. Renal vascular damage was determined as pulsatility index by colour-flow Doppler ultrasonography of both kidneys (Aloka SSD-2000 scanner, Aloka, Tokyo, Japan) [22]. The pulsatility index, which represents vascular impedance, was calculated as A-B/mean, where A is the peak systolic frequency, B is the end diastolic frequency, and the mean is the time-averaged frequency. The pulsatility index was calculated as an average value obtained with eight waveforms on the renal interlobar arteries of both kidneys. Pulmonary function, including vital capacity (VC) and diffusion capacity for carbon monoxide (Dlco), was also tested. When the Dlco and VC were <75% and <80%, respectively, of the normal values, they were considered to be abnormal.
ELISA for anti-MMP-3 antibody
ELISAs were conducted as previously described [23]. Ninety-six-well plates (EIA/RIA plate, Costar, Cambridge, MA, USA) were coated with human recombinant MMP-3 (1 µg/ml; R&D Systems, Inc., Minneapolis, MN, USA) at 4°C overnight. The wells were blocked with 2% bovine serum albumin and 1% gelatin in Tris-buffered saline for 1 h at 37°C and the serum samples (100 µl) diluted to 1 : 100 were added to triplicate wells for 90 min at 20°C. After washing four times, the bound antibodies were detected with alkaline phosphatase-conjugated goat antihuman IgG or IgM antibodies (Cappel, Aurora, OH, USA), using p-nitrophenyl phosphate (Sigma-Aldrich, St Louis, MO, USA) as substrate. The optical density (OD) of the wells was subsequently determined.
Immunoblotting
Human recombinant MMP-3 (1 µg/lane; R&D Systems, Inc.) was subjected to electrophoresis on 10–20% gradient sodium dodecyl sulphate-polyacrylamide slab gels. The proteins were electrotransferred from gels to nitrocellulose sheets for immunoblotting analysis. The nitrocellulose sheets were cut into strips and incubated overnight with serum samples diluted 1 : 50. The strips were then incubated for 1·5 h with alkaline phosphatase-conjugated goat antihuman IgG antibody (Cappel), and colour was developed using an amplified alkaline phosphatase immunoblot assay kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Ten SSc patients positive for IgG anti-MMP-1 antibody by ELISA, nine SSc patients positive for either antitopoisomerase I antibody or anticentromere antibody, but not for IgG anti-MMP-3 antibody by ELISA, and five healthy individuals were evaluated.
MMP-3 activity inhibition assay
IgG was purified from serum samples using magnetic beads coated with recombinant Protein G covalently coupled to the surface (Dynal Biotech ASA, Oslo, Norway). Final IgG concentration was measured by spectrophotometer (gene Quont II, Amsterdam Bioscience, Inc., Piscataway, NJ, USA). MMP-3 activity was determined by a stromelysin (MMP-3) activity kit (Yagai, Yamagata, Japan), according to the manufacturer's protocol. Briefly, 60 ng of MMP-3 was incubated with 40 µg of purified IgG for 20 min at 20°C. The enzymatic activity of MMP-3 was measured using fluorescein isothiocyanate-labelled acetyl casein solution as a substrate. The fluorescence of substrate cleaved by MMP-3 was measured with excitation and emission at 495 nm and 520 nm, respectively. MMP-3 untreated with purified IgG served as positive control. Ten SSc patients positive for anti-MMP-3 antibody by ELISA, 10 SSc patients positive for either antitopoisomerase I antibody, anticentromere antibody or anti-U1RNP antibody, but not for IgG anti-MMP-3 antibody by ELISA, and 10 healthy individuals were assessed.
