Abstract
Despite advances in our understanding of tumour immunology there is no therapy of proven survival benefit for advanced melanoma. Nevertheless, disease progression is slow in a small proportion of patients with metastatic melanoma, suggesting a contribution to outcome from host factors. Recent data have indicated the importance of the heat shock protein receptor CD91 in immune responses to, and progression of, infectious disease. Here we investigate the relationship between CD91 expression and outcome in malignancy. Rare melanoma patients were recruited with advanced disease that was progressing unusually slowly. CD91 expression on their monocytes was compared with control patients with more typical rapidly advancing metastatic disease. Th1 and Th2 cytokines, as well as innate and adaptive immune subsets, were also measured in the two groups. A significant increase in median CD91 expression levels was observed in slow progressors (P = 0·006). There were no differences in other immune subset markers or inflammatory cytokines. The ability of CD91 to internalize and cross-present tumour antigens through the major histocompatibility complex class I pathway may maintain CD8-positive cytotoxic T cell responses and contribute to slow progression of advanced melanoma.
Keywords: CD91, skin cancer/melanoma
INTRODUCTION
Advances in tumour immunology have led to an improved understanding of the immune responses to malignancy [1]. No interindividual differences in host immunity have been identified so far that might account for the observed variation in host melanocyte-specific immunity. In an earlier study, the large cell surface dimer CD91 was found to be up-regulated on monocytes of human immunodeficiency virus (HIV) type-1 infected individuals that do not develop AIDS (termed long-term non-progessors, LTNP), while other immune markers and cytokine levels remain generally unchanged [2]. Furthermore we have shown recently that functionally impaired dendritic cells from immunosuppressed patients with cancer can respond successfully to tumour antigens via CD91 [3].
The high efficiency stimulation of cytotoxic T cells has been attributed in many cases to direct binding to, and internalization of, heat shock protein (HSP)–peptide complexes via the CD91 molecule (also called α2-macroglobulin receptor, or the low-density lipoprotein-related protein). CD91 has been identified as a common receptor for the HSPs hsp70, hsp90, gp96, hsp110 and calreticulin [4,5]. Alternatively, CD91 function unrelated to HSPs may be important; CD91 has been identified as the receptor for α-defensins [6], molecules known to be secreted from activated T cells of LTNP [7]. Such CD91-mediated pathways may provide a mechanism for the maintenance of cytotoxic T cell responses in LTNP [8,9].
There is currently no therapy of proven survival benefit in metastatic malignant melanoma [10], and yet a small proportion of patients with AJCC stage IV disease live very significantly longer than the median survival of 12 months. In order to investigate possible mechanisms for this variable outcome we applied ‘lessons learnt from HIV’[11] to patients with metastatic melanoma by measuring innate and adaptive immune subsets, Th1 and Th2 cytokines and levels of CD91 on monocytes.
PATIENTS AND METHODS
Patients and samples
Consenting individuals with non-progressive metastatic melanoma (n = 8) were recruited and defined as slow progressors based on survival for (i) 6 years or more following diagnosis of cutaneous metastases; (ii) 6 years or more following diagnosis of lymph node involvement that was unresectable or beyond the regional nodes; or (iii) 2 years or more following diagnosis of visceral metastases (lung, liver or bowel). The next eight consecutive patients with cutaneous metastases or unresectable primary melanoma, but who did not meet the criteria for slow progression, were recruited as controls. All individuals were enrolled between May and October 2003. The study received appropriate ethical approval and all volunteers were from St George's Hospital NHS Trust, London.
Each patient provided 20 ml of blood which was collected into heparinized vacutainers and processed within 2 h of drawing. Plasma was collected and peripheral blood mononuclear cells (PBMCs) were isolated on a Ficoll-Histopaque® gradient (Sigma, Poole, UK).
Flow cytometry
Total lymphocyte and subset analysis was performed using whole blood stained with murine antihuman monoclonal antibodies (TetraOne, Beckman Coulter, High Wycombe, UK) to CD3 (total lymphocyte), CD4 (T helper subset), CD8 (cytotoxic T subset), CD16/56 (natural killer) and CD19 (B cell) and were evaluated on an Epics XL-MCL (Beckman Coulter) flow cytometer.
CD91 receptor levels were performed on 5 × 105 washed PBMCs stored previously in liquid nitrogen. These were labelled for 30 min at 4°C with fluorescein isothiocyanate (FITC)-conjugated anti-α2-macroglobulin α-chain (BioMac, Leipzig, Germany) and phycoerythrin (PE)-conjugated anti-CD14 (Pharmingen, Oxford, UK). A minimum of 100 000 cells were acquired in the live gate and analysed on a Becton Dickinson FacsCaliber using CellQuest® software. Positive staining for each marker was determined by comparison to appropriate isotype-matched controls and identical settings were used on each sample.
Cytokines
Plasma samples were stored at −80°C and analysed together on single plates for each cytokine to reduce variability in results, using quantitative sandwich immunoassay kits. Enzyme-linked immunosorbent assays (ELISAs) were performed for tumour necrosis factor (TNF)-α (Roche, Mannheim, Germany), interferon (IFN)-γ, monocyte chemotactic protein-1 (MCP-1) and interleukin (IL)-2, IL-4, IL-6 and IL-12 (Diaclone, Besançon, France).
Between-group comparisons were made using Mann–Whitney U-tests on the