Abstract
There is accumulating evidence that haem oxygenase (HO)-1 plays a protective role in various disorders. The beneficial efficacy of HO-1 induction therapy has been shown in renal diseases such as glomerulonephritis, interstitial nephritis and drug induced nephrotoxicity. However, involvement of HO-1 in the development of autoimmune renal diseases remains uncertain. To assess the clinical efficacy of HO-1 induction therapy for lupus glomerulonephritis, MRL/lpr mice were intraperitoneally injected with 100 µmol/kg hemin, a potent HO-1 inducer, or PBS as controls, once a week from 6 weeks of age to 21–24 weeks-old. We found that treatment with hemin led to a significant reduction of proteinuria and remarkable amelioration of glomerular lesions accompanied by decreased immune depositions. In addition, the circulating IgG anti-double-stranded DNA antibody level was significantly decreased in hemin treated mice when compared with controls. A single intraperitoneal injection with hemin resulted in reduction of inducible nitric oxide synthase expression in the kidney and spleen, and serum interferon-γ level. Our results suggest that HO-1 induction therapy ameliorates lupus nephritis by suppressing nitric oxide (NO) dependent inflammatory responses and attenuating production of pathogenic autoantibodies.
Keywords: haem oxygenase-1 (HO-1), MRL/lpr, inducible nitric oxide synthase (iNOS), interferon-gamma (IFN-γ)
INTRODUCTION
Haem oxygenase (HO) is the rate-limiting enzyme that catalyses haem into carbon monoxide (CO), Fe2+, and biliverdin. The inducible form of HO-1, 32 kD heat shock protein, is expressed in response to various stimuli such as hydrogen peroxide, heat, heavy metal ions, hyperoxia, hypoxia, endotoxin and inflammatory cytokines, whereas HO-2, another isozyme of HO, is constitutively expressed [1]. Recent studies have shown that HO-1 plays a protective role in the development of various diseases including inflammatory diseases. The actions are mediated by haem degradation products and their metabolic derivatives [1–4]. Induction and forced expression of HO-1 suppress synthesis of inflammatory and proinflammatory cytokines such as interleukin (IL)-1, IL-6, IL-8 and tumour necrosis factor (TNF), and stimulate that of anti-inflammatory cytokine, IL-10, in most of pathological conditions, though several exceptions have been documented in some disease models [5]. The modulating effects on cytokine production mainly rely on CO [6]. In addition, CO suppresses expressions of inducible nitric oxide synthase (iNOS) and cyclooxygenase (COX)-2, resulting in reduction of nitric oxide (NO) and prostaglandins (PGs), respectively, both of which are critical chemical mediators of inflammation [5]. Biliverdin, another haem degradation product, is subsequently converted to bilirubin by biliverdin reductase, whereas Fe2+ stimulates ferritin synthesis [7]. Both bilirubin and ferritin function as antioxidants [8]. Thus, multiple biochemical actions of haem degradation products and their metabolic derivatives contribute to the cytoprotective functions of HO.
We have previously reported that adenovirus vector mediated gene transfer of HO-1 cDNA suppressed lipopolysaccharide (LPS)-induced lung injury [9], influenza viral pneumonia [10], bleomycin-induced pulmonary fibrosis [11] and pseudomonas chronic respiratory infection [12] in murine models. Similarly, favourable outcomes of therapies using chemical inducers or gene HO-1 have been shown in various diseases including respiratory diseases, cardiovascular diseases, renal diseases, liver injuries, ocular diseases and organ transplantation of animal models [8,13,14].
In a patient with congenital HO-1 deficiency and HO-1-targeted mice mesangioproliferative glomerulonephritis is one of the most characteristic pathological features [13,15,16]. Accordingly, protective roles of HO-1 have been shown in ischemic renal injury, cisplatin induced nephrotoxicity, acute glomerulonephritis and rejection of renal transplantation [17–21]. Anti-glomerular basement membrane antibody-mediated glomerulonephritis was ameliorated by HO-1 induction therapy, in which iNOS was suggested as a major target [22]. Because NO is also involved in lupus nephritis of MRL/MP-lpr/lpr (MRL/lpr) mice [23], which spontaneously develop a systemic lupus erythematosus (SLE) like autoimmune disease characterized by polyclonal B cell activation associated with synthesis of various autoantibodies including nephritogenic IgG anti-double-stranded DNA (anti-dsDNA) antibody [24], we here examined effects of HO-1 induction therapy for the autoimmune mice.
To study the effects of HO-1 induction on lupus nephritis in MRL/lpr, we monitored renal and immunological parameters in MRL/lpr receiving weekly intraperitoneal administration with hemin as an HO-1 inducer. The results showed that the induction of endogenous HO-1 successfully suppressed pathological injury of glomeruli and inhibited deposition of immune complexes. The therapeutic effects were associated with significant reduction of renal iNOS expression, and circulating levels of serum IgG anti-dsDNA antibody and interferon (IFN)-γ in hemin-treated mice. Thus, the data suggest that HO-1 induction therapy protects autoimmune glomerulonephritis through multiple mechanisms.
MATERIALS AND METHODS
Animal
Female MRL/lpr mice from SLC (Shizuoka, Japan) were intraperitoneally administered with 100 µmol/kg hemin (Sigma-Aldrich, St. Louis, MO, USA) once a week (n = 16) or PBS as controls (n = 16) from age of 6 week to 21–24 week or death. In some experiments, the mice were sacrificed at 24 or 48 h after a single injection with hemin or PBS.