MMP-3 mRNA expression by real-time polymerase chain reaction (PCR)
MMP-3 mRNA expression was assessed by real-time PCR in lesional skin tissues from five early dSSc patients (all females, age 45·8 ± 6·0 years, disease duration 1·8 ± 1·1 years) positive for anti-MMP-3 antibody levels by ELISA, five early dSSc patients (all females, age 56·2 ± 12·4 years, disease duration 1·2 ± 1·2 years) negative for anti-MMP-3 antibody and six normal individuals. Skin biopsies were performed on the extensor surface of the forearm from early dSSc patients. Biopsy specimens were frozen immediately in liquid nitrogen and stored at −80°C. Total RNA was isolated from frozen tissue with Qiagen RNeasy spin columns (Qiagen Ltd, Crawley, UK) and was reversely transcribed into cDNA. MMP-3 mRNA expression was analysed using a real-time PCR quantification method (Applied Biosystems, Foster City, CA, USA). Sequence-specific primers and probes were designed by pre-developed TaqMan® assay reagents or Assays-On-Demands™ (Applied Biosystems). Real-time PCR (one cycle at 50°C for 2 min, at 95°C for 10 min; 40 cycles at 92°C for 15 s, at 60°C for 60 s) was performed on ABI Prism 7000 Sequence Detector (Applied Biosystems). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used to normalize mRNA. To compare target gene and housekeeping (GAPDH) gene mRNA expression, relative expression of real-time PCR products was determined using the ΔΔCt method [24]. One of the control samples was chosen as a calibrator sample. Each sample was performed in duplicate and the mean Ct was used in the equation.
Cross-reactivity of anti-MMP-3 antibody with MMP-1
IgG was purified from 40 µl of serum samples from five dSSc patients positive for anti-MMP-3 antibody but negative for anti-MMP-1 antibody by ELISA, and five dSSc patients positive for anti-MMP-1 antibody but negative for anti-MMP-3 antibody by ELISA. Purified IgG was preincubated with 5 µg of either human recombinant MMP-1 (Techne Corp., Minneapolis, MN, USA) or human recombinant MMP-3 (R&D Systems, Inc.) for 30 min at room temperature. ELISA for both anti-MMP-3 antibody and anti-MMP-1 antibody were performed as described above.
Statistical analysis
Statistical analysis was performed using the Mann–Whitney U-test for comparison of antibody levels and Bonferroni's test for multiple comparisons. Spearman's rank correlation coefficient was used to examine the relationship between two continuous variables.
RESULTS
Anti-MMP-3 autoantibody levels in SSc by ELISA
The presence and levels of anti-MMP-3 autoantibodies in serum samples from patients with collagen diseases and normal controls were assessed by ELISA (Fig. 1). In total, patients with SSc, IgG anti-MMP-3 antibody levels were significantly higher than those found in active SLE patients (P < 0·0005), DM patients (P < 0·05) or normal controls (P < 0·0001), while IgM anti-MMP-3 antibody levels tended to be higher than those in normal controls (P = 0·054), but were similar to those in SLE or DM patients. Regarding the SSc subsets, IgG anti-MMP-3 antibody levels in patients with dSSc were increased significantly compared with patients with lSSc (P < 0·001), those with SLE (P < 0·0001), those with DM (P < 0·0001) or normal controls (P < 0·0001). IgM anti-MMP-3 antibody levels in patients with dSSc were also elevated significantly relative to patients with lSSc (P < 0·05) or normal controls (P < 0·05). Patients with lSSc exhibited significantly higher levels of IgG anti-MMP-3 antibody than those in normal controls (P < 0·0001) or SLE patients (P < 0·0001), but had normal IgM anti-MMP-3 antibody levels. IgG anti-MMP-3 antibody levels correlated positively with IgM anti-MMP-3 antibody levels in total patients with SSc (r = 0·288, P < 0·005). However, IgG and IgM anti-MMP-3 antibody levels did not correlate with serum levels of other autoantibodies, including antibodies against topoisomerase I and centromere. Thus, IgG anti-MMP-3 autoantibody levels were increased in SSc, but not in other collagen diseases including SLE and DM.
Fig. 1.
Anti-MMP-3 antibody levels in serum samples from patients with lSSc, dSSc, SLE, or DM and normal controls (CTL). Anti-MMP-3 antibody levels were determined by an ELISA using human recombinant MMP-3. The short bar indicates the mean value in each group. A broken line indicates the cut-off value (mean ± 2 s.d. of the control samples).
Frequency of anti-MMP-3 antibody positivity and its clinical correlation in SSc
Absorbance values higher than the mean ± 2 s.d. (0·536 for IgG anti-MMP-3 antibody and 0·498 for IgM anti-MMP-3 antibody) of the control serum samples were considered to be positive in this study (Fig. 1). IgG or IgM anti-MMP-3 antibody was found in 52% of total patients with SSc. IgG or IgM anti-MMP-3 antibody was detected in 71% of patients with dSSc, while it was positive in only 33% of patients with lSSc (Table 1). By contrast, IgG or IgM anti-MMP-3 antibody was detected in only two healthy individuals (8%).
Table 1.
Frequency of anti-MMP-3 antibody positivity in collagen diseases and normal controlsa
| Anti-MMP-3 antibody | |||
|---|---|---|---|
| IgG | IgM | IgG or IgM | |
| SSc (n = 58) | 25 (43) | 12 (21) | 30 (52) |
| lSSc (n = 30) | 8 (27) | 2 (7) | 10 (33) |
| dSSc (n = 28) | 17 (61) | 10 (36) | 20 (71) |
| SLE (n = 22) | 4 (18) | 4 (18) | 5 (23) |
| DM (n = 14) | 2 (14) | 2 (14) | 3 (21) |
| Normal (n = 24) | 2 (8) | 0 | 2 (8) |
Values are the number (%) of patients with anti-MMP-3 antibody that was determined by an ELISA using human recombinant MMP-3.
The direct correlation of anti-MMP-3 antibody levels with the extent of skin sclerosis, renal vascular damage and lung fibrosis was then assessed. IgG anti-MMP-3 antibody levels (P ≤ 0·001; Fig. 2a) and IgM anti-MMP-3 antibody levels (P ≤ 0·01; Fig. 2b) correlated positively with modified Rodnan TSS, a semiquantitative measure of skin sclerosis [20]. Similarly, the positive association of IgG anti-MMP-3 antibody levels with renal vascular resistance, which was determined as pulsatility index value in renal interlobar arteries by colour flow Doppler scans [22], was observed (P ≤ 0·05; Fig. 2c). Furthermore, IgG anti-MMP-3 antibody levels correlated negatively with %VC (P ≤ 0·05; Fig. 2d). Thus, IgG anti-MMP-3 antibody levels correlated with the extent of fibrosis in the skin, lung and blood vessels, while IgM anti-MMP-3 antibody levels correlated with skin fibrosis only.
Fig. 2.
The correlation of IgG (a) and IgM (b) anti-MMP-3 antibody levels against modified Rodnan total skin thickness score (modified Rodnan TSS) and correlation of IgG anti-MMP-3 antibody levels against pulsatility index (c) or %VC (d). The anatomical areas were rated as 0 (normal), 1 + (mild skin thickening), 2 + (moderate), and 3 + (severe) and modified Rodnan TSS was derived by summation of the scores from all 17 areas. The pulsatility index is a parameter for renal vascular resistance determined by colour-flow Doppler ultrasonography of the renal interlobar arteries of both kidneys. Anti-MMP-3 antibody levels were determined by an ELISA using human recombinant MMP-3.
Immunoblotting analysis for anti-MMP-3 antibody
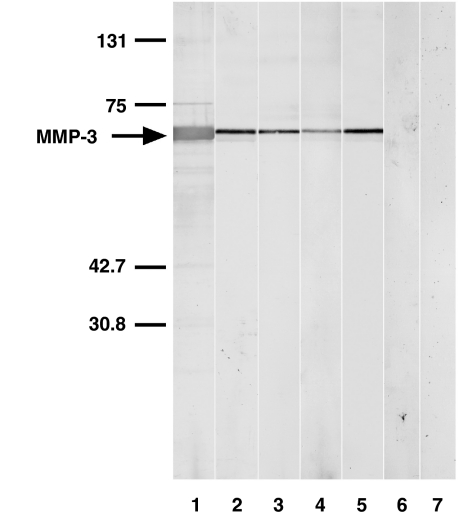
The presence of anti-MMP-3 antibody was evaluated by immunoblotting using human recombinant MMP-3. Serum samples from SSc patients positive for IgG anti-MMP-3 antibody by ELISA exhibited reactivity with MMP-3 (∼52 kDa) by immunoblotting (lanes 2–5, Fig. 3). By contrast, serum samples from the SSc patients positive for antitopoisomerase I antibody or anticentromere antibody, but not for IgG anti-MMP-3 antibody by ELISA, did not react with MMP-3 (lane 6 and data not shown). Similarly, no reactivity with MMP-3 was observed using serum samples from normal individuals (lane 7). Thus, the presence of anti-MMP-3 autoantibody in patients with SSc was confirmed by immunoblotting analysis.
Fig. 3.
Representative immunoblotting of human recombinant MMP-3 with sera from patients with SSc positive for IgG anti-MMP-3 antibody by ELISA. Lane 1, colloidal gold-stained MMP-3; lanes 2–5, serum samples from patients with SSc positive for IgG anti-MMP-3 antibody by ELISA; lane 6, a serum sample from the SSc patients positive for antitopoisomerase I antibody, but not for IgG anti-MMP-3 antibody by ELISA; and lane 7, a normal human serum. Markers for molecular weights (kDa) are shown to the left. The results represent those obtained with 10 SSc positive for IgG anti-MMP-3 antibody by ELISA, nine SSc patients positive for either antitopoisomerase I antibody or anticentromere antibody but not for IgG anti-MMP-3 antibody by ELISA, and six healthy individuals.
Inhibition of MMP-3 activity by anti-MMP-3 antibody
It was assessed whether anti-MMP-3 autoantibody was able to inhibit MMP-3 activity. MMP-3 activity was determined by measuring the amount of substrate fragment cleaved by MMP-3. MMP-3 activity was not inhibited by IgG isolated from healthy individuals (Fig. 4). By contrast, IgG isolated from serum samples of SSc patients positive for IgG anti-MMP-3 antibody by ELISA significantly inhibited MMP-3 activity to 26% compared to normal controls (P < 0·0005). Serum samples that were negative for anti-MMP-3 antibody but contained autoantibodies against topoisomerase I or centromere did not significantly inhibit MMP-3 activity. Thus, IgG anti-MMP-3 antibody from patients with SSc was able to inhibit MMP-3 activity.
Fig. 4.
Inhibition of MMP-3 activity by anti-MMP-3 antibody from patients with SSc. IgG was purified from serum samples of SSc patients positive for IgG anti-MMP-3 antibody by ELISA [anti-MMP-3 (+)], those positive for either antitopoisomerase I antibody or anticentromere antibody but not for IgG anti-MMP-3 antibody by ELISA [anti-MMP-3 (–)], and normal control. Purified IgG was incubated with MMP-3, and MMP-3 activity was determined by measuring the amount of substrate fragment cleaved by MMP-3. MMP-3 enzymatic activity was shown as percentage of untreated MMP-3 that was defined as 100%. Each histogram shows the mean (± s.d.) results obtained for 10 subjects from each group.
MMP-3 mRNA expression in SSc lesional skin
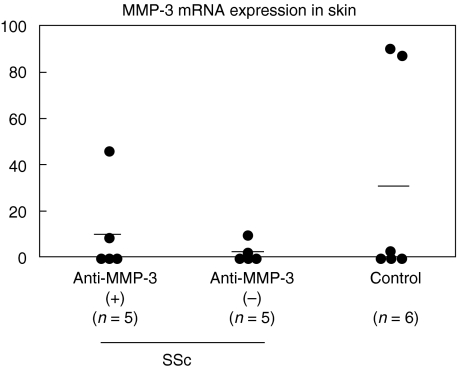
A previous study reported that cultured fibroblasts from patients with early SSc exhibited higher levels of MMP-3 mRNA expression [6], while another study reported that collagenase activity, including MMP-3, was significantly decreased in SSc skin fibroblasts [25]. Therefore, to assess whether MMP-3 expression was altered in SSc sclerotic skin, skin MMP-3 mRNA expression was assessed by real-time PCR. MMP-3 mRNA expression was detected in 40% (2/5) of early dSSc patients with anti-MMP-3 antibodies and 40% (2/5) of those without anti-MMP-3 antibodies. A similar frequency (50%, 3/6) was found in skin from healthy individuals. There was no significant difference in MMP-3 mRNA expression levels between dSSc skin lesion (6·7 ± 14·5) and normal skin (30·5 ± 46·9). In addition, there was no significant difference in MMP-3 mRNA expression between early dSSc patients positive for anti-MMP-3 antibody (11·1 ± 20·2) and those negative for the antibody (2·4 ± 4·2, Fig. 5). Thus, SSc sclerotic skin expressed normal MMP-3 mRNA, irrespective of the presence or absence of anti-MMP-3 antibody.
Fig. 5.
MMP-3 mRNA expression in SSc lesional skin. MMP-3 mRNA levels were determined by real-time RT-PCR. The short bar indicates the mean value in each group.
Cross-reactivity of anti-MMP-3 antibody with MMP-1
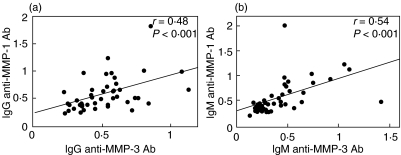
IgG anti-MMP-1 antibody levels correlated positively with IgG anti-MMP-3 antibody (P < 0·001, Fig. 6). A similar correlation was found between IgM anti-MMP-1 antibody levels and IgM anti-MMP-3 levels (P < 0·0001). However, IgG anti-MMP-3 antibody levels were not decreased significantly after incubation with MMP-1 (OD value, 0·89 ± 0·27), but reduced significantly after incubation with MMP-3 (0·58 ± 0·29, P < 0·05) relative to those without preincubation (0·90 ± 0·19). Similarly, IgG anti-MMP-1 antibody levels were not significantly decreased after incubation with MMP-3 (OD value, 0·98 ± 0·15), but reduced significantly after incubation with MMP-1 (0·59 ± 0·20, P < 0·05) relative to those without preincubation (0·97 ± 0·12). Thus, anti-MMP-3 antibody did not cross-react with MMP-1.
Fig. 6.
The correlation of IgG anti-MMP-3 antibody levels against IgG anti-MMP-1 antibody (a) and correlation of IgM anti-MMP-3 antibody levels against IgM anti-MMP-1 antibody (b). Both autoantibody levels were determined by an ELISA using human recombinant MMP-3 and MMP-1, respectively.
DISCUSSION
In this study, autoantibody against MMP-3 was detected in ∼50% of SSc patients, especially ∼70% of patients with dSSc, a more severe form. Furthermore, IgG and IgM anti-MMP-3 antibody levels were higher in dSSc patients than in lSSc patients. In addition, IgG anti-MMP-3 antibody levels correlated with the extent of fibrosis in skin, lung and renal blood vessels. The presence of anti-MMP-3 antibody in sera from patients with SSc was also confirmed by immunoblotting analysis. These results suggest that anti-MMP-3 autoantibody is a serological marker that reflects the severity of SSc.
Target autoantigens in SSc are generally intracellular molecules critical for cell mitosis. Furthermore, there is little evidence that autoantibodies are able to penetrate into viable cells. Therefore, autoantibodies in SSc do not appear to play a direct role in the pathogenesis by inducing tissue damage. We have shown that autoantibody to MMP-1 may be involved in the accumulation of ECM in SSc by reducing the ECM degradation [10]; however, increased deposition of type V collagen, decorin, osteonectin, elastin and fibrillin could not be explained by this antibody. In the current study, IgG anti-MMP-3 antibody present in sera from SSc patients could inhibit MMP-3 enzymatic activity that degrades these ECMs. Although anti-MMP-3 antibody levels correlate positively with anti-MMP-1 antibody levels, there was no cross-reactivity, suggesting that anti-MMP-1 antibodies and anti-MMP-3 antibodies are independent autoantibody systems. Because MMPs-1 and -3 degrade different ECMs, these two autoantibodies might co-operate to augment fibrosis in SSc. Collectively, the results of this study suggest that anti-MMP-3 autoantibody may contribute to the development of fibrosis by inhibiting MMP-3 activity and reducing the turnover of ECM.
A role for reduced ECM turnover in the pathogenesis of fibrosis has been suggested by studies showing that SSc skin fibroblasts in culture produced decreased amounts of MMP-1 and MMP-3, and increased amounts of TIMP-1 compared with those produced by control skin fibroblasts [25–28]. In addition, elevated serum TIMP-1 levels have been shown to correlate with disease activity and the early phase in SSc patients [29,30]. Activated skin fibroblasts were reported to express elevated levels of TIMP-3 mRNA in culture and in vivo[31]. However, cultured fibroblasts from patients with early SSc exhibited higher levels of MMP-1, MMP-3 and TIMP-1 mRNA expression than those from normal controls, while TIMP-1 mRNA expression remained increased, but MMP-1 and MMP-3 mRNA expression was decreased in fibroblasts from patients with mid-stage SSc [6]. In the present study, there was no significant difference in the frequency and amount of MMP-3 mRNA expression between SSc sclerotic skin and normal skin. These results suggest that the relative increase in anti-MMP-3 autoantibody may be associated with more efficient inhibition of MMP-3 enzymatic activity in SSc lesional skin.
Connective tissue growth factor (CTGF) has been suggested to play an important role in the development of various forms of fibrosis, such as hepatic fibrosis, atherosclerosis and myocardial fibrosis [32–34]. CTGF is a cystein-rich peptide originally identified from cultured human umbilical endothelial cell supernatants [35]. CTGF exhibits platelet-derived growth factor (PDGF)-like chemotactic and mitogenic activities on mesenchymal cells, and appears to be antigenically related to PDGF A and B chain peptides [35]. In SSc, CTGF mRNA is overexpressed on sclerotic lesions [36–38]. Furthermore, the serum CTGF level is elevated significantly in SSc patients compared to that in normal controls, which is specific to SSc [39]. CTGF has been shown to be the substrate of MMPs including MMP-1 and MMP-3 [40]. It is possible that autoantibody against MMP-3 may inhibit the cleavage of CTGF, leading to the overexpression of CTGF and excessive fibrosis in SSc. Damage to microvasculature, such as digital ulcers and renal crisis, is a characteristic manifestation in SSc [41]. The angiogenic activity of vascular endothelial growth factor (VEGF)165, one of the VEGFs, is suppressed by complex formation with CTGF and is recovered through the selective degradation of CTGF by MMPs including MMP-3. These results suggest that anti-MMP-3 antibody may also contribute to SSc vasculopathy through inhibition of angiogenesis as well as vascular damage due to fibrosis. Thus, anti-MMP-3 antibody may be related to various aspects of SSc disease expression, including skin sclerosis, lung fibrosis, renal vasculopathy and autoimmunity. However, ∼50% of SSc patients did not show elevated levels of anti-MMP-3 antibody; thus, the significance and role of anti-MMP-3 antibody in SSc remain unknown in this study. To clarify these issues, further study will be required.
REFERENCES
- 1.Jimenez SA, Hitraya E, Varga J. Pathogenesis of scleroderma. Collagen Rheum Dis Clin North Am. 1996;22:647–74. doi: 10.1016/s0889-857x(05)70294-5. [DOI] [PubMed] [Google Scholar]
- 2.Lovell CR, Nicholls AC, Duance VC, Bailey AJ. Characterization of dermal collagen in systemic sclerosis. Br J Dermatol. 1979;100:359–69. doi: 10.1111/j.1365-2133.1979.tb01635.x. [DOI] [PubMed] [Google Scholar]
- 3.LeRoy EC. Increased collagen synthesis by scleroderma skin fibroblasts in vitro: a possible defect in the regulation or activation of the scleroderma fibroblast. J Clin Invest. 1974;54:880–9. doi: 10.1172/JCI107827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peltonen J, Kahari L, Uitto J, Jimenez SA. Increased expression of type VI collagen genes in systemic sclerosis. Arthritis Rheum. 1990;33:1829–35. doi: 10.1002/art.1780331211. [DOI] [PubMed] [Google Scholar]
- 5.Xu WD, Leroy EC, Smith EA. Fibronectin release by systemic sclerosis and normal dermal fibroblasts in response to TGF-beta. J Rheumatol. 1991;18:241–6. [PubMed] [Google Scholar]
- 6.Kuroda K, Shinkai H. Gene expression of types I and III collagen, decorin, matrix metalloproteinases and tissue inhibitors of metalloproteinases in skin fibroblasts from patients with systemic sclerosis. Arch Dermatol Res. 1997;289:567–72. doi: 10.1007/s004030050241. [DOI] [PubMed] [Google Scholar]
- 7.Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eeckhout Y, Vaes G. Further studies on the activation of procollagenase, the latent precursor of bone collagenase. Effects of lysosomal cathepsin B, plasmin and kallikrein, and spontaneous activation. Biochem J. 1977;166:21–31. doi: 10.1042/bj1660021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Apte SS, Olsen BR, Murphy G. The gene structure of tissue inhibitor of metalloproteinases (TIMP)-3 and its inhibitory activities define the distinct TIMP gene family. J Biol Chem. 1995;270:14313–8. doi: 10.1074/jbc.270.24.14313. [DOI] [PubMed] [Google Scholar]
- 10.Sato S, Hayakawa I, Hasegawa M, Fujimoto M, Takehara K. Function blocking autoantibodies against matrix metalloproteinase-1 in patients with systemic sclerosis. J Invest Dermatol. 2003;120:542–7. doi: 10.1046/j.1523-1747.2003.12097.x. [DOI] [PubMed] [Google Scholar]
- 11.Okano Y. Antinuclear antibody in systemic sclerosis (scleroderma) Rheum Dis Clin North Am. 1996;22:709–35. doi: 10.1016/s0889-857x(05)70297-0. [DOI] [PubMed] [Google Scholar]
- 12.Sato S, Hamaguchi Y, Hasegawa M, Takehara K. Clinical significance of anti-topoisomerase I antibody levels determined by ELISA in systemic sclerosis. Rheumatology (Oxford) 2001;40:1135–40. doi: 10.1093/rheumatology/40.10.1135. [DOI] [PubMed] [Google Scholar]
- 13.Saito E, Fujimoto M, Hasegawa M, et al. CD19-dependent B lymphocyte signaling thresholds influence skin fibrosis and autoimmunity in the tight-skin mouse. J Clin Invest. 2002;109:1453–62. doi: 10.1172/JCI15078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Subcommittee for Scleroderma Criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Preliminary criteria for the classification of systemic sclerosis (scleroderma) Arthritis Rheum. 1980;23:581–90. doi: 10.1002/art.1780230510. [DOI] [PubMed] [Google Scholar]
- 15.LeRoy EC, Black C, Fleischmajer R, et al. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol. 1988;15:202–5. [PubMed] [Google Scholar]
- 16.Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–7. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 17.Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH. Derivation of the SLEDAI: a disease activity index for lupus patients. Arthritis Rheum. 1992;35:630–40. doi: 10.1002/art.1780350606. and the Committee on Prognosis Studies in SLE. [DOI] [PubMed] [Google Scholar]
- 18.Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts) N Engl J Med. 1975;292:344–8. doi: 10.1056/NEJM197502132920706. [DOI] [PubMed] [Google Scholar]
- 19.Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts) N Engl J Med. 1975;292:403–7. doi: 10.1056/NEJM197502202920807. [DOI] [PubMed] [Google Scholar]
- 20.Clements PJ, Lachenbruch PA, Seibold JR, et al. Skin thickness score in systemic sclerosis: an assessment of inter-observer variability in three independent studies. J Rheumatol. 1993;20:1892–6. [PubMed] [Google Scholar]
- 21.Steen VD, Powell DL, Medsger TA. Clinical correlations and prognosis based on serum autoantibodies in patients with systemic sclerosis. Arthritis Rheum. 1988;31:196–203. doi: 10.1002/art.1780310207. [DOI] [PubMed] [Google Scholar]
- 22.Nishijima C, Sato S, Hasegawa M, et al. Renal vascular damage in Japanese patients with systemic sclerosis. Rheumatology (Oxford) 2001;40:406–9. doi: 10.1093/rheumatology/40.4.406. [DOI] [PubMed] [Google Scholar]
- 23.Sato S, Hasegawa M, Fujimoto M, Tedder TF, Takehara K. Quantitative genetic variation in CD19 expression correlates with autoimmunity. J Immunol. 2000;165:6635–43. doi: 10.4049/jimmunol.165.11.6635. [DOI] [PubMed] [Google Scholar]
- 24.Meijerink J, Mandigers C, van de Locht L, Tonnissen E, Goodsaid F, Raemaekers J. A novel method to compensate for different amplification efficiencies between patient DNA samples in quantitative real-time PCR. J Mol Diagn. 2001;3:55–61. doi: 10.1016/S1525-1578(10)60652-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takeda K, Hatamochi A, Ueki H, Nakata M, Oishi Y. Decreased collagenase expression in cultured systemic sclerosis fibroblasts. J Invest Dermatol. 1994;103:359–63. doi: 10.1111/1523-1747.ep12394936. [DOI] [PubMed] [Google Scholar]
- 26.Bou-Gharios G, Osman J, Black C, Olsen I. Excess matrix accumulation in scleroderma is caused partly by differential regulation of stromelysin and TIMP-1 synthesis. Clin Chim Acta. 1994;231:69–78. doi: 10.1016/0009-8981(94)90255-0. [DOI] [PubMed] [Google Scholar]
- 27.Kirk TZ, Mark ME, Chua CC, Chua BH, Mayes MD. Myofibroblasts from scleroderma skin synthesize elevated levels of collagen and tissue inhibitor of metalloproteinase (TIMP-1) with two forms of TIMP-1. J Biol Chem. 1995;270:3423–8. doi: 10.1074/jbc.270.7.3423. [DOI] [PubMed] [Google Scholar]
- 28.Kikuchi K, Kadono T, Furue M, Tamaki K. Tissue inhibitor of metalloproteinase 1 (TIMP-1) may be an autocrine growth factor in scleroderma fibroblasts. J Invest Dermatol. 1997;108:281–4. doi: 10.1111/1523-1747.ep12286457. [DOI] [PubMed] [Google Scholar]
- 29.Kikuchi K, Kubo M, Sato S, Fujimoto M, Tamaki K. Serum tissue inhibitor of metalloproteinases in patients with systemic sclerosis. J Am Acad Dermatol. 1995;33:973–8. doi: 10.1016/0190-9622(95)90289-9. [DOI] [PubMed] [Google Scholar]
- 30.Young-Min SA, Beeton C, Laughton R, et al. Serum TIMP-1, TIMP-2, and MMP-1 in patients with systemic sclerosis, primary Raynaud's phenomenon, and in normal controls. Ann Rheum Dis. 2001;60:846–51. [PMC free article] [PubMed] [Google Scholar]
- 31.Mattila L, Airola K, Ahonen M, et al. Activation of tissue inhibitor of metalloproteinases-3 (TIMP-3) mRNA expression in scleroderma skin fibroblasts. J Invest Dermatol. 1998;110:416–21. doi: 10.1046/j.1523-1747.1998.00138.x. [DOI] [PubMed] [Google Scholar]
- 32.Oemar BS, Werner A, Garnier JM, et al. Human connective tissue growth factor is expressed in advanced atherosclerotic lesions. Circulation. 1997;95:831–9. doi: 10.1161/01.cir.95.4.831. [DOI] [PubMed] [Google Scholar]
- 33.Paradis V, Dargere D, Vidaud M, et al. Expression of connective tissue growth factor in experimental rat and human liver fibrosis. Hepatology. 1999;30:968–76. doi: 10.1002/hep.510300425. [DOI] [PubMed] [Google Scholar]
- 34.Chen MM, Lam A, Abraham JA, Schreiner GF, Joly AH. CTGF expression is induced by TGF-beta in cardiac fibroblasts and cardiac myocytes: a potential role in heart fibrosis. J Mol Cell Cardiol. 2000;32:1805–19. doi: 10.1006/jmcc.2000.1215. [DOI] [PubMed] [Google Scholar]
- 35.Bradham DM, Igarashi A, Potter RL, Grotendorst GR. Connective tissue growth factor: a cysteine-rich mitogen secreted by human vascular endothelial cells is related to the SRC induced immediate early gene product CEF-10. J Cell Biol. 1991;114:1285–94. doi: 10.1083/jcb.114.6.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holmes A, Abraham DJ, Sa S, Shiwen X, Black CM, Leask A. CTGF and SMADs, maintenance of scleroderma phenotype is independent of SMAD signaling. J Biol Chem. 2001;276:10594–601. doi: 10.1074/jbc.M010149200. [DOI] [PubMed] [Google Scholar]
- 37.Shi-wen X, Pennington D, Holmes A, et al. Autocrine overexpression of CTGF maintains fibrosis: RDA analysis of fibrosis genes in systemic sclerosis. Exp Cell Res. 2000;259:213–24. doi: 10.1006/excr.2000.4972. [DOI] [PubMed] [Google Scholar]
- 38.Igarashi A, Okochi H, Bradham DM, Grotendorst GR. Regulation of connective tissue growth factor gene expression in human skin fibroblasts and during wound repair. Mol Biol Cell. 1993;4:637–45. doi: 10.1091/mbc.4.6.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sato S, Nagaoka T, Hasegawa M, et al. Serum levels of connective tissue growth factor are elevated in patients with systemic sclerosis: association with extent of skin sclerosis and severity of pulmonary fibrosis. J Rheumatol. 2000;27:149–54. [PubMed] [Google Scholar]
- 40.Hashimoto G, Inoki I, Fujii Y, Aoki T, Ikeda E, Okada Y. Matrix metalloproteinases cleave connective tissue growth factor and reactivate angiogenic activity of vascular endothelial growth factor 165. J Biol Chem. 2002;277:36288–95. doi: 10.1074/jbc.M201674200. [DOI] [PubMed] [Google Scholar]
- 41.Campbell PM, LeRoy EC. Pathogenesis of systemic sclerosis: a vascular hypothesis. Semin Arthritis Rheum. 1975;4:351–68. doi: 10.1016/0049-0172(75)90017-7. [DOI] [PubMed] [Google Scholar]