Abstract
Sepsis is associated with depression of T cell-dependent immune reactivity with proinflammatory cytokines, such as tumour necrosis factor (TNF)-α, playing an important role. Recent investigations describe an association between these immunological alterations and disturbances of the endocrine system, related most frequently to sex steroid hormones. Dehydroepiandrosterone (DHEA), one of the most abundant adrenal sex steroid precursors, seems to have a protective immunological effect towards septic insults. In this study, both the role of TNF-receptor I (RI) and possible interactions in the protective role of DHEA were investigated in a murine model of polymicrobial sepsis. Polymicrobial sepsis was induced by caecal ligation and puncture (CLP) in a murine model. The effects of DHEA on survival, clinical parameters and cellular immunity (T lymphocytes and natural killer (NK) cells) were investigated. CLP was performed in genetically modified TNF-RI knock-out (TNF-RI–/–) and genetically unmodified (wild-type, WT) mice. DHEA application was associated with a decrease in the mortality rate in WT animals. A mortality rate of 91·7% was observed in TNF-RI–/– mice after CLP. This mortality rate was reduced to 37·5% by the application of DHEA. In sham-operated TNF-RI–/– animals, a significantly higher proportion of NK cells within the lymphocyte population was measured compared with the corresponding WT group. After CLP, a significant increase in the percentage cell count of NK cells was recorded in WT mice. Overall, following DHEA application in WT mice, an alteration in the cellular immune response was characterized by a reduction in the percentage counts of CD4+, CD8+ and NK cells. In the group of TNF-RI–/– mice treated with DHEA, no increase in the percentage cell count of NK cells was observed after CLP. No data for cell analysis were available from the CLP-TNF-RI–/– mice treated with saline, due to the high mortality rate in these animals. DHEA reduces the complications of sepsis in a TNF-RI-independent manner. Our study suggests that NK cells are involved in the protective mechanism of DHEA in WT mice. It would therefore seem that DHEA represents a feasible alternative therapy for the dysregulated immune system in sepsis.
Keywords: dehydroepiandrosterone (DHEA), lymphocytes, mortality sepsis, tumour necrosis factor receptor I (TNF-RI)
INTRODUCTION
Host reaction to systemic sepsis is associated with a wide variety of modifications to the immune system. An overall decrease in T cell proliferation is observed [1] and profound depression of T cell-dependent immunity, quantified by delayed-type hypersensitivity, has been demonstrated following induction of polymicrobial sepsis in a rodent model [2]. Reduced synthesis and secretion of interleukin (IL)-2 by a subpopulation of CD4+ lymphocytes, Th1 cells, is observed [3], along with reduced IL-2 surface receptor expression and increased receptor shedding [4]. IL-2 has been shown to act as an autocrine stimulator of Th1 cells, leading to activation of CD8+ and CD56+ lymphocytes.
Increased concentrations of other cytokines, such as tumour necrosis factor (TNF)-α, seem to play an important role in the pathogenesis of sepsis. Plasma TNF-α concentrations correlate with severity and outcome and persistantly elevated plasma levels are associated with a poor prognosis in septic patients [5]. In many cases TNF-α acts via the TNF-RII receptor, mediating such functions as cell proliferation [6]. Following subcutaneous injection of TNF-α, TNF-RII knock-out mice (TNF-RII–/–) demonstrate reduced tissue necrosis when compared to wild-type mice following toxic stimulus. TNF-α has also been shown to stimulate synthesis of IL-2 and interferon (IFN)-γ by T lymphocytes in vitro [7]. These alterations in immune function have been associated with increased activation of the endocrine system, mediated by increased concentrations of circulating cytokines. Moreover, sepsis-induced hormonal dysregulation leads to modifications of cellular immune function, suggesting that this altered immunological response could be influenced by therapeutic hormonal manipulation [8–10].
The immunological effect of dehydroepiandrosterone (DHEA, 5-androsten-3-ol-17-one), an adrenal sex steroid hormone precursor, in sepsis has been well documented [11–13]. By stimulating IFN-γ and IL-2 production in Th1 cells and inhibiting Th2 cell synthesis of IL-4 and IL-6, DHEA counteracts a form of antigen-specific immune suppression [14]. DHEA has also been shown to modulate prednisone-induced immune suppression, resulting in higher survival rates following Gram-negative sepsis. This improvement in cellular immune function is associated with reversal of sepsis-related delayed-type hypersensitivity depression [15]. The administration of DHEA to healthy volunteers has been associated with reduced plasma levels of CD4+ and increased CD8+ and CD56+ cell counts [16]. In a rodent, DHEA administration was associated with a smaller subsequent fall in both CD8+ cell counts and DTH reactivity following induction of sepsis [17].
Based on these previous findings, our study sought to answer the following questions: (1) are sepsis-induced immunological malfunctions improved by administration of DHEA as suggested by previous studies? (2) If so, are these improvements mediated by the TNF receptor?
MATERIALS AND METHODS
Animal care
The study was approved by the Institutional Review Board of our university. The experiments were performed in 48 male C57BL/6 TNF-RI receptor knock-out (TNF-RI–/–) mice aged 8–10 weeks and weighing 20 ± 3 g. Forty male C57BL/6 mice of similar weight without TNF receptor knock-out (wild-type; WT) were used as a control group.
The animals were bred and raised in the central animal facility of our institution. Throughout the entire study, pelleted mouse feed (Altromin 1324) and water were available ad libitum. Lighting was provided on a 12-h cycle and the room temperature was maintained at a constant 20 ± 2°C.
Caecal ligation and puncture
Following anaesthetic by subcutaneous injection of ketamine (100 mg/kg) and xylazine (16 mg/kg), polymicrobial sepsis was induced by caecal ligation and puncture (CLP). The caecum was exposed via midline laparotomy and unilateral puncture performed twice using an 18-gauge needle. Caecal contents were expressed subsequently by pressure to ensure bacteria were delivered to the peritoneal cavity and the abdomen closed in two layers. The mice were then warmed to 36°C using a heating lamp. For the control group, a sham laparotomy was performed without caecal puncture.
Experimental groups
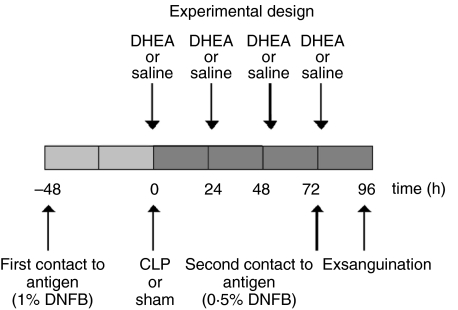
Animals were subjected subsequently to subcutaneous injection of DHEA or placebo. The DHEA, dissolved in normal saline containing 0·1% ethanol, was administered at a dose of 25 mg/kg immediately after the operation (sham or CLP) and once daily at 24, 48 and 72 h. Mice in the two remaining groups received the same volume of 0·9% saline subcutaneously, also containing 0·1% ethanol (Fig. 1). Equal volumes were administered in each case. Thus, by employing these four experimental approaches in WT and TNF-RI–/– mice (Table 1), eight groups were created in total.
Fig. 1.
Time-course of experiments. DHEA: dehydroepiandrosterone; saline: control, control for DHEA; CLP: caecal ligation and puncture; sham: only laparotomy; control for CLP
Table 1.
Distribution of experimental groups
| Experimental groups | |||
|---|---|---|---|
| WT | TNF-RI–/– | ||
| Sham | |||
| Saline | 10 animals | period of 96 h | 10 animals |
| DHEA | 10 animals | 10 animals | |
| CLP | |||
| Saline | 11 animals | 12 animals | |
| DHEA | 9 animals | 16 animals | |
WT: wild-type; C57BL/6 mice; TNF-RI: tumour necrosis factor receptor I; DHEA: dehydroepiandrosterone; saline: control for DHEA; CLP: caecal ligation and puncture; sham: only laparotomy; control for CLP.
Delayed-type hypersensitivity (DTH) reaction
The DTH reaction was quantified by measuring the degree of pinnal swelling following local sensitization using dinitrofluorobenzene (DNFB, Sigma-Aldrich GmbH, Deisenhofen, Germany). Baseline values for pinnal thickness were taken from the right ear, using an engineering micrometer (Oditest, Kroeplin, Germany). DNFB was diluted in acetone and olive oil to concentrations of 0·5% and 1·0%. For sensitization, animals were anaesthetized lightly using ethylether. The dorsal skin was shaved and 50 µl of DNFB (1·0%) applied 48 h prior to operation. DNFB (0·5%) was reapplied subsequently on the dorsal skin of the right ear at 72 h following the CLP or sham operation. Pinnal swelling was measured again 24 h later.
Clinical parameters
Body temperature, weight and mortality were recorded 48 h before and at 12, 24, 48, 72, 84 and 96 h following procedures. Temperature was measured rectally using a digital thermometer (Greisinger Electronic, Bonn, Germany). Differences between current and preoperative body weight were calculated at each time-point.
Blood collection
All animals were sacrificed at 96 h after the CLP or sham operation. Following deep anaesthetic using subcutaneous injections of ketamine (100 mg/kg) and xylazine (16 mg/kg), the mice were fixed in a supine position and killed by exsanguination via cardiac puncture. Syringes were primed with heparin (Hoffmann-La Roche, Grenzach-Whylen, Germany) to avoid premature blood clotting. The samples were centrifuged for 5 min at 13·000 g and the cellular components resuspended in 1 ml saline (0·9%) prior to evaluation of CD4+, CD8+ and natural killer (NK) cells.
Phenotyping by flow cytometry
Erythrocytes in the blood samples were lysed using a solution containing 8·3 g NH4Cl, 0·1 g EDTA and 1·0 g KHCO3 in 1 l of distilled water. The leucocyte fraction was washed twice with, then suspended in, phosphate-buffered saline (PBS) (pH 7·4). One hundred µl of the resulting suspension was added to 5 µg of appropriate antibody in separate polypropylene tubes. Selected antigens included CD4, CD8 and CD56 (Ly49c or a mouse equivalent, Pharmingen, Freiburg, Germany). The CD4-FITC and CD8-PE antibodies were used together in one tube in order to detect CD4+, CD8+ and CD4+ CD8+ populations. Cells were incubated with these antibodies for 20 min at 4°C. After washing twice with PBS, the cells were resuspended in a binding buffer and quantified immediately. Measurement was performed using a FACScan cytometer (Becton Dickson, Heidelberg, Germany). An identical sample, free of antibodies, was used as a negative control in order to adjust forward and side scatter and the fluorescence channels. A sample containing an irrelevant antimouse antibody was used to check for non-specific staining effects. Five thousand lymphocytes per sample were counted using lymphocyte gating. The gate was set using the negative control. By means of the evaluation software winmdi the relevant ratios of lymphocyte subsets were determined.
Statistics
Statistical analysis was performed using spss software (SPSS version 11·5, Chicago, IL, USA). Significance was assumed at the P < 0·05 level. Comparison between groups (CLP versus sham; DHEA versus saline) was performed using one-way analysis of variance (anova) and a post-hoc Tukey test. Survival rates were compared using the χ2 test. Results are expressed as mean ±standard error of the mean (s.e.m.).
RESULTS
Survival rate
All sham-operated animals survived the experiment until sacrifice. In the group of WT mice, five of 11 animals died after CLP and saline application (mortality rate 45·5%). Following CLP and application of DHEA, one of nine mice died (mortality rate 11·1%). In C57Bl/6 TNF-RI–/– mice, 11 of 12 CLP-operated animals who did not receive DHEA died (mortality rate 91·7%). The application of DHEA reduced the mortality rate in these mice to 37·5% (six of 16 died). Because analysing the data of the only surviving animal of the TNF-RI–/– CLP + saline group did not appear reasonable, this group was excluded from the following result descriptions.
Body weight
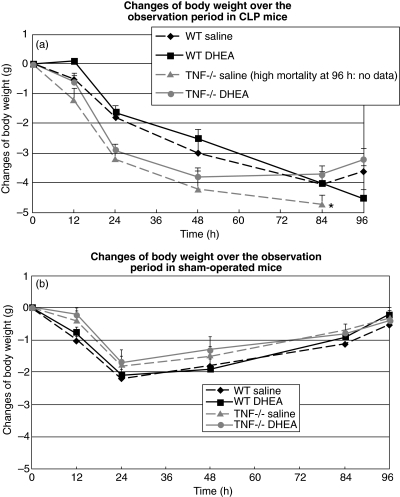
All animals suffered a weight loss (average 2·1 ± 0·2 g) in the first 24 h postoperatively. Sham-operated animals regained weight, until they regained their approximate initial weight by the end of the experiment. The observed initially greater loss of body weight in WT animals is related probably to their higher average initial weight (Fig. 2a).
Fig. 2.
Body weight of wild-type (WT) and TNF-RI knock-out (TNF-RI–/–) mice over the observation period. CLP: caecal ligation and puncture; DHEA: dehydroepiandrosterone; comparison between groups (CLP versus sham; DHEA versus saline) was performed using one-way analysis of variance (anova) and a post-hoc Tukey test; *P < 0·05.
At 72 h following induction of sepsis, significant weight loss was recorded in the CLP animals. WT and the TNF-RI–/– animals treated with saline demonstrated a more pronounced loss of body weight when compared with those who had received DHEA injections (P ≤ 0·05) (Fig. 2a).
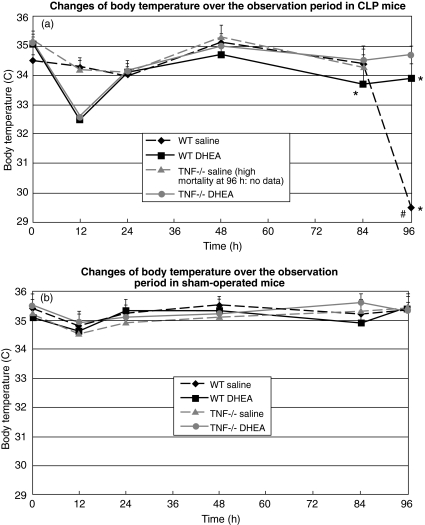
Body temperature
The initial average body temperature of all animals prior to operation was 35·3 ± 0·2°C. Sham-operated animals had a slightly reduced temperature at 12 h post-procedure compared to baseline (average body temperature: 34·7 ± 0·1°C). By 24 h, temperature returned to normal values (Fig. 3a).
Fig. 3.
Body temperature of wild-type (WT) and TNF-RI knock-out (TNF-RI–/–) mice over the observation period. CLP: caecal ligation and puncture; DHEA: dehydroepiandrosterone; comparison between groups (CLP versus sham; DHEA versus saline) was performed using one-way analysis of variance (anova) and a post-hoc Tukey test; statistical significance *P < 0·05, WT versus equivalent TNF−/− saline and WT DHEA versus TNF−/−. #Statistical significance (P < 0·05), WT saline versus WT DHEA.
In contrast, at 12 h after the induction of sepsis, the average body temperature of CLP animals treated with DHEA was significantly decreased, and was significantly lower than that in both the sham-operated animals and the CLP animals treated with saline. At 12, 24, 48 and 72 h no significant difference in body temperature was observed between treated and non-treated CLP groups. However, at 96 h the average body temperature of untreated WT-CLP mice had fallen to 29·5 ± 0·4°C, while body temperature in other groups was only slightly below baseline (P < 0·05, t-test) (Fig. 3a).
Phenotyping of lymphocytes
CD4+ lymphocytes
At the 96-h time-point, no differences in total leucocyte counts were observed between the different groups, nor were differences detected between the different strains.
Application of DHEA was associated with a significant decrease in CD4+ cell counts in both TNF-RI–/– and WT groups when compared to the corresponding saline control groups (P = 0·007). CLP resulted in a small but insignificant change in the CD4+ lymphocyte count when compared to the respective sham groups given the same medication (P = 0·6).
Comparison of mean cell counts from all experimental groups revealed a significant difference between the WT sham mice given saline (24·4% ± 1·2%) and WT sham mice given DHEA (18·5% ± 1·7%) (P = 0·015, t-test).
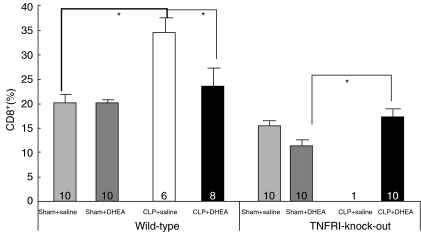
CD8+ lymphocytes
The influence of CLP and DHEA administration on CD8+ cell counts was statistically significant (P < 0·05) (Fig. 4). CLP resulted in an increase in CD8+ cell counts. There was no observed difference in the cell count ratios from WT sham-operated animals whether or not they received DHEA (20·3% ± 1·3% versus 20·5% ± 0·8%). An increase to 34·9% ± 2·9% was observed in the WT animals subjected to CLP receiving saline (P < 0·05 versus WT sham + saline). This increase was reduced significantly by administration of DHEA (23·8% ± 3·9%, P = 0·042, t-test). In TNF-RI–/– mice treated with DHEA, the induction of sepsis by CLP led to a significant increase of CD8+ cell counts (TNF-RI–/– CLP + DHEA: 17·7% ± 1·4% versus TNF-RI–/– sham + DHEA: 11·6% ± 1·2%, P = 0·005, t-test) (Fig. 4).
Fig. 4.
Percentage part of CD8+ lymphocytes in blood of wild-type (WT) and TNF-RI knock-out (TNF-RI–/–) mice. CLP: caecal ligation and puncture; DHEA: dehydroepiandrosterone; comparison between groups (CLP versus sham; DHEA versus saline) was performed using one-way analysis of variance (anova) and a post-hoc Tukey test; statistical significance *P < 0·05.
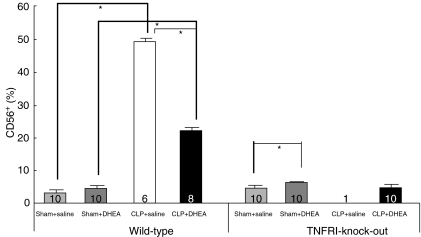
NK cells
DHEA treatment significantly increased the proportion of NK cells in sham-operated animals in both the WT and TNF-RI–/– animals (P < 0·05) (Fig. 5). This DHEA-induced increase was small compared to that induced by CLP in WT mice. Polymicrobial sepsis resulted in a highly significant increase in NK cell counts in WT groups compared to respective WT-sham groups. DHEA treatment was observed to reduce NK cell counts in WT mice that underwent CLP compared to the WT CLP + saline control group [WT CLP + saline: 49·5 ± 0·7% versus WT CLP + DHEA: 22·4 ± 0·8% (P < 0·05)] (Fig. 5).
Fig. 5.
Percentage part of CD56+ (NK) lymphocytes in blood of wild-type (WT) and TNF-RI knock-out (TNF-RI–/–) mice. CLP: caecal ligation and puncture; DHEA: dehydroepiandrosterone; comparison between groups (CLP versus sham; DHEA versus saline) was performed using one-way analysis of variance (anova) and a post-hoc Tukey test; statistical significance *P < 0·05.
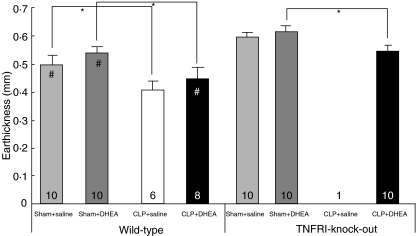
DTH reaction
Significant oedema formation was observed in all animals. The average baseline pinnal thickness was 0·24 mm ± 0·002 mm for all animals. Twenty-four h after second antigen exposure, an average increase in pinnal thickness of 0·53 mm ± 0·01 mm was observed. The application of DHEA resulted in slightly increased oedema formation compared to the groups receiving saline in both WT and TNF-RI–/– mice. Univariate analysis revealed DHEA administration to have a significant influence on the increase of oedema formation (P = 0·049). After induction of sepsis, a significantly less severe response to the second antigen contact compared to the respective sham group was observed in WT and TNF-RI–/– animals (P ≤ 0·0005) (Fig. 6).
Fig. 6.
Delayed type of hypersensitivity reaction (DTH reaction) of wild-type (WT) and TNF-RI knock-out (TNF-RI–/–) mice. CLP: caecal ligation and puncture; DHEA: dehydroepiandrosterone; comparison between groups (CLP versus sham; DHEA versus saline) was performed using one-way analysis of variance (anova) and a post-hoc Tukey test; statistical significance *P < 0·05. Statistical significance #P < 0·05, WT versus equivalent TNF-RI−/− groups.
TNF-RI–/– animals demonstrated a significantly higher reactivity to the second antigen contact compared to equivalent WT mice. This was observed in both sham-operated animals treated with DHEA or saline and in CLP mice after saline application (Fig. 6).
DISCUSSION
The main results of this study can be summarized as follows. (1) In accordance with previous studies, DHEA administration resulted in a decreased mortality rate in WT mice with sepsis induced by CLP [17]. Moreover, DHEA also reduced the mortality rate in TNF-RI–/– mice; this has not been described previously. (2) The TNF-RI was shown to be essential for survival in sepsis, confirming previous work [18]. All TNF-RI–/– animals not treated with DHEA died after CLP. (3) Polymicrobial sepsis (CLP) was shown to increase subpopulations of CD4+, CD8+ and NK cells, most significantly in NK cells in both WT animals and in TNF-RI–/– mice treated with DHEA. This rise was attenuated in WT animals by administration of DHEA. This has not been demonstrated previously. Data of TNF-RI–/– mice treated with saline were not available due to the high mortality rate in this group. (4) The DTH reaction was diminished following CLP. DHEA treatment partially negated this phenomenon, resulting in an improved reaction. This is in accordance with previous reports [17].
Because a number of mice in each CLP group died prior to the end of the observation period (96 h), blood samples for determination of the T lymphocyte subpopulations was not possible in all animals. It should be recognized that this might limit the significance of our data, as the most profoundly affected animals died and their results are therefore not considered.
Considering the results of previous studies, it has been proposed that DHEA acts by decreasing plasma TNF-α levels [15,19]. However, in the current study a significant improvement in survival rates was also demonstrated in TNF-RI–/– mice following DHEA administration, demonstrating clearly that its main effect on mortality is not via the TNF-RI. It has been shown that the TNF-RI is responsible for mediating most of the effects of TNF-α [20]; it might therefore be suggested that DHEA has a function independent of TNF-α. However, TNF-RI and -RII are considered to exert a broad range of immunological effects. It was supposed that TNF-RI might have protective effects in sepsis, whereas TNF-RII might mediate shock and proliferation of immune cells [6,21]. Moreover, the role of other receptors for TNF-α has not been elucidated fully. It is likely that, in specific situations, TNF-α exerts its effects by means of these alternative receptors and it may be that DHEA interacts via these mechanisms.
This study confirms that an immune function mediated through the TNF-RI is essential for survival in polymicrobial sepsis. The mortality of TNF-RI–/– mice treated with saline was almost 100%. A previous study reported increased survival rates in p55/p75–/– mice following CLP [18]. However, there is some evidence from previous work that TNF-α has a protective role in sepsis: mice with anti-TNF-α-antibodies died after induction of polymicrobial sepsis by CLP [22], this situation being remedied somewhat by administration of recombinant TNF-α, improving survival rates [23]. In another study, all TNF-α knock-out mice died after CLP within 10 h [24]. Increased mortality rates of TNF-RI–/– mice were also observed despite increased endotoxin tolerance, following specific bacterial infections, e.g. after intraperitoneal injection of Streptococcus pneumoniae, Escherichia coli or Listeria monocytogenes. In addition, in order to mount an appropriate immune response to viral infection, both the 55 kDa and the 75 kDa TNF receptors have been found to be essential [25,26]. TNF-α is thought to be one of the main mediators of septic shock in humans and because increased serum concentrations of TNF-α are associated with poor outcome, several controlled therapeutic trials have been performed in septic patients using anti-TNF-α or TNF receptor antibodies. These interventions did not, however, prove effective and, in several studies, inferior outcomes in treated patients have been reported [27,28]. This finding may be explained at least partially by the protective effect of TNF-α, mediated via the TNF-RI, as demonstrated in our study.
Interestingly, despite reducing the overall mortality, DHEA administration caused only a slight improvement in such parameters as body weight and core temperature. Only after 96 h following induction of sepsis was a significant difference in body temperature observed between animals treated with saline and DHEA in the WT group. More significant differences in body weight were observed between WT and TNF-RI–/– mice. This may have been due to the fact that TNF-RI–/– mice were less able to tolerate sepsis than WT animals. This is also consistent with the observed differences in survival times. No significant difference in body weight between animals treated with saline and DHEA were recorded in this study.
While Th1 cells play a major role in the cellular immune response (delayed, specific immunity) through secretion of IL-2 and IFN-γ, Th2 cells induce the production of antibodies by B lymphocytes via secretion of IL-4 and IL-10. During sepsis, initially cells of the Th1 phenotype predominate. Following this, increasing concentrations of Th2 cells are observed, accompanied by a rising plasma IL-10 concentration and a falling concentration of IL-2 and IFN-γ (known as the Th1–Th2 shift) [29].
In our study, contact hypersensitivity was determined by means of right pinnal swelling following prior sensitization and second antigen contact; this method is well established [30]. Sepsis led to a decreased response in all animals. This response was improved slightly by the application of DHEA over placebo, without reaching statistical significance. However, univariate analysis revealed DHEA administration to have a significant influence. Improvement in the delayed hypersensitivity reaction (Type IV) in NMRI mice was more distinct in previous studies from our research group [17,31]; a similar effect of DHEA administration was also observed after burn injuries [32]. It is assumed that DHEA has a stimulatory effect on Th1 cells, leading to increased activation [33]. Specific DHEA receptors have been isolated from T cells [34] and the overall Th1/Th2 ratio has been shown to be dependent on DHEA-sulphatase activity, an enzyme that converts DHEAS to DHEA [35]. Moreover, it has been demonstrated that a single dose of DHEA can neutralize the T cell-inhibitory effect of glucocorticoids [36]. TNF-RI also appears to play a role in the local sensitivity reaction. TNF-RI–/– animals demonstrate florid reactivity to the second antigen contact compared to equivalent WT mice. Other groups, demonstrating an inhibitory effect of TNF-α on dermal Langerhans cells, report similar results [37]. Absence or delay of the specific humoral immune response, such as Th1–Th2 shifts, impairs B cell activation. A similar role for TNF-α has been described in worm-infected mice [38]. The clinical relevance of the dermal immune reaction has been demonstrated previously in a clinical study, where it was shown that surgical patients demonstrating an anergic skin immune response suffered an increased incidence of sepsis and higher mortality [39]. However, the specific role played by TNF-α in the delayed immune response is unknown.
Individual subpopulations of T lymphocytes underwent different reactions to the induction of sepsis and the administration of DHEA. DHEA application reduced slightly the proportion of CD4+ cells in all groups. This is in accordance with previous observations in a healthy population [40]. In prior studies, a significant fall in CD8+ cell concentrations was recorded following CLP. In contrast, we observed a marked increase in the proportion of CD8+ cells following induction of sepsis. However, the previous observations were made in NMRI mice at different time-points from those in our experiments (48 h) [17]. As a result of polymicrobial sepsis, marked overall suppression of immune responsiveness in lymphocytes was demonstrated by reduced cytokine synthesis and an increased rate of apoptosis. This immune suppression is associated with increased concentrations of T suppressor cells. In previous studies, the increased number of apoptotic cells was limited mainly to CD4+ cells and it was accepted that the increased apoptosis rate of lymphocytes is induced by the Fas-ligand (FasL) [41]. However, some groups have assumed that FasL induces the apoptosis in specific cells by induction of TNF [42]. Further studies are needed to clarify the role of TNF in apoptosis regulation in our mice model.
Little is known about the role of NK cells in the pathogenesis of ‘multiple organ dysfunction syndrome’ (MODS) and sepsis. In an experimental study it was observed that haemorrhage resulted in massive increases in circulating NK cell numbers [43]. Furthermore, it was demonstrated that mortality was reduced after depletion of NK cells in cytokine-induced shock and by a systemic response to endotoxin, the Shwartzman reaction [44]. This is induced by endotoxin application following prior intradermal sensitization. These results verify the negative effects of increased NK cell proliferation. Activation of macrophages by NK cells is one possible mechanism [45].
One of the key findings in our study is that CLP increased significantly the relative proportion of NK cells in WT animals. DHEA treatment reduced this effect significantly. This suggests that NK cells are of major importance in the protective mechanism of DHEA. It should also be noted that TNF-RI–/– mice had lower concentrations of NK cells and of CD8+ cells compared to WT animals in the corresponding group. To our knowledge, these phenotypic differences have not been demonstrated previously, and may be responsible for the increased mortality rate in TNF-RI–/– mice.
CONCLUSION
It appears that TNF-α offers protection during the initial phase of polymicrobial sepsis via the TNF-RI. DHEA also had positive effects in TNF-RI–/– animals, suggesting a TNF-RI-independent mechanism. This work supports the idea that DHEA may represent a reasonable therapeutic option for the dysregulated immune system, as has been suggested previously [46,47]. Further investigations are required to clarify the functional mechanisms of DHEA.
REFERENCES
- 1.Levy EM, Alharbi SA, Grindlinger G, Black PH. Changes in mitogen responsivness of lymphocyte subsets after traumatic injury: relation to development of sepsis. Clin Immunol Immunopathol. 1984;32:224–8. doi: 10.1016/0090-1229(84)90123-5. [DOI] [PubMed] [Google Scholar]
- 2.Pape HC, Remmers D, Grotz M, Tscherne H. Reticuloendothelial system activity and organ failure in multiply injured patients. Arch Surg. 1999;134:421–7. doi: 10.1001/archsurg.134.4.421. [DOI] [PubMed] [Google Scholar]
- 3.Gadd MA, Hansbrough JF, Hoyt DB, Ozkan AN. Defective T cell surface antigen expression after mitogen stimulation. An index of lymphocyte dysfuction after controlled murine injury. Ann Surg. 1989;209:112–9. doi: 10.1097/00000658-198901000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoyt DB, Ozkan AN, Hansbrough JF, Marshall L, van Berkum-Clark M. Head injury: an immunologic deficit in T cell activation. J Trauma. 1990;30:759–65. [PubMed] [Google Scholar]
- 5.Blackwell TS, Christman JW. Sepsis and cytokines: current status. Br J Anaesth. 1996;77:110–5. doi: 10.1093/bja/77.1.110. [DOI] [PubMed] [Google Scholar]
- 6.Grell M, Becke FM, Wajant H, Mannel DN, Scheurich P. TNF receptor type 2 mediates thymocyte proliferation independently of TNF receptor type 1. Eur J Immunol. 1998;28:257–63. doi: 10.1002/(SICI)1521-4141(199801)28:01<257::AID-IMMU257>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 7.Joseph SB, Miner KT, Croft M. Augmentation of naive, Th1 and Th2 effector CD4 responses by IL-6, IL-1 and TNF. Eur Immunol. 1998;28:277–89. doi: 10.1002/(SICI)1521-4141(199801)28:01<277::AID-IMMU277>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 8.Zellweger R, Wichmann MW, Ayala A, Stein S, Demaso CM, Chaudry IH. Females in proestrus state maintain splenic immune functions and tolerate sepsis better than males. Crit Care Med. 1997;25:106–10. doi: 10.1097/00003246-199701000-00021. [DOI] [PubMed] [Google Scholar]
- 9.Kahlke V, Angele MK, Ayala A, et al. Immune dysfunction following trauma-haemorrhage: influence of gender and age. Cytokine. 2000;12:69–77. doi: 10.1006/cyto.1999.0511. [DOI] [PubMed] [Google Scholar]
- 10.Straub RH, Lehle K, Herfarth H, et al. Dehydroepiandrosterone in relation to other adrenal hormones during an acute inflammatory stressful disease state compared with chronic inflammatory disease: role of interleukin-6 and tumor necrosis factor. Eur J Endocrinol. 2002;146:365–74. doi: 10.1530/eje.0.1460365. [DOI] [PubMed] [Google Scholar]
- 11.Okabe T, Haji M, Takayanagi R, et al. Up-regulation of high-affinty dehydroepiandrosterone binding activity by dehydroepiandrosterone in activated human T-lymphocytes. J Clin Endocrinol Metab. 1995;80:2993–8. doi: 10.1210/jcem.80.10.7559886. [DOI] [PubMed] [Google Scholar]
- 12.Beishuizen A, Thijs LG, Vermes I. Decreased levels of dehydroepiandrosterone sulphate in severe critical illness: a sign of exhausted adrenal reserve. Crit Care Med. 2002;6:434–8. doi: 10.1186/cc1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marx C, Petros S, Bornstein SR, et al. Adrenocortical hormones in survivors and nonsurvivors of severe sepsis: diverse time course of dehydroepiandrosterone, dehydroepiandrpsterone-sulphate, and cortisol. Crit Care Med. 2003;31:1382–8. doi: 10.1097/01.CCM.0000063282.83188.3D. [DOI] [PubMed] [Google Scholar]
- 14.Catania RA, Angele MK, Ayala A, Ciuffi WG, Bland KI, Chaudry IH. Dehydroepiandrosterone restores immune function following trauma-haemorrhage by a direct effect on T-lymphocytes. Cytokine. 1999;11:443–50. doi: 10.1006/cyto.1998.0458. [DOI] [PubMed] [Google Scholar]
- 15.Danenberg HD, Alpert G, Lustig S, Ben-Nathan D. Dehydroepiandrosterone protects mice from endotoxin toxicity and reduces tumor necrosis factor production. Antimicrob Agents Chemother. 1992;36:2275–9. doi: 10.1128/aac.36.10.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Casson PR, Andersen RN, Herrod HG, et al. Oral dehydroepiandrosterone in physiologic doses modulates imune function in postmenopausal women. Am J Obstet Gynecol. 1993;169:1536–9. doi: 10.1016/0002-9378(93)90431-h. [DOI] [PubMed] [Google Scholar]
- 17.van Griensven M, Dahlweid M, Giannoudis PV, et al. Dehydroepiandrosterone (DHEA) modulates the activity and the expression of lymphocyte subpopulations induced by cecal ligation and puncture. Shock. 2002;18:445–51. doi: 10.1097/00024382-200211000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Leon LR, White AA, Kluger MJ. Role of IL-6 and TNF in thermoregulation and survival during sepsis in mice. Am J Physiol. 1998;275:R269–77. doi: 10.1152/ajpregu.1998.275.1.R269. [DOI] [PubMed] [Google Scholar]
- 19.Di Santo E, Foddi MC, Ricciardi-Castagnoli P, Mennini T, Ghezzi P. DHEAS inhibits TNF production in monocytes, astrocytes and microglial cells. Neuroimmunomodulation. 1996;3:285–8. doi: 10.1159/000097282. [DOI] [PubMed] [Google Scholar]
- 20.Bazzoni F, Beutler B. The tumor necrosis factor ligand and receptor families. N Engl J Med. 1996;334:1717–25. doi: 10.1056/NEJM199606273342607. [DOI] [PubMed] [Google Scholar]
- 21.Pfeffer K. Biological functions of tumor necrosis factor cytokines and their receptors. Cytokine Growth Factor Rev. 2003;14:185–91. doi: 10.1016/s1359-6101(03)00022-4. [DOI] [PubMed] [Google Scholar]
- 22.Echtenacher B, Falk W, Mannel DN, Krammer PH. Requirement of endogenous tumor necrosis factor/cachectin for recovery from experimental peritonitis. J Immunol. 1990;145:3762–6. [PubMed] [Google Scholar]
- 23.Sheppard BC, Fraker DL, Norton JA. Prevention and treatment of endotoxin and sepsis lethality with recombinant human tumor necrosis factor. Surgery. 1989;106:156–61. [PubMed] [Google Scholar]
- 24.Calandra T, Baumgartner JD, Grau GE, Wu MM, Lambert PH, Schellekens J. Prognostic values of tumor necrosis factor/cachectin, interleukin-1, interferon-α, and interferon-gamma in the serum of patiens with septic shock. J Infect Dis. 1990;161:928–37. doi: 10.1093/infdis/161.5.982. [DOI] [PubMed] [Google Scholar]
- 25.Acton RD, Dahlberg PS, Uknis ME, et al. Differential sensitivity to Escherichia coli infection in mice lacking tumor necrosis factor p55 or interleukin-1 p80 receptors. Arch Surg. 1996;131:1216–21. doi: 10.1001/archsurg.1996.01430230098017. [DOI] [PubMed] [Google Scholar]
- 26.O'Brien DP, Briles DE, Szalai AJ, Tu AH, Sanz I, Nahm MH. Tumor necrosis factor alpha receptor I is important for survival from Streptococcus pneumoniae infections. Infect Immun. 1999;67:595–601. doi: 10.1128/iai.67.2.595-601.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abraham E, Anzueto A, Gutierrez G, et al. Double-blind randomised controlled trial of monoclonal antibody to human tumour necrosis factor in treatment of septic shock. NORASEPT II Study Group. Lancet. 1998;351:929–33. [PubMed] [Google Scholar]
- 28.Fisher CJJR, Agosti JM, Opal SM, et al. Treatment of septic shock with the tumor necrosis factor receptor: Fc fusion protein. The Soluble TNF Receptor Sepsis Study Group. N Engl J Med. 1996;334:1697–702. doi: 10.1056/NEJM199606273342603. [DOI] [PubMed] [Google Scholar]
- 29.Ayala A, Deol ZK, Lehmann DL, Herdon CD, Chaudry IH. Polymicrobial sepsis but not low-dose endotoxin infusion causes decreased splenocyte IL-2/IFN-gamma release while increasing IL-4/IL-10 production. J Surg Res. 1994;56:579–85. doi: 10.1006/jsre.1994.1092. [DOI] [PubMed] [Google Scholar]
- 30.Dhabhar FS, McEwen BS. Acute stress enhances while chronic stress suppresses cell-mediated immunity in vivo: a potential role for leukocyte trafficking. Brain Behav Immun. 1997;11:286–306. doi: 10.1006/brbi.1997.0508. [DOI] [PubMed] [Google Scholar]
- 31.Oberbeck JR, Dahlweid FM, Koch R, et al. Dehydroepiandrosterone decreases mortality rate and improves cellular immune function during polymicrobial sepsis. Crit Care Med. 2001;29:380–4. doi: 10.1097/00003246-200102000-00029. [DOI] [PubMed] [Google Scholar]
- 32.Araneo BA, Shelby J, Li GZ, Ku W, Daynes RA. Administration of dehydroepiandrosterone to burned mice preserves normal immunologic competence. Arch Surg. 1993;128:318–25. doi: 10.1001/archsurg.1993.01420150074014. [DOI] [PubMed] [Google Scholar]
- 33.Rook GA, Hernandez-Pando R, Lightman SL. Hormones, peripherally activated prohormones and regulation of the Th1/Th2 balance. Immunol Today. 1994;15:301–3. doi: 10.1016/0167-5699(94)90075-2. [DOI] [PubMed] [Google Scholar]
- 34.Meikle AW, Dorchuck RW, Araneo BA, et al. The presence of a dehydroepiandrosterone-specific receptor binding complex in murine T cells. J Steroid Biochem Molec Biol. 1992;42:293–304. doi: 10.1016/0960-0760(92)90132-3. [DOI] [PubMed] [Google Scholar]
- 35.Daynes RA, Araneo BA, Dowell TA, Huang K, Dudley D. Regulation of murine lymphokine production in vivo. The lymphoid tissue microenvironment exerts regulatory influences over T helper cell function. J Exp Med. 1990;171:979–96. doi: 10.1084/jem.171.4.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blauer KL, Poth M, Rogers WM, Bernton EW. Dehydroepiandrosterone antagonizes the suppressive effects of dexamethasone on lymphocyte proliferation. Endocrinology. 1991;129:3174–9. doi: 10.1210/endo-129-6-3174. [DOI] [PubMed] [Google Scholar]
- 37.Kondo S, Wang B, Fujisawa H, et al. Effect of gene-targeted mutation in TNF receptor (p55) on contact hypersensitivity and ultraviolet B-induced immunosuppression. J Immunol. 1995;155:3801–5. [PubMed] [Google Scholar]
- 38.Artis D, Humphreys NE, Bancroft AJ, Rothwell NJ, Potten CS, Grencis RK. Tumor necrosis factor alpha is a critical component of interleukin 13-mediated protective T helper cell type 2 responses during helminth infection. J Exp Med. 1999;190:953–62. doi: 10.1084/jem.190.7.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Christou NV. Host-defence mechanisms in surgical patients. a correlative study of the delayed hypersensitivity skin-test response, granulocyte function and sepsis. Can J Surg. 1985;28:39–46. [PubMed] [Google Scholar]
- 40.Casson PR, Andersen RN, Herrod HG, et al. Oral dehydroepiandrosterone in physiologic doses modulates immune function in postmenopausal women. Am J Obstet Gynecol. 1993;169:1536–9. doi: 10.1016/0002-9378(93)90431-h. [DOI] [PubMed] [Google Scholar]
- 41.Ayala A, Chung CS, Xu YX, Evans TA, Redmond KM, Chaudry IH. Increased inducible apoptosis in CD4+ T lymphocytes during polymicrobial sepsis is mediated by Fas ligand and not endotoxin. Immunology. 1999;97:45–55. doi: 10.1046/j.1365-2567.1999.00765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ksontini R, Colagiovanni DB, Josephs MD, et al. Disparate roles for TNF-alpha and Fas ligand in concanavalin A-induced hepatitis. J Immunol. 1998;160:4082–9. [PubMed] [Google Scholar]
- 43.Oberbeck R, Nickel E, van Griensven M, et al. The effect of dehydroepiandrosterone on hemorrhage-induced suppression of cellular immune function. Intensive Care Med. 2002;28:963–8. doi: 10.1007/s00134-002-1292-8. [DOI] [PubMed] [Google Scholar]
- 44.Carson WE, Yu H, Dierksheide J, et al. A fatal cytokine-induced systemic inflammatory response reveals a critical role for NK cells. J Immunol. 1999;162:4943–51. [PubMed] [Google Scholar]
- 45.Unanue ER. Macrophages, NK cells and neutrophils in the cytokine loop of Listeria resistance. Res Immunol. 1996;147:499–505. doi: 10.1016/s0923-2494(97)85214-5. [DOI] [PubMed] [Google Scholar]
- 46.van Vollenhoven RF, Engleman EG, McGuire JL. Dehydroepiandrosterone in systemic lupus erythematosus: results of a double blind, placebo-controlled, randomized clinical trial. Arthritis Rheum. 1995;38:1826–31. doi: 10.1002/art.1780381216. [DOI] [PubMed] [Google Scholar]
- 47.Knöferl MW, Angele MK, Catania RA, Diodato MD, Bland KI, Chaudry IH. Immunomodulatory effects of dehydroepiandrosterone in prostrus female mice after trauma-hemorrhage. J Appl Physiol. 2003;95:529–35. doi: 10.1152/japplphysiol.01201.2002. [DOI] [PubMed] [Google Scholar]