Abstract
The inflammatory response to ozone in atopic asthma suggests that soluble mediators of inflammation are released in response to oxidant stress. Antioxidants may alleviate additional oxidative stress associated with photochemical oxidant pollution. This study investigates the impact of antioxidant supplementation on the nasal inflammatory response to ozone exposure in atopic asthmatic children. We conducted a randomized trial using a double-blinded design. Children with asthma (n = 117), residents of Mexico City, were given randomly a daily supplement of vitamins (50 mg/day of vitamin E and 250 mg/day of vitamin C) or placebo. Nasal lavages were performed three times during the 4-month follow-up and analysed for content of interleukin-6 (IL-6), IL-8, uric acid and glutathione (GSx). IL-6 levels in the nasal lavage were increased significantly in the placebo group after ozone exposure while no increase was observed in the supplement group. The difference in response to ozone exposure between the two groups was significant (P = 0·02). Results were similar for IL-8, but with no significant difference between the groups (P = 0·12). GSx decreased significantly in both groups. Uric acid decreased slightly in the placebo group. Our data suggest that vitamin C and E supplementation above the minimum dietary requirement in asthmatic children with a low intake of vitamin E might provide some protection against the nasal acute inflammatory response to ozone.
Keywords: antioxidants, childhood asthma, cytokines/interleukins, ozone
INTRODUCTION
Exposure to oxidants from different sources has been related to asthma incidence, increased frequency of respiratory symptoms and bronchial response [1,2]. In controlled human studies, mild atopic asthmatics exposed to short-term peak ambient levels of ozone experience an acute inflammatory response in the lower airways and a decrement in lung function [3,4]. Recent studies examining the inflammatory response in bronchoalveolar lavage (BAL) fluid and bronchial wash in mild atopic asthmatics have shown that short-term exposure to ozone induces polymorphonuclear leucocytes (PMNL) influx in the lower airway, paralleled by the release of soluble mediators of inflammation including albumin, total protein, myeloperoxidase (MPO), lactate deshydrogenase and cytokines [including interleukin-8 (IL-8) and granulocyte-macrophage colony-stimulating factor (GM-CSF)] and eosinophil cationic proteins [5,6].
Although the role of reactive oxygen species (ROS) in the inflammatory and immunological cascade characteristics of asthma is not well understood, antioxidants could prove useful as an adjuvant treatment for asthma, particularly in subjects exposed to additional oxidative stress associated with photochemical oxidant pollution [3,7]. Both experimental and epidemiological studies have suggested that antioxidant supplementation could modulate the acute change in lung functions observed among people exposed to photo-oxidants [8,9]. To date only one controlled human study conducted in healthy volunteers has investigated the role of dietary antioxidants on the inflammatory responses of the lung to ozone exposure [9].
The Metropolitan Area of Mexico City experiences significant air pollution problems related in particular to high ozone levels. We have reported recently that supplementation with antioxidants modulates the impact of ozone exposure on small airways of moderate to severe asthmatic children residing in Mexico City [10]. In this paper we investigated the impact of antioxidant supplementation on the inflammatory response of the airways due to ozone exposure in the same asthmatic population.
MATERIALS AND METHODS
Study population
The study design has been presented elsewhere [10]. Briefly, children were recruited through the allergy clinic of the Hospital Infantil Federico Gomez. All children were followed for their asthma at this clinic and were classified according to the NLBI [11] as intermittent, mild persistent, moderate persistent or severe. One hundred and sixty children were recruited and followed for 12 weeks, divided into groups of 40 and treated in a sequential manner. Parents were asked to sign a consent form allowing their child to participate. The protocol was reviewed and approved by the ethical committees of the Hospital Infantil de Mexico and the Instituto Nacional de Salud Publica, Mexico.
Children were assigned randomly to received either supplement (vitamin C; 250 mg/day and vitamin E 50 mg/day), or placebo in a doubled-blinded manner, informing neither the health personnel at the clinic or the patients of the assignment code. Placebo and supplement were presented in similar pills (provided by Roche Laboratory). During the 12 weeks of follow-up, children performed two spirometric tests per week. Blood samples were obtained at baseline, after 6 weeks of follow-up and at the end of the follow-up (12 weeks) to check compliance with the supplementation. Nasal lavages were obtained from only 120 children (2nd, 3rd, 4th groups) at baseline and at 6 and 12 weeks of the follow-up. Two participants did not complete the follow-up because the mother found it too cumbersome to visit the asthma clinic twice a week and nasal lavage samples could not be obtained from one child. These three children were excluded from the analysis
Exposure assessment
We obtained measurements of particulates (with a mass median diameter of less than 10 µm, PM10), ambient ozone and climatic variables (relative humidity and minimum, maximum and daily average temperature), from the Mexican government's air monitoring stations. Concentrations of SO2 and NO2 were also recorded but levels were low. In order to locate the nearest monitoring station, no further than 5 km away, each participating child's home was plotted on a map. Information on environmental tobacco smoke exposure (ETS) was obtained from the baseline questionnaire and included questions on smokers in the home (mother, father, others) and number of cigarettes smoked per day in the home and in the presence of the child.
Nasal lavage samples processing
Nasal lavage was obtained as described by Noah et al. [12] using a disposable syringe filled with sterile, normal saline solution at room temperature. This device was used five times in each nostril while occluding the other nostril; then the subject was instructed to exhale through the lavaged side into a specimen cup and the returned fluid pooled. The total input of saline solution was approximately 5 ml and the return was close to 40%. The lavage fluid was passed through filters to remove the mucus and cell debris and frozen immediately and kept at −70°C for mediator's assays. None of the samples were contaminated with blood.
Cytokine analysis
Soluble components were normalized per ml of nasal lavage fluid. IL-8 and IL-6 levels were measured using enzyme-linked immunosorbent assay (ELISA) kits purchased from R&D Systems (Minneapolis, MN, USA). Total proteins and albumin levels were determined using a centrifugal chemical analyser purchased from Roche Laboratories COBAS-FARA, Branchburg (NJ, USA) at the laboratory of Dr Robert Devlin, US EPA Human Studies Division, NC, USA.
Biochemical analysis
Blood samples were collected in EDTA vacutainer tubes, covered immediately with aluminium foil and centrifuged to obtain plasma. Aliquots were stored at −70°C and shipped on dry ice to the laboratory of Dr Gary Hatch at the US EPA for analysis. α-Tocopherol (vitamin E) in the plasma was extracted for lipids, then measured by high performance liquid chromatography (HPLC) with electrochemical detection using methods described previously [13]. A second aliquot of plasma was treated with 4% perchloric acid to a final concentration of 1% then it was vortex centrifuged (27 000 g for 20 min). Supernatants were assayed for total glutathione (reduced plus oxidized) using a COBAS-FARA II clinical analyser (Roche Diagnostics, Branchburg, NJ, USA) using the glutathione reductase recycling method [14].
In nasal lavage, total glutathione was determined using the method already described. Urate and vitamin C were measured on the perchloric acid treated nasal lavage, using HPLC-electrochemistry [15]. Vitamin C levels were mostly under the detection limit, probably because of improper handling of the sample, and therefore could not be studied.
Statistical analysis
We studied the effect of ozone exposure on the levels of IL-6 and IL-8 in subjects assigned to receive a placebo or supplement as well as on the levels of uric acid and GSx in nasal fluid. We used the daily maximum of 8-h moving average as exposure to ozone as well as cumulative exposure over several days prior to nasal lavage. Pearson correlation was determined between levels of air contaminants and various climatic variables [16]. Comparisons of cytokine and antioxidant levels in nasal lavage and blood were performed on log-transformed data to normalize the distribution. Levels at baseline, 6 weeks and 12 weeks were compared between the placebo and supplement groups using t-test for independent samples. In addition, we evaluated the change in levels within each group comparing levels at baseline and at 12 weeks using the paired t-test [16].
We analysed data using mixed effect models for longitudinal data to determine the association between air pollutant exposure and levels of inflammatory markers, defining each child as the group variable. This type of model assumes that the relationship between variables has the same structure for each individual but allows the regression coefficients to vary. The model determines the conditional expected value of the response variable based on individual characteristics [17]. We adjusted models for potential confounding factors including participants' age and exposure to environmental tobacco smoke (ETS), atopy, temperature (mean temperature), relative humidity, asthma severity, use of corticoids and uric acid levels in nasal lavage. In the final models, we adjusted only for age, asthma severity (mild or moderate and severe), week of the study, total protein (to adjust for dilution), use of corticoids and uric acid levels in the nasal lavage, because the inclusion of other variables did not modify the results. We compared regression coefficients using a t-test [16] to estimate the effect of the supplementation and all analyses were conducted using stata software (version 7·0) [18]. Two participants did not complete the follow-up because the mothers found visiting the asthma clinic twice a week too inconvenient and were excluded from the analysis.
RESULTS
Study population
Table 1 presents the characteristics of the population according to the groups assigned either a placebo or a supplement. There was no significant difference between the two groups except for atopy, which was more frequent among children assigned to the placebo group (95% in the placebo group and 81% in the supplement group, P = 0·03). Skin tests showed positive results most frequently for dermatophagoides, cat and Blatella germanica.
Table 1.
Characteristics of the study population; asthmatic children residing in Mexico City, 1998–2000
| Characteristics | Supplement (n = 59) | Placebo (n = 58) | P |
|---|---|---|---|
| Gender (% male) | 64·4 | 63·8 | 0·94 |
| Age (years) | 8·9 | 9·2 | 0·52 |
| Time residing in Mexico City (years) | 8·6 | 8·7 | 0·77 |
| Age at asthma diagnosis (years) | 3·3 | 3·1 | 0·50 |
| Number of crises of asthma in the last 12 months | 2·6 | 2·5 | 0·49 |
| Asthma severity | |||
| Mild (%) | 47·5 | 58·6 | 0·23 |
| Moderate and severe (%) | 52·5 | 41·4 | 0·23 |
| Chronic cough in the last 12 months (%) | 82·8 | 69·0 | 0·16 |
| Wheezing in the last 12 months (%)* | 52·6 | 46·2 | 0·61 |
| Rhinitis (%) | 38·9 | 43·1 | 0·65 |
| Antibiotics use in last 12 months (%) | 57·9 | 42·1 | 0·11 |
| Use of inhaled medicine ever (%) | 91·2 | 80·7 | 0·11 |
| Smoking at home | |||
| Mother (%) | 22·8 | 15·5 | 0·32 |
| Father (%) | 48·2 | 43·6 | 0·63 |
| Others (%) | 19·0 | 21·0 | 0·79 |
| Atopy (%) | 81·0 | 94·6 | 0·03 |
| Animals at home (%)† | 58·6 | 50·9 | 0·41 |
| Dog (%) | 46·6 | 38·6 | 0·39 |
| Cat (%) | 5·2 | 12·3 | 0·18 |
| Birds (%) | 17·2 | 15·8 | 0·84 |
| Cockroach (%)† | 15·8 | 25·0 | 0·22 |
Twice or more during the year.
Proportion of homes for which parents reported having pets or pests.
At baseline participants' mean reported dietary intakes of vitamin C and vitamin E were 97·0 mg (SE = 2·0) and 5·1 mg (SE = 1·6), respectively. Intake of these nutrients was similar in the supplement and placebo groups.
Exposure data
During the study period (from May 17, 1999 to April 19, 2000), ozone 8-h moving average ranged from 11·1 to 142·5 p.p.b., with a mean of 66·2 p.p.b. The maximum of PM10 8-h moving average ranged from 16·6 µg/m3 to 180·9 µg/m3 with a mean of 77·3 µg/m3. Ozone and PM10 were negatively related to minimum temperature (r = −0·17, P < 0·001; r = −0·28, P < 0·001, respectively).
Cytokine and antioxidant levels in the nasal fluids
At baseline, IL-6 and IL-8 were slightly higher in the supplement group [mean (SE): 48·6 (23·4) versus 25·3 (10·5) pg/ml for IL-6, P = 0·21 and 825·8 (140·8) versus 732·6 (131·6) pg/ml for IL-8, P = 0·25], due probably to the higher proportion of children with moderate and severe asthma in this group. Both IL-6 and IL-8 decreased in subsequent weeks in the placebo and supplement groups. However, the only significant decrease between baseline and 12 weeks was observed for IL-6 in the supplement group [mean (SE): 48·6 (23·4) versus 11·4 (2·3) pg/ml P < 0·05]. Uric acid also decreased in both groups, more so in the placebo group (P < 0·01), while GSx increased significantly in both groups (P < 0·01).
Blood data
At baseline plasma α-tocopherol levels were similar among the placebo and supplement group [mean (SE): 3·02 (0·14) mg/ml in the placebo group versus 3·02 (0·134) mg/ml in the supplement group]. Plasma α-tocopherol levels were significantly higher in the group receiving supplement than in the placebo group at 12 weeks follow-up [mean (SE): 2·97 (0·12) mg/ml in the placebo group versus 4·14 (0·21) ng/ml in the supplement group, P < 0·01].
Inflammatory response to air pollutant exposure
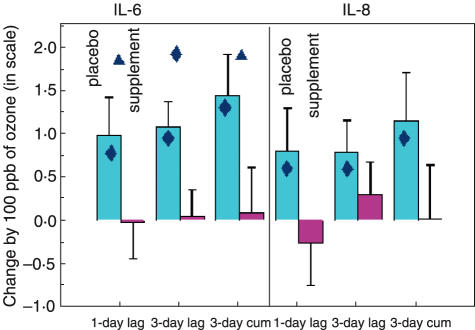
Table 2 presents the results of the statistical analyses of IL-6 and IL-8 and ozone concentrations (8-h moving average) on different lags prior to the nasal lavage for the supplement and placebo group. After adjustment for age, week of the study, use of corticoids, uric acid, total protein levels in nasal lavage and severity of asthma, ozone levels were positively associated with IL-6 levels in the placebo group, while no significant increase was observed in the supplement group. An increase of 1·07 pg/ml [95% confidence interval (CI) 0·48–1·65] was observed in the placebo group in relation with 100 ppb ozone exposure with a 3-day lag, while in the supplement group there was no significant change (0·04 pg/ml, 95% CI −0·56–0·64). The difference of the ozone effect on IL-6 levels between the two groups (placebo and supplement) was highly significant for ozone exposure 3 days prior to the nasal lavage and when considering a cumulative exposure to ozone over 3 days (P = 0·02) (Fig. 1). For IL-8 levels, a significant increase was observed in the placebo group with the maximum effect 3 days after exposure (P = 0·04) and when considering a cumulative exposure to ozone over 3 days (P = 0·04). No significant change was observed in the supplement group. However, the difference in the ozone effect on IL-8 levels between the two groups was not significant.
Table 2.
Change in IL-6, IL-8 (log-transformed) related to ozone (100 ppb) concentrations with a 1 and 3 day lags and accumulated over 3 days prior to nasal lavage
| Variables* | Subgroup | Beta† (SE) | P-value |
|---|---|---|---|
| IL-6 | |||
| 1-day lag¶ | Placebo | 0·98 (0·44) | 0·02 |
| Supplement | −0·02 (0·42) | 0·97 | |
| 3-day lag‡ | Placebo | 1·07 (0·30) | 0·00 |
| Supplement | 0·04 (0·31) | 0·90 | |
| 3-day cumulative§ | Placebo | 1·44 (0·47) | 0·00 |
| Supplement | 0·09 (0·52) | 0·86 | |
| IL-8 | |||
| 1-day lag | Placebo | 0. 80 (0·49) | 0·10 |
| Supplement | −0·26 (0·49) | 0·60 | |
| 3-day lag | Placebo | 0·78 (0·37) | 0·04 |
| Supplement | 0·29 (0·38) | 0·45 | |
| 3-day cumulative | Placebo | 1·15 (0·55) | 0·04 |
| Supplement | 0·018 (0·62) | 0·98 | |
Variables are log-transformed to improve normality.
The IL-6 models include 56 subjects and 112 observations in the placebo group and 57 subjects and 120 observations in the supplement group. The IL-8 models include 58 subjects and 119 observations in the placebo group and 59 subjects and 126 observations in the supplement group. Adjusted for age, week of the study, asthma severity, total protein content of nasal lavage, uric acid content in nasal lavage and use of corticoids. Each beta coefficient refers to the change in the IL-6 or IL-8 levels (ln-transformed) in nasal lavage for an increased of 100 ppb of ozone (8-h moving average). The P-value refers to the significance of this change. The effect of supplementation is determined comparing the coefficients of the placebo and supplement groups (multiplied by 100) using t-test. Significance levels are:
P = 0·01;
0·01 < P = 0·05;
0·05 < P = 0·10.
Fig. 1.
Change in IL-6 and IL-8 (ln scale) in nasal lavage related to 100 ppb ozone in the placebo and supplement groups of asthmatic children with different lags prior to exposure. The significance of changes per group are presented within the bars, and the significance of changes between groups are presented on the upper part of the graph.  P ≤ 0·05
P ≤ 0·05  0·05 < P ≤ 0·10.
0·05 < P ≤ 0·10.
GSx in nasal lavage decreased in both groups in relation to ozone exposure. Cumulative ozone exposure was related to a larger depletion. When we estimated the impact of 3 consecutive days of ozone exposure, we observed that for 100 ppb increment in the 3-day mean of ozone (8-h moving average) the decrease in GSx was –0·35 (95% CI –0·66 to –0·04) in the placebo group and –0·33 (95% CI –0·60 to –0·06) in the supplement group. However, we did not observe significant differences in the changes of GSx between the placebo and supplement groups. Uric acid in nasal lavage was negatively related to ozone levels in the placebo group but changes were non significant (Table 3). These results remained similar after adjustment for PM10 ambient levels.
Table 3.
Change in GSx and uric acid (log-transformed) related to ozone (100 ppb) concentrations with a 2-day lag and accumulated over 3 days prior to nasal lavage
| Variables* | Subgroup | Beta† (SE) | P-value |
|---|---|---|---|
| GSx | |||
| 2-day lag | Placebo | −0·13 (0·15) | 0·39 |
| Supplement | −0·29 (0·13) | 0·03 | |
| 3-day lag | Placebo | −0·27 (0·10) | 0·01 |
| Supplement | −0·06 (0·09) | 0·51 | |
| 3-day cumulative | Placebo | −0·35 (0·16) | 0·03 |
| Supplement | −0·33 (0·14) | 0·02 | |
| Uric acid | |||
| 2-day lag | Placebo | −0·25 (0·38) | 0·51 |
| Supplement | 0·08 (0·41) | 0·85 | |
| 3-day lag‡ | Placebo | −0·34 (0·27) | 0·20 |
| Supplement | 0·41 (0·28) | 0·14 | |
| 3-day cumulative | Placebo | −0·42 (0·40) | 0·30 |
| Supplement | 0·34 (0·46) | 0·46 | |
Variables are log-transformed to improve normality.
The model for GSx includes 58 subjects and 159 observations in the placebo group and 59 subjects and 159 observations in the supplement group, adjusted for week of the study, asthma severity, total protein content in nasal lavage, and plasma GSx levels. The model for uric acid includes 59 subjects and 142 observations in the placebo group and 58 subjects and 143 observations in the supplement group. Adjusted for week of the study, asthma severity, total protein content in nasal lavage. Each beta coefficient refers to the change in the GSx or uric acid levels (ln transformed) in nasal lavage for an increased of 100 ppb of ozone (8-h moving average). The P-value refers to the significance of this change. The effect of supplementation is determined comparing the coefficients of the placebo and supplement groups (multiplied by 100) using a t-test. Significance levels are:
0·05 < P = 0·10.
DISCUSSION
In this double-blind intervention study, we observed that children who received placebo had a significant increase in IL-6 and IL-8 in nasal lavage in response to ozone exposure, while children receiving antioxidant supplement did not. To our knowledge this is the first study conducted in asthmatic children suggesting that antioxidant supplementation might decrease the nasal inflammatory response to air pollutants as a marker of lower airway inflammation.
Controlled human exposure and field studies have shown that levels of ozone found in ambient air induced transient functional and inflammatory changes in the lung, particularly in asthmatics [4,19]. It is believed that the effects of ozone are mediated through oxidant damage to cell structures [3,20,21] with a possible role for oxidant-initiated activation of cell signalling processes. Augmented production of ROS has been found in adults and children with very severe asthma and acute exacerbation of asthma [7]. Recent studies examining the inflammatory response in bronchoalveolar lavage (BAL) fluid and bronchial wash have shown that short-term exposure of mild atopic asthmatics to ozone, induces polymorphonuclear leucocytes (PMNL) influx in the lower airway, paralleled by the release of soluble mediators of inflammation including albumin, total protein, myeloperoxidase (MPO), lactate deshydrogenase and cytokines (including IL-8 and GM-CSF) and eosinophil cationic proteins [5–7,22,23]. Krishna et al. report an increase in IL-8 related to exposure to 200 ppb of ozone [22]. Similarly, Hiltermann reports an increase in IL-8, as well as changes in eosinophils in sputum and BAL of asthmatics after ozone exposure (0·4 ppm for 2 h) [5]. After repeated exposure to ozone, studies have suggested that the decrement in lung function observed after single exposure attenuated, suggesting an adaptation of lung function; however, the airway inflammation persisted despite attenuation of some inflammatory markers [6,23,24]. Jorres et al. reported that after 200 ppb of ozone exposure on 4 consecutive days, concentration of total protein, IL-6 and IL-8, and GSx remained elevated while the effect on lung function was abolished [23].
Both experimental and epidemiological studies have also suggested that antioxidant supplementation could modulate the acute change in lung function observed among people exposed to photo-oxidants [8,9]. We have shown recently among these children that antioxidant supplementation modulated the adverse effect of ozone on lung function [10]. However, there are few data on the impact of antioxidant supplementation on the production of inflammatory mediators in asthmatic subjects and there is no evidence of a relationship between the intensity of the inflammatory response and the functional decrement after exposure to ozone [25].
To date, only one study has evaluated the impact of antioxidant supplementation on both functional and inflammatory response. Samet et al. reported recently that antioxidant supplementation among healthy volunteers modulated the adverse effect of ozone on pulmonary function and did not affect the inflammatory response as represented by the percentage of neutrophils and the concentration of IL-6 [9]. Our population is not directly comparable to that of Samet, given that it was composed of asthmatic children who were chronically exposed to high ozone levels and were deficient in vitamin E [10]. Our results suggest that in addition to modulating the adverse effect of ozone on lung function [10], antioxidant supplementation could modulate the inflammatory response, although the mechanisms by which antioxidants modulate the functional and inflammatory responses may differ. Studies have shown that vitamin E supplementation could decrease the production of inflammatory cytokines and chemokines [26] and that other antioxidants, such as pyrrolidine dithiocarbamate and N-acetyl-cysteine, could inhibit the secretion of IL-8 after diesel exposure [27]. A recent animal study has also shown that guinea pigs deficient in vitamin C and glutathione were more susceptible to the effect of residual oil fly ash, with unusual BAL cellular changes and lower antioxidants concentrations in BAL [28].
Decreases in uric acid and GSx have been observed after ozone exposure, suggesting that it is consumed by ozone [29,30]. However, in some studies an adaptation of RTFL antioxidants is observed including increases in GSx, uric acid from plasma RTLF and mobilization of α-tocopherol from non-lung tissue to the plasma [4]. In our study we observed a significant decreases of GSx in both study groups with cumulative exposure being related to a larger depletion. Unfortunately, we did not have information on the ratio of reduced to oxidized glutathione (GSH/GSSG), which would have provided more information relating to mechanisms. Uric acid in nasal lavage was negatively related to ozone exposure in the placebo group; however, changes were non-significant. Because children in our study were chronically exposed to high ozone levels and that the mechanism underpinning repletion capacity of the lining fluid of the upper airway are not known [30], these results are difficult to interpret.
Several factors need to be considered in the interpretation of our results. At baseline the characteristics of the two groups were similar and by using a double-blinded intervention we were able to avoid information bias. The exposure estimation of participating children was based on the monitoring network and not on personal measurement, possibly causing some misclassification of exposure. This will tend to underestimate the potential protective effect of antioxidant supplementation. We did not count with information of allergens indoor and outdoors; however, for a given child exposure to allergens is unlikely to vary with ozone ambient levels and therefore would not confound the association. Similarly, it is unlikely that ETS exposure be correlated with ozone levels. In addition, accounting for ETS exposure and atopy in the analysis did not affect our results.
We used nasal lavage to sample upper airway RTLF as it represented a relatively non-invasive procedure and has been proven to be a reliable method to study acute inflammatory response to inhaled pollutants [31] and reflect the acute inflammatory effect of ozone in the lower lung [32]. At baseline, antioxidant intake and plasma concentrations of vitamin E were similar in the supplement and placebo group. Participants reported intake of vitamin C close to the recommended dietary daily allowance (RDA); however, vitamin E intake was lower than the RDA [30,33] compared to other populations, as observed in the serum [34]. During this study, plasma vitamin E levels in the supplement group rose by 37%, as in other supplementation studies [9,10], indicating that participants were taking the supplement, of close to five times the RDA, for vitamin C and vitamin E.
Our data suggest that vitamin C and vitamin E supplementation above the RDA in asthmatic children with a low intake of vitamin E provides some protection against the acute inflammatory response to ozone on their lungs, but the optimum dose still needs to be determined.
Acknowledgments
This study was supported by the Mexican Sciences and Technology Council, no. 26206-M, the National Center for Environmental Health of the Centers for Disease Control and Prevention, Atlanta GA, USA. The authors thank the Monitoring Network of Mexico City for providing data on air pollutants, the Roche Laboratory for providing the supplements and placebo used in the study, the participants and their families and Caroline Karslake and Irma Soyachi for their assistance in the preparation of this document.
REFERENCES
- 1.US Department of Health and Human Services. Healthy people 2000: national health promotion and diseases prevention objectives. Washington, DC: US Department of health and Human Services; 1991. p. 32. DHHS publication no. PHS. 91–50212. [Google Scholar]
- 2.Bascom R, Bromberg PA, Costa DA, et al. Health effects of outdoor air pollution. Part I of II. Am J Respir Crit Care Med. 1996;153:3–50. doi: 10.1164/ajrccm.153.1.8542133. [DOI] [PubMed] [Google Scholar]
- 3.Pryor WA. Can vitamin E protect humans against the pathological effects of ozone in smog? Am J Clin Nutr. 1991;53:702–22. doi: 10.1093/ajcn/53.3.702. [DOI] [PubMed] [Google Scholar]
- 4.Mudway IS, Kelly FJ. Ozone and the lung: a sensitive issue. Mol Aspects Med. 2000;21:1–48. doi: 10.1016/s0098-2997(00)00003-0. [DOI] [PubMed] [Google Scholar]
- 5.Hiltermann JT, Lapperre TS, Van Bree L, Steerenberg PA, Brahim JJ. Ozone-induced inflammation assessed in sputum and bronchial lavage fluid from asthmatics: a new noninvasive tool in epidemiologic studies on air pollution and asthma. Free Radic Biol Med. 1999;27:1448–54. doi: 10.1016/s0891-5849(99)00191-4. December (11–12) [DOI] [PubMed] [Google Scholar]
- 6.Christian DL, Chen LL, Scannell CH, Ferrando RE, Welch BS, Balmes JR. Ozone-induced inflammation is attenuated with multiday exposure. Am J Respir Crit Care Med. 1988;158:532–7. doi: 10.1164/ajrccm.158.2.9709023. [DOI] [PubMed] [Google Scholar]
- 7.Dworski RTSS. Oxidant stress in asthma. Thorax. 2000;55:51–3. doi: 10.1136/thorax.55.suppl_2.S51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grievink L, Smit HA, Brunekreef B. Anti-oxidants and air pollution in relation to indicators of asthma and COPD: a review of the current evidence. Clin Exp Allergy. 2000;30:1344–54. doi: 10.1046/j.1365-2222.2000.00835.x. [DOI] [PubMed] [Google Scholar]
- 9.Samet JM, Hatch GE, Horstman D, et al. Effect of antioxidant supplementation on ozone-induced lung injury in human subjects. Am J Crit Care Med. 2001;164:819–25. doi: 10.1164/ajrccm.164.5.2008003. [DOI] [PubMed] [Google Scholar]
- 10.Romieu I, Sienra-Monge JJ, Ramirez-Aguilar M, et al. Antioxidants supplementation and lung function among asthmatic children exposed to high levels of air pollutants. Am J Respir Crit Care Med. 2002;166:703–9. doi: 10.1164/rccm.2112074. [DOI] [PubMed] [Google Scholar]
- 11.US Department of Health and Human Services. 1998. NIH/NLBI. Guia de bolsillo para el tratamiento y la prevencion del asma. NIH publication no. 96–3659B, Bethesda, MD. [DOI] [PubMed]
- 12.Noah TL, Henderson FW, Henry MM, Peden DB, Devlin RB. Nasal lavage cytokines in normal, allergic, and asthmatic school-age children. Am J Respir Crit Care Med. 1995;152:290–6. doi: 10.1164/ajrccm.152.4.7551384. [DOI] [PubMed] [Google Scholar]
- 13.Vandewoude M, Claeys M, DeLeeus I. Determination of alpha-tocopherol in human plasma by high performance liquid chromatography with electrochemical detection. J Chromatogr. 1984;311:176–82. doi: 10.1016/s0378-4347(00)84706-4. [DOI] [PubMed] [Google Scholar]
- 14.Anderson ME. Determination of glutathione and glutathione disulfide in biological samples. Meth Enzymol. 1985;113:548–55. doi: 10.1016/s0076-6879(85)13073-9. [DOI] [PubMed] [Google Scholar]
- 15.Kutnink MA, Hawkes WA, Schaus EE, Omaye ST. An internal standard method for unattended high-performance liquid chromatography analysis of ascorbic acid in blood components. Anal Biochem. 1987;166:424–30. doi: 10.1016/0003-2697(87)90594-x. [DOI] [PubMed] [Google Scholar]
- 16.Zar J. Biostatistical analysis. New Jersey: Prentice Hall; 1996. [Google Scholar]
- 17.Diggle P, Liang K, Zeger S. Analysis of longitudinal data. New York: Oxford University Press; 1994. [Google Scholar]
- 18.Stata Corporation. stata software. College Station, TX: Stata Corporation; 1996. release 7.0. [Google Scholar]
- 19.Horstman DH, Ball BA, Brown J, Gerrity T, Folinsbee LJ. Comparison of pulmonary responses of asthmatic and nonasthmatic subjects performing light exercise while exposed to a low level of ozone. Toxicol Ind Health. 1995;11:369–85. doi: 10.1177/074823379501100401. [DOI] [PubMed] [Google Scholar]
- 20.Finkel T. Oxygen radicals signaling. Curr Opin Cell Biol. 1998;10:248–53. doi: 10.1016/s0955-0674(98)80147-6. [DOI] [PubMed] [Google Scholar]
- 21.Blomberg A. Airway inflammatory and antioxidant responses to oxidative and particulate air pollutants − experimental exposure studies in humans. Clin Exp Allergy. 2000;30:310–7. doi: 10.1046/j.1365-2222.2000.00814.x. [DOI] [PubMed] [Google Scholar]
- 22.Krishna MT, Madden J, Teran LM, Biscione GL, Lau LC, Withers NJ. Effects of 0·2 ppm ozone on biomarkers of inflammation in bronchoalveolar lavage fluid and bronchial mucosa of healthy subjects. Eur Respir J. 1998;11:1294–300. doi: 10.1183/09031936.98.11061294. [DOI] [PubMed] [Google Scholar]
- 23.Jorres RA, Holtz O, Zachgo W, Timm P, Koschyk S, Muller B. The effects of repeated ozone exposures in inflammatory markers in bronchoalvelolar lavage fluid and mucosal biopsies. Am J Respir Crit Care Med. 2000;161:1855–61. doi: 10.1164/ajrccm.161.6.9908102. [DOI] [PubMed] [Google Scholar]
- 24.Van Bree L, Dormans JA, Koren HS, Devlin RB, Rombout PJ. Attenuation and recovery of pulmonary injury in rats following short-term, repeated daily exposure to ozone. Inhal Toxicol. 2002;14:883–900. doi: 10.1080/08958370290084674. [DOI] [PubMed] [Google Scholar]
- 25.Blomberg A, Mudway IS, Norderhall C, Hendenstrom H, Kelly FJ, Frew AJ. Ozone-induced lung function decrements do not correlate with early airway inflammatory or antioxidant responses. Eur Resp J. 1999;13:1418–28. doi: 10.1183/09031936.99.13614299. [DOI] [PubMed] [Google Scholar]
- 26.Wu D, Koga T, Martin KR, Meydani M. Effect of vitamin E on human aortic endothelial cell production of chemokines and adhesion to monocytes. Atherosclerosis. 1999;147:297–307. doi: 10.1016/s0021-9150(99)00199-9. [DOI] [PubMed] [Google Scholar]
- 27.Abe S, Takizawa H, Sugawara I, Kudoh S. Diesel exhaust (DE-induced cytokine expression in human bronchial epithelial cells: a study with a new cell exposure system to freshly generated DE in vitro. Am J Respir Cell Biol. 2000;22:296–303. doi: 10.1165/ajrcmb.22.3.3711. [DOI] [PubMed] [Google Scholar]
- 28.Norwood J, Jr, Ledbetter AD, Doerfler DL, Hatch G. Residual oil fly ash inhalation in guinea pigs: influence of absorvate and glutathione depletion. Toxicol Sci. 2001;61:144–53. doi: 10.1093/toxsci/61.1.144. [DOI] [PubMed] [Google Scholar]
- 29.Mudway IS, Housley D, Eccles R, Richards RJ, Datta AK, Tetley TD. Differential depletion of human respiratory tract antioxidants in response to ozone challenge. Free Radic Biol Med. 1996;25:499–513. doi: 10.3109/10715769609149072. [DOI] [PubMed] [Google Scholar]
- 30.Mudway IS, Blomberg A, Frew AJ, Holgate ST, Sandstrom T, Kelly FJ. Antioxidant consumption and repletion kinetics in nasal lavage fluid following exposure of healthy human volunteers to ozone. Eur Respir J. 1999;13:1429–33. doi: 10.1183/09031936.99.13614399. [DOI] [PubMed] [Google Scholar]
- 31.Koren HS, Hatch GE, Graham DE. Nasal lavage as a tool in assessing acute inflammation in response to inhaled pollutants. Toxicology. 1990;60:15–25. doi: 10.1016/0300-483x(90)90159-e. [DOI] [PubMed] [Google Scholar]
- 32.Graham DE, Koren HS. Biomarkers of inflammation in ozone-exposed humans. Comparison of the nasal and bronchoalveolar lavage. Am Rev Respir Dis. 1990;142:152–6. doi: 10.1164/ajrccm/142.1.152. [DOI] [PubMed] [Google Scholar]
- 33.Food and Nutrition Board. 10. Washington, DC: National Academy, Press; 1989. Recommended dietary allowance, [Google Scholar]
- 34.Traber MG. Vitamin E. In: Shills ME, Olson JA, Shike M, Ross CA, editors. Modern nutrition in health and disease. 9. Baltimore: Williams & Wilkins; 1998. pp. 347–62. [Google Scholar]