Sera collection and isolation of organs
To examine circulating antibodies, sera were collected from the MRL/lpr mice during weekly treatment with hemin. For assessment of cytokines, sera were collected at 48 h after a single administration with hemin or PBS. Mice were sacrificed by cardiac punctures under anaesthesia with kethamin (Sigma) and xylazine (Sigma), and then the spleen and kidneys were surgically removed.
Cell culture
Spleen cells were suspended in RPMI1640 HEPES modification (Sigma) with 10% FCS (Equitech-Bio, Kerrville, TX, USA), 4·1 mm l-glutamine (Sigma), 100 U/ml penicillin and 0·1 mg/ml streptomycin (Sigma). Then, 1 × 107/ml of the cells were cultured in 12-well plates (Sumitomo, Osaka, Japan) with or without 100 µm hemin at 37°C and 5% CO2.
Western analysis
Cellular proteins were extracted from freshly isolated spleen, kidney and cultured cells by adding lysis buffer containing 137 mm NaCl, 20 mm Tris-HCl, 50 mm NaF, 1 mm EDTA, 1% Triton-X, and protease inhibitor (Sigma). Each lysate was resolved by 4%-20% of gradient polyacrylamide gel (Daiichi Kagaku, Tokyo, Japan) for electrophoresis and transferred onto a polyvinylidene difluoride membrane. After blocking with 5% skim milk, the membrane was probed with rabbit anti-HO-1 polyclonal antibody (StressGen Biotechnologies, Victoria, BC, Canada) for 1 h at room temperature, followed by incubation with horse raddish peroxidase (HRP)-conjugated anti-rabbit Igs (Amersham Biosciences, Piscataway, NJ, USA) for 30 min. To detect iNOS, the membrane was serially incubated with rabbit anti-NOS2 antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 1 h, biotin-labelled goat anti-rabbit IgG antibody (KPL, Gaithrsburg, MD, USA) for 30 min, and HRP-conjugated streptavidin for 30 min (Pierce, Rockford, IL, USA). Blots were developed using ECL chemiluminescent detection system (Amersham Life Sciences, Little Chalfont, UK) and exposed to Kodak Biomax film for 1–5 min (Kodak Imaging Systems, Rochester, NY, USA).
Measurement of urinary protein excretion
Urine was collected from individual mice for 24 h. Protein concentration in the urine was determined by using the Bio-Rad protein assay kit, according to the Bradford method (Biorad, Hercules, CA).
Assessment of kidney pathology
MRL/lpr mice, which received weekly hemin treatment until 21-week-old, were sacrificed to take out the kidneys. One kidney was fixed with 10% formalin, embedded in paraffin, sectioned, and stained with Periodic Acid Schiff (PAS), while the other was snap-frozen for immunofluorescent studies. Two renal immunopathologists independently read and interpreted the slides without prior knowledge of the treatment modality. Sixty glomeruli per mouse were evaluated by the score system as follows; score 0 represents no abnormality, whereas score 1, 2, 3 and 4 represent mild, moderate, moderately severe and severe abnormality with crescent formation and necrosis, respectively, as previously described [25].
For immunofluorescence study, the snap-frozen kidneys were sectioned by a cryostat and fixed in cold acetone for 20 min. After blocking with 10% normal goat serum (Nichirei, Tokyo, Japan) containing PBS for 30 min, the samples were incubated with alkaline phosphatase conjugated anti-mouse IgG (Southern Biotechnology Associates, Birmingham, AL, USA) for 1 h, and then with Alexa Flour 488 conjugated donkey anti-goat IgG (H + l) (Wako, Osaka, Japan) for another 1 h. The sections were subsequently analysed by laser fluorescence microscopy (LSM-GB200, Olympus, Tokyo, Japan).
Glomerular immunodeposits were also evaluated quantitatively by immunohistochemical technique. In brief, the formalin fixed sections were pretreated with proteinase K (Sigma) followed by incubation with alkaline phosphatase conjugated anti-mouse IgG for 1 h. The signals were visualized by HISTOFINE (Nichirei). Glomerular IgG deposits were graded from 0 to 3; 0: none, 1: minor, 2: moderate, and 3: severe deposition [26].
ELISA
Total IgG, M, and A were determined by using individual ELISA kits (Bethyl, Montogomery, TX, USA). ELISA kits for IgG anti-dsDNA antibody and IgG rheumatoid factor (RF) were purchased from Shibayagi, Gunma, Japan. Concentrations of IFN-γ, IL-4, IL-6, IL-10 and TNF were measured with specific ELISA kits, respectively (R & D Systems, Minneapolis, MI, USA).
Flow cytometry
Spleen cells (1 × 106) were incubated for 30 min at 4°C with the following fluorescein isothiocyanate or phycoerythrin conjugated monoclonal anti-mouse antibodies; CD3, CD4, CD8 and CD19 (PharMingen, San Diego, CA, USA). We analysed cells located in the lymphocyte region based on FSC and SSC by FACS Calibur using the Cell Quest program (Becton Dickinson, Mountain View, CA, USA).
Statistical analysis
Comparisons of two independent data sets were made by using Mann–Whitney U and χ2 tests. A P-value < 0·05 was considered statistically significant.
RESULTS
HO-1 induction by intraperitoneal injection with hemin
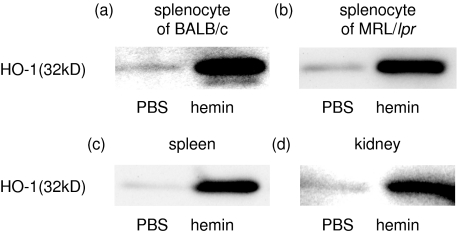
We first examined HO-1 expression in the spleen from MRL/lpr mice by immunoblotting technique using specific anti-HO-1 antibody. Freshly isolated spleen cells from MRL/lpr mice expressed as little HO-1 as those from BALB/c mice, whereas substantial amounts of HO-1 were induced by in vitro treatment for 24 h with 100 µm hemin, a potent HO-1 inducer (Fig. 1a,b). To confirm HO-1 induction by in vivo hemin treatment, we examined HO-1 expression in the spleens and kidneys from mice at 24 h after a single intraperitoneal injection with 100 µmol/kg of hemin or PBS. The dose administered was selected based on the results of our previous study [9], which showed that this dosage successfully led to HO-1 dependent protection against LPS-induced acute lung injury in mice without adverse effects. As expected, a single intraperitoneal administration with hemin induced substantial amounts of HO-1 in the kidneys as well as the spleen (Fig. 1c,d).
Fig. 1.
Hemin-induced HO-1 expression in the spleen and kidney. HO-1 expression is determined by immunoblotting technique. Splenocytes from BALB/c (a) and MRL/lpr (b) were treated in vitro with or without 100 µm hemin for 24 h. The spleens (c) and kidneys (d) were recovered from MRL/lpr mice at 24 h after receiving a single intraperitoneal injection with hemin (100 µmol/kg) or PBS. All samples were obtained from mice at 16 weeks of age. Representative results of more than three individual experiments are shown. Both in vitro and in vivo treatment with hemin led to remarkable enhancement of HO-1 expression in the spleen and kidney.
Effects of weekly treatment with hemin on lupus nephritis
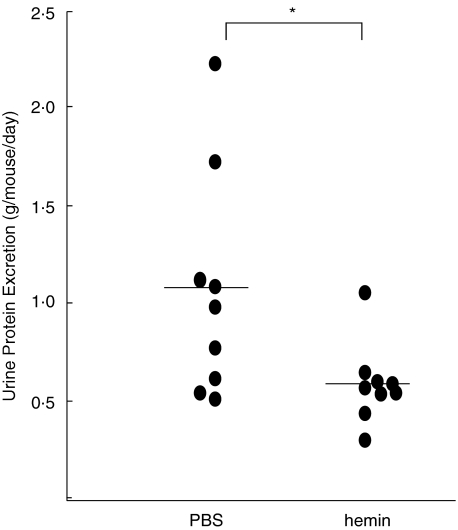
To examine the effects of HO-1 induction on lupus nephritis, MRL/lpr mice were intraperitoneally injected with 100 µmol/kg of hemin or PBS, as a control, once a week from 6 weeks to 24 weeks of age. Urine was collected from individual mice at 21 weeks of age. The urinary protein levels were quantitatively determined. The results showed that weekly hemin treatment significantly reduced proteinuria when compared with controls (P < 0·05, Fig. 2).
Fig. 2.
Suppressive effects of weekly hemin treatment on proteinuria in MRL/lpr mice. Amounts of daily urinary protein excretion were determined in 21 week-old MRL/lpr mice which had received 100 µmol/kg of hemin (n = 9) or PBS (n = 9) weekly from 6 weeks of age. Dots indicate 24 h urinary protein excretion (g/mouse/day) of individual mice. Bars represent means of individual groups. Weekly hemin treatment significantly reduced amounts of urinary protein excretion (*P < 0·05 by Mann–Whitney U-test).
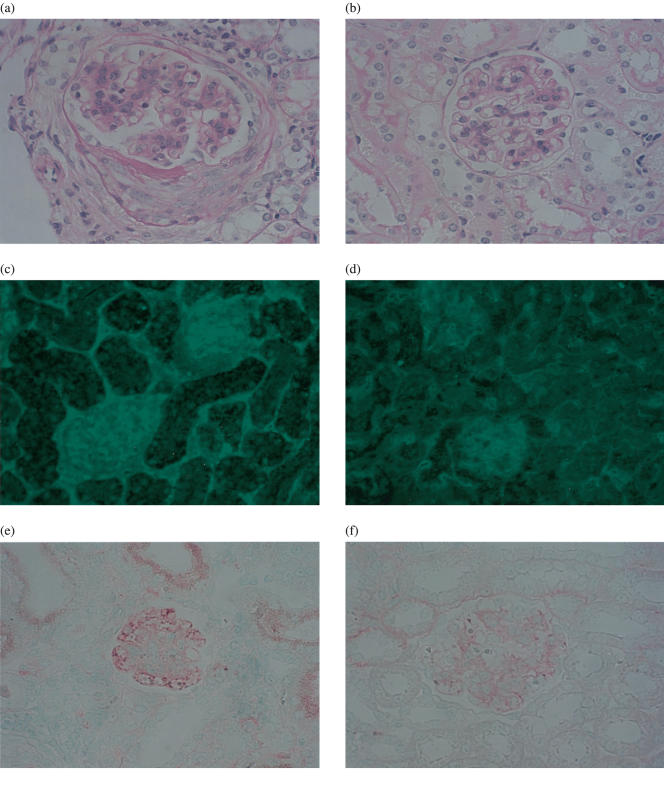
We next assessed histopathological findings in the kidney from 8 hemin treated mice and 9 PBS treated mice sacrificed at 21 week-old. Damage of individual glomeruli was graded from score 0–4. The mean pathological score in individual mice was significantly lower in hemin treated mice than controls (control versus hemin; P < 0·05 by Mann–Whitney U-test, Table 1, Fig. 3a,b). We compared frequency of each score in all glomeruli evaluated between individual groups (Table 1). In the hemin treated mice, most of glomeruli had mild injuries (score 1 and 2), but not intact (score 0), and frequency of severely damaged glomeruli classified into score 3 or score 4 was significantly fewer than controls (control versus hemin, P < 0·001, by χ2 test, Table 1). While 18·3% of glomeruli were judged as score 4 showing advanced lesions such as glomerular hyalinization, crescent formation and necrosis in control mice, no glomerulus was categorized into score 4 in hemin treated mice.
Table 1.
Histopathological glomerular damage score in weekly hemin-treated and control MRL/lpr mice at 21 weeks of age
| Frequency of score (%)ठ| ||||||||
|---|---|---|---|---|---|---|---|---|
| Treatment | No. of mice | No. of glomeruli | Mean score†* | 0 | 1 | 2 | 3 | 4 |
| PBS | 9 | 520 | 2·3 ± 0·3 | 0 | 27·1 | 36·0 | 18·6 | 18·3 |
| Hemin | 8 | 480 | 1·5 ± 0·1 | 0 | 57·9 | 38·8 | 3·3 | 0 |
Sixty glomeruli per mouse were studied, except for one PBS treated mouse in which 40 glomeruli were available. Damage of individual glomeruli was evaluated by the score system grading from score 0–4.
The pathological score was determined for every mouse by calculating the average of the glomerular scores examined. Mean score represents average ± SD of the pathological score in individual groups. The pathological score was significantly lower in hemin treated mice than controls
P < 0·05 by Mann–Whitney U-test.
Frequency of each score was calculated in all glomeruli studied from individual groups. Glomerular damage was significantly milder in hemin treated mice than controls
P < 0·001, by χ2 test.
Fig. 3.
Amelioration of renal histological and immunopathological findings by hemin treatment in MRL/lpr mice. Representative PAS staining renal sections from (a) control mice (n = 9) and (b) hemin treated mice (n = 8) are shown. Histopathological glomerular damage of (a) was judged as score 4, whereas that of (b) was score 1. Representative immunofluorescent sections using anti-mouse IgG antibodies are shown in control (c) and hemin treated mouse (d). Glomerular immunodepositions were massive in a control mouse (c), but scant in hemin treated mouse (d). Representative immunohistochemical sections are shown in control (e) and hemin treated mouse (f). Glomerular IgG deposition of (e) was judged as score 3, whereas that of (f) was score 1. Original magnifications of (a–f) are × 400.
Furthermore, immunofluorescent studies using anti-IgG antibodies demonstrated that glomerular immunodeposits were remarkably reduced by weekly hemin treatment (Fig. 3c,d). Glomerular immunodeposits were quantitatively evaluated by the immunohistochemical technique to minimize differences among every experiment (Fig. 3e,f). We found that glomerular immune deposition score was significantly lower in hemin treatment mice than controls (hemin 0·84 ± 0·30, control 1·70 ± 0·20, P < 0·05 by Mann–Whitney U-test). The results indicated that weekly hemin treatment suppressed immune complex mediated glomerulonephritis in MRL/lpr mice, leading to clinical ameliorations.
Hemin treatment suppresses iNOS expression in the kidney
NO has been shown to play a critical role in the development of lupus nephritis [23]. Because iNOS is a possible therapeutic target in HO-1 induction therapy, we here examined its expression in the spleen and kidney from MRL/lpr mice (21 week-old) at 48 h after a single intraperitoneal injection with 100 µmol/kg of hemin. Immunoblotting analysis revealed that iNOS expressions in both the organs were remarkably decreased by hemin treatment (Fig. 4). These data are consistent with the hypothesis that reduction of iNOS expression was partly involved in favourable outcomes of HO-1 induction therapy for MRL/lpr mice.
Fig. 4.
Reduction of iNOS expression in the spleen and kidneys by hemin. Hemin (100 µmol/kg) or PBS was intraperitoneally given to 21-week-old MRL/lpr mice. 48 h later, iNOS expression in the spleen and kidneys were examined by immunoblotting technique. Representative results of more than three individual experiments are shown. Hemin treatment led to substantial reduction of iNOS expressions in the both organs.
Effects of hemin treatment on immunological parameters
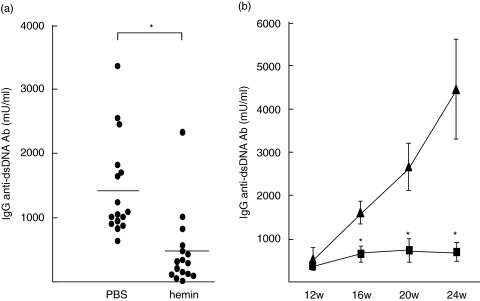
Significant reduction of glomerular immunodeposits in the hemin treated mice raised the possibility that HO-1 induction suppressed synthesis of nephritogenic antibody, IgG anti-dsDNA antibody. We compared serum levels of specific and nonspecific antibodies between MRL/lpr mice receiving weekly hemin and those receiving PBS by using ELISA systems. The results demonstrated that titres of IgG anti-dsDNA antibody were significantly lower in 16 week-old mice receiving weekly hemin than in controls (Fig. 5a,b). While titres of the pathogenic antibodies increased with age in control mice, those in hemin treated mice remained as low as those in young mice (Fig. 5b). Nevertheless, serum IgG RF levels were not different between the two groups at 16 weeks of age (control group 54·4 ± 24·3 U/ml, hemin group 27·0 ± 7·8 U/ml, control versus hemin; NS), or at 20 weeks of age (control group 78·7 ± 32·9 U/ml, hemin group 52·3 ± 25·6 U/ml, control versus hemin; NS). In addition, serum levels of IgG, IgM and IgA were comparable between both the groups at any time (data not shown). Significant differences were not found in serum levels of IgG subclasses either (data not shown). These findings suggested that HO-1 induction selectively inhibited IgG anti-dsDNA antibody synthesis, resulting in reduction of glomerular immunodeposits and subsequent glomerular injuries.
Fig. 5.
Suppressive effects of hemin treatment on autoantibody synthesis in MRL/lpr mice. Serum IgG anti-dsDNA antibody levels in MRL/lpr mice treated with hemin (100 µmol/kg) or PBS every week from 6-week-old were determined by ELISA. (a) Serum IgG anti-dsDNA antibody levels at 16 weeks of age were significantly lower in MRL/lpr mice treated with hemin (n = 16) than those with PBS (n = 16) (*P < 0·05 by Mann–Whitney U-test). (b) A significant reduction was also observed at 20 and 24 weeks of age (▴) PBS (n = 11); (▪) hemin (n = 11), *P < 0·05 by Mann–Whitney U-test). The data shown are the mean ± SEM.
We also investigated effects of HO-1 induction on other immunological parameters in MRL/lpr. Abnormal accumulation of double negative T cells, so-called lpr cells, is characteristic of MRL/lpr mice and elimination of the population leads to clinical remission [27]. To examine whether HO-1 induction affects lymphocyte populations in MRL/lpr mice, we determined frequency of B cells and lpr cells in the spleen from the mice treated weekly with hemin or PBS at 24 weeks of age by using flowcytometric analysis. Frequencies of the lymphocyte subsets examined including lpr cells, did not differ between the two groups (Table 2).
Table 2.
Effects of weekly hemin treatment on lymphocyte subsets in the spleen
| Treatment | CD3+ CD4-CD8- | CD19+ |
|---|---|---|
| PBS (n = 7) | 48·6 ± 3·8 | 7·7 ± 2·6 |
| Hemin (n = 7) | 47·9 ± 3·9 | 10·7 ± 2·1 |
Frequency of B cells and lpr cells in the spleen lymphocytes from mice treated weekly with hemin or PBS at 24-week-old was determined by the flowcytometric analysis using anti-CD3, CD4, CD8 and CD19 mAbs. Results are expressed as mean ± SEM.
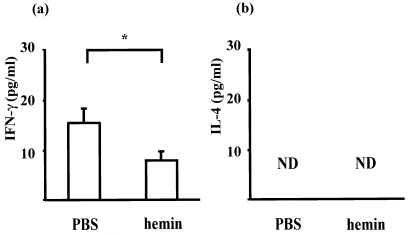
Dysregulation of cytokine profiles is another feature of MRL/lpr mice [28–30]. Relative predominance of Th1 to Th2 is associated with autoimmune diseases in MRL/lpr mice [29,31]. To assess the effects of hemin administration on Th1/Th2 balance in MRL/lpr mice, serum levels of IL-4 and IFN-γ were measured by ELISA at 48 h after hemin (100 µmol/kg) or PBS was intraperitoneally given to 21-week-old mice. We found that hemin treatment reduced serum concentration of IFN-γ and that IL-4 was undetectable in all samples studied (Fig. 6). We also measured serum IL-6, IL-10, and TNF, all of which have been reported to be involved in manifestations of MRL/lpr and can be modulated by HO-1 induction [5,6,32,33]. However, there were no differences in these cytokines between the two groups. These data suggest that a relative Th1 shift is partly corrected by chemical induction of HO-1 in MRL/lpr mice. The immunomodulatory effects of HO-1 induction may partly contribute to reduction of the nephritogenic antibody and glomerular injuries in MRL/lpr mice.
Fig. 6.
Modulation of hemin treatment on cytokine profiles in MRL/lpr mice. Concentrations of IFN-γ and IL-4 were determined by ELISA of sera from MRL/lpr mice (21-week-old) at 48 h after a single intraperitoneal injection with 100 µmol/kg hemin (n = 7) or PBS (n = 7). Results are expressed as mean ± SEM (*P < 0·05 by Mann–Whitney U-test). The serum levels of IFN-γ were significantly decreased by hemin treatment (a), whereas no difference was found in IL-4 (b).
DISCUSSION
This study is the first to demonstrate that chemical induction of HO-1 results in amelioration of lupus nephritis in MRL/lpr mice and is associated with suppression of iNOS expression in the kidney and reduction of circulating levels of IgG anti-dsDNA antibody and IFN-γ. These data suggest that not only anti-inflammatory but also immunomodulatory effects of HO-1 induction prevent the development of autoimmune nephritis.
NO generation system is considered as one of the major targets of HO-1 induction therapy, especially for glomerular diseases, because excessive NO output is involved in glomerular injuries [22]. This is the case for lupus nephritis in MRL/lpr mice. Beneficial effects of NOS inhibitors have been demonstrated [23]. Both L-NMMA, a nonspecific NOS inhibitor, and L-NIL, a specific iNOS inhibitor, ameliorated the renal diseases without affecting deposition of immune complexes on glomeruli. Rather, NOS inhibitors suppressed NO dependent inflammatory reactions subsequent to immunodeposits as shown in kidney, skin and lung diseases of animal models [23,34,35]. For example, NOS inhibitors remarkably suppressed macrophage accumulation and activation at the sites of immune deposits [34,35].
In this study, unlike NOS inhibitors, hemin treatment significantly reduced not only serum IgG anti-dsDNA antibody level but also the glomerular immunodeposits in MRL/lpr mice. Reilly et al. [23] reported that L-NMMA, a nonspecific NOS inhibitor, showed moderately suppressive effects on serum level of anti-dsDNA antibody, but not on antiglomerular basement membrane antibody, whereas L-NIL, a specific iNOS inhibitor, did not affect levels of either autoantibodies. Because CO selectively suppresses iNOS [7], it is likely that alternative mechanisms operate in the reduction of anti-dsDNA antibody synthesis in hemin treated mice. Therefore, we investigated additional immunological effects of HO-1 induction.
Besides polyclonal B cell activation with synthesis of various autoantibodies, relative Th1 cytokine predominance is one of the characteristic features in MRL/lpr mice [31]. Serum IFN-γ level and its mRNA expression in the spleen and other organs are increased in MRL/lpr mice [29,36]. IFN-γ or IFN-γ receptor deficient MRL/lpr mice develop much less serious disease manifestations [37]. All of these findings indicate that IFN-γ plays a critical role in this disease. In the present study, treatment with hemin led to a significant decrease in serum IFN-γ concentrations of MRL/lpr mice, suggesting that suppressive effects on IFN-γ production are partly involved in the reduction of IgG anti-dsDNA antibody level and directly contribute to improvement of renal lesions in HO-1 induction therapy. Although the relationship between HO-1 expression and IFN-γ synthesis has been controversial, up-regulation of HO-1 led to reduction of IFN-γ synthesis in some experimental systems [38–41]. Treatment with cobalt protoporphyrin, an HO-1 inducer, decreases production of IL-10, IFN-γ and TNF in mouse allogeneic mixed lymphocyte reaction [38]. The blockade of selectin-P-selectin glycoprotein ligand-1 (PSGl−1) interactions prolongs survival of cardiac allografts by suppressing Th1 type cytokines associated with increased HO-1 expression [39]. In addition, HO-1 inducers modulate intracellular signal transduction through IFN-γ receptors [40]. Biliverdin, which is converted into bilirubin, one of the haem degradation products, also interferes with T cell signalling [41]. The molecular targets are nuclear factor of activated T cells (NFAT) and nuclear factor κB (NF-κB), both of which are involved in transcription of Th1 cytokines such as IL-2 and IFN-γ[41]. These findings indicate that HO-1 induction therapy counteracts functions of IFN-γ by inhibiting the synthesis as well as the postreceptor intracellular signalling.
Possible implications of superoxides in SLE have been demonstrated [42–44]. Rokutan et al. [42] showed increased oxygen intermediates in the organs from MRL/lpr mice, whereas reduced serum levels of antioxidants and radical scavengers have been reported in human SLE [43]. Treatment with antioxidants, such as vitamin E, suppressed circulating anti-dsDNA antibody levels and the development of renal disease [44,45]. Therefore, it is plausible that antioxidant effects are implicated in therapeutic outcomes of HO-1 induction for MRL/lpr mice, because haem degradation products eventually lead to synthesis of biliverdin and ferritin as potent antioxidants [8]. Besides our findings in this study, more complex immunomodulatory and anti-inflammatory effects of HO-1 induction may contribute to amelioration of renal disease of MRL/lpr.
Collectively, our data demonstrate that HO-1 induction therapy ameliorates lupus nephritis, possibly through multiple mechanisms, including suppression of NO synthesis, inhibition of antibody synthesis and modulation of cytokine production. Thus, pharmacological induction of HO-1 as well as genetic overexpression of HO-1 may be novel therapeutic strategies for lupus nephritis and other autoimmune diseases.
Acknowledgments
This work was partly supported by grants from the Yokohama City University Center of Excellence Program of the Ministry of Education, Culture, Sports, Science and Technology of Japan (to Y. Ishigatsubo), and 2004 grant in aid for scientific research project No. 16590991 from the Ministry of Education, Culture, Sports, and Technology of Japan (to M. Takeno).
REFERENCES
- 1.Maines MD. The heme oxygenase system: a regulator of second messenger gases. Annu Rev Pharmacol Toxicol. 1997;37:517–54. doi: 10.1146/annurev.pharmtox.37.1.517. [DOI] [PubMed] [Google Scholar]
- 2.Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN. Bilirubin is an antioxidant of possible physiological importance. Science. 1987;235:1043–6. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- 3.Choi AM, Alam J. Heme oxygenase-1: function, regulation, and implication of a novel stress-inducible protein in oxidant-induced lung injury. Am J Respir Cell Mol Biol. 1996;15:9–19. doi: 10.1165/ajrcmb.15.1.8679227. [DOI] [PubMed] [Google Scholar]
- 4.Oberle S, Schwartz P, Abate A, Schroder H. The antioxidant defense protein ferritin is a novel and specific target for pentaerithrityl tetranitrate in endothelial cells. Biochem Biophys Res Commun. 1999;261:28–34. doi: 10.1006/bbrc.1999.0941. [DOI] [PubMed] [Google Scholar]
- 5.Nakao A, Moore BA, Murase N, et al. Immunomodulatory effects of inhaled carbon monoxide on rat syngeneic small bowel graft motility. Gut. 2003;52:1278–85. doi: 10.1136/gut.52.9.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Otterbein LE, Bach FH, Alam J, et al. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med. 2000;6:422–8. doi: 10.1038/74680. [DOI] [PubMed] [Google Scholar]
- 7.Otterbein LE, Soares MP, Yamashita K, Bach FH. Heme oxygenase-1. unleashing the protective properties of heme. Trends Immunol. 2003;24:449–55. doi: 10.1016/s1471-4906(03)00181-9. [DOI] [PubMed] [Google Scholar]
- 8.Morse D, Choi AM. Heme oxygenase-1: The ‘emerging molecule’ has arrived. Am J Respir Cell Mol Biol. 2002;27:8–16. doi: 10.1165/ajrcmb.27.1.4862. [DOI] [PubMed] [Google Scholar]
- 9.Inoue S, Suzuki M, Nagashima Y, et al. Transfer of heme oxygenase 1 cDNA by a replication-deficient adenovirus enhances interleukin 10 production from alveolar macrophages that attenuates lipopolysaccharide-induced acute lung injury in mice. Hum Gene Ther. 2001;12:967–79. doi: 10.1089/104303401750195926. [DOI] [PubMed] [Google Scholar]
- 10.Hashiba T, Suzuki M, Nagashima Y, Suzuki S, Inoue S, Matsuse T, Ishigatubo Y. Adenovirus-mediated transfer of heme oxygenase-1 cDNA attenuates severe lung injury induced by the influenza virus in mice. Gene Ther. 2001;8:1499–507. doi: 10.1038/sj.gt.3301540. [DOI] [PubMed] [Google Scholar]
- 11.Tsuburai T, Suzuki M, Nagashima Y, et al. Adenovirus-mediated transfer and overexpression of heme oxygenase 1 cDNA in lung prevents bleomycin-induced pulmonary fibrosis via a Fas-Fas ligand-independent pathway. Hum Gene Ther. 2002;13:1945–60. doi: 10.1089/10430340260355356. [DOI] [PubMed] [Google Scholar]
- 12.Tsuburai T, Kaneko T, Nagashima Y, Ueda A, Tagawa A, Shinohara T, Ishigatsubo Y. Pseudomonas aeruginosa-induced neutrophilic lung inflammation is attenuated by adenovirus-mediated transfer of the heme oxygenase 1 cDNA in mice. Hum Gene Ther. 2004;15:273–85. doi: 10.1089/104303404322886129. [DOI] [PubMed] [Google Scholar]
- 13.Ohta K, Yachie A, Fujimoto K, et al. Tubular injury as a cardinal pathologic feature in human heme oxygenase-1 deficiency. Am J Kid Dis. 2000;35:863–70. doi: 10.1016/s0272-6386(00)70256-3. [DOI] [PubMed] [Google Scholar]
- 14.Nath KA, Balla G, Vercellotti GM, Balla J, Jacob HS, Levitt MD, Rosenberg M. Induciton of heme oxygenase is a rapid, protective response in rhabdomyolysis in the rat. J Clin Invest. 1992;90:267–70. doi: 10.1172/JCI115847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poss KD, Tonegawa S. Reduced stress defense in heme oxygenase 1-deficient cells. Proc Natl Acad Sci USA. 1997;94:10925–30. doi: 10.1073/pnas.94.20.10925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yachie A, Niida Y, Wada T, et al. Oxidative stress causes enhanced endothelial cell injury in human heme oxygenase-1 deficiency. J Clin Invest. 1999;103:129–35. doi: 10.1172/JCI4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shimizu H, Takahashi T, Suzuki T, et al. Protective effect of heme oxygenase induction in ischemic acute renal failure. Crit Care Med. 2000;28:809–17. doi: 10.1097/00003246-200003000-00033. [DOI] [PubMed] [Google Scholar]
- 18.Shiraishi F, Curtis LM, Truong L, Poss K, Visner GA, Madsen K, Nick HS, Agarwal A. Heme oxygenase-1 gene ablation or expression modulates cisplatin-induced renal tubular apoptosis. Am J Physiol Renal Physiol. 2000;278:F726–36. doi: 10.1152/ajprenal.2000.278.5.F726. [DOI] [PubMed] [Google Scholar]
- 19.Datta PK, Koukouritaki SB, Hopp KA, Lianos EA. Heme oxygenase-1 induction attenuates inducible nitric oxide synthase expression and proteinuria in glomerulonephritis. J Am Soc Nephrol. 1999;10:2540–50. doi: 10.1681/ASN.V10122540. [DOI] [PubMed] [Google Scholar]
- 20.Mosley K, Wembridge DE, Cattell V, Cook HT. Heme oxygenase is induced in nephrotoxic nephritis and hemin, a stimulator of heme oxygenase synthesis, ameliorates disease. Kidney Int. 1998;53:672–8. doi: 10.1046/j.1523-1755.1998.00798.x. [DOI] [PubMed] [Google Scholar]
- 21.Magee CC, Azuma H, Knoflach A, Denton MD, Chandraker A, Iyer S, Buelow R, Sayegh M. In vitro and in vivo immunomodulatory effects of RDP1258, a novel synthetic peptide. J Am Soc Nephrol. 1999;10:1997–2005. doi: 10.1681/ASN.V1091997. [DOI] [PubMed] [Google Scholar]
- 22.Datta PK, Gross EJ, Lianos EA. Interactions between inducible nitric oxide synthase and heme oxygenase-1 in glomerulonephritis. Kidney Int. 2002;61:847–50. doi: 10.1046/j.1523-1755.2002.00231.x. [DOI] [PubMed] [Google Scholar]
- 23.Reilly CM, Farrelly LW, Viti D, et al. Modulation of renal disease in MRL/lpr mice by pharmacologic inhibition of inducible nitric oxide synthase. Kidney Int. 2002;61:839–46. doi: 10.1046/j.1523-1755.2002.00230.x. [DOI] [PubMed] [Google Scholar]
- 24.Theofilopoulos AN, Dixon FJ. Murine models of systemic lupus erythematosus. Adv Immunol. 1985;37:269–390. doi: 10.1016/s0065-2776(08)60342-9. [DOI] [PubMed] [Google Scholar]
- 25.Watson ML, Rao JK, Gilkeson GS, et al. Genetic analysis of MRL-lpr mice: relationship of the Fas apoptosis gene to disease manifestations and renal disease-modifying loci. J Exp Med. 1992;176:1645–56. doi: 10.1084/jem.176.6.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Watanabe H, Garnier G, Circolo A, et al. Modulation of renal disease in MRL/lpr mice genetically deficient in the alternative complement pathway factor B. J Immunol. 2000;164:786–94. doi: 10.4049/jimmunol.164.2.786. [DOI] [PubMed] [Google Scholar]
- 27.Morse HC, III, Davidson WF, Yetter RA, Murphy ED, Roths JB, Coffman RL. Abnormalities induced by the mutant gene lpr: expansion of a unique lymphocyte subset. J Immunol. 1982;129:2612–5. [PubMed] [Google Scholar]
- 28.Kiberd BA. Interleukin-6 receptor blockage ameliorates murine lupus nephritis. J Am Soc Nephrol. 1993;4:58–61. doi: 10.1681/ASN.V4158. [DOI] [PubMed] [Google Scholar]
- 29.Peng SL, Moslehi J, Craft J. Roles of interferon-γ and interleukin-4 in murine lupus. J Clin Invest. 1997;99:1936–46. doi: 10.1172/JCI119361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwarting A, Tesch G, Kinoshita K, Maron R, Weiner HL, Kelley VR. IL-12 drives IFN-γ-dependent autoimmune kidney disease in MRL-Fas lpr mice. J Immunol. 1999;163:6884–91. [PubMed] [Google Scholar]
- 31.Takahashi S, Fossati L, Iwamoto M, Merino R, Motta R, Kobayakawa T, Izui S. Imbalance towards Th1 predominance is associated with acceleration of lupus–like autoimmune syndrome in MRL mice. J Clin Invest. 1996;97:1597–604. doi: 10.1172/JCI118584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lemay S, Mao C, Singh AK. Cytokine gene expression in the MRL/lpr model of lupus nephritis. Kidney Int. 1996;50:85–93. doi: 10.1038/ki.1996.290. [DOI] [PubMed] [Google Scholar]
- 33.Yamamoto K, Loskutoff DJ. Expression of transforming growth factor-beta and tumor necrosis factor-alpha in the plasma and tissues of mice with lupus nephritis. Laboratory Invest. 2000;80:1561–70. doi: 10.1038/labinvest.3780166. [DOI] [PubMed] [Google Scholar]
- 34.Mulligan MS, Warren JS, Smith CW, Anderson DC, Yeh CG, Rudolph AR, Ward PA. Lung injury after deposition of IgA immune complexes: Requirements for CD18 and 1-arginine. J Immunol. 1992;148:3086–92. [PubMed] [Google Scholar]
- 35.Mulligan MS, Hevel JM, Marletta MA, Ward PA. Tissue injury caused by deposition of immune complexes is 1-arginine dependent. Proc Natl Acad Sci USA. 1991;88:6338–42. doi: 10.1073/pnas.88.14.6338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haas C, Ryffel B, Le Hir M. IFN-γ is essential for the development of autoimmune glomerulonephritis in MRL/lpr mice. J Immunol. 1997;158:5484–91. [PubMed] [Google Scholar]
- 37.Balomenos D, Rumold R, Theofilopoulos AN. Interferon-γ is required for lupus-like disease and lymphoaccumulation in MRL-lpr mice. J Clin Invest. 1998;101:364–71. doi: 10.1172/JCI750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Woo J, Iyer S, Cornejo MC, Mori N, Gao L, Sipos I, Maines M, Buelow R. Stress protein-induced immunosuppression: inhibition of cellular immune effector functions following overexpression of haem oxygenase (HSP 32) Transpl Immunol. 1998;6:84–93. doi: 10.1016/s0966-3274(98)80022-1. [DOI] [PubMed] [Google Scholar]
- 39.Coito AJ, Shaw GD, Li J, Ke B, Ma J, Busuttil RW, Kupiec-Weglinski JW. Selectin–mediated interactions regulate cytokine networks and macrophage heme oxygenase-1 induction in cardiac allograft recipients. Laboratory Invest. 2002;82:61–70. doi: 10.1038/labinvest.3780395. [DOI] [PubMed] [Google Scholar]
- 40.Weiss G, Lutton JD, Fuchs D, et al. Comparative effects of heme and metalloporphyrins on interferon-γ-mediated pathways in monocytic cells (THP-1) Proc Soc Exp Biol Medical. 1993;202:470–5. doi: 10.3181/00379727-202-43561. [DOI] [PubMed] [Google Scholar]
- 41.Yamashita K, McDaid J, Ollinger R, et al. Biliverdin, a natural product of heme catabolism, induces tolerance to cardiac allografts. FASEB J. 2004;00:000–000. doi: 10.1096/fj.03-0839fje. [February 20 Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 42.Rokutan K, Hosokawa T, Nakamura K, Koyama K, Aoike A, Kawai K. Increased superoxide anion production and glutathione peroxidase activity in peritoneal macrophages from autoimmune-prone MRL/Mp-lpr/lpr mice. Int Arch Allergy Appl Immunol. 1988;87:113–9. doi: 10.1159/000234660. [DOI] [PubMed] [Google Scholar]
- 43.Blount S, Griffiths HR, Lunec J. Reactive oxygen species damage to DNA and its role in systemic lupus erythematosus. Mol Aspects Med. 1991;12:93–105. doi: 10.1016/0098-2997(91)90005-7. [DOI] [PubMed] [Google Scholar]
- 44.Weimann BJ, Weiser H. Effects of antioxidant vitamins C, E, and beta-carotene on immune functions in MRL/lpr mice and rats. Ann NY Acad Sci. 1992;669:390–2. doi: 10.1111/j.1749-6632.1992.tb17132.x. [DOI] [PubMed] [Google Scholar]
- 45.Weimann BJ, Hermann D. Inhibition of autoimmune deterioration in MRL/lpr mice by vitamin E. Int J Vitam Nutr Res. 1999;69:255–61. doi: 10.1024/0300-9831.69.4.255. [DOI] [PubMed] [Google Scholar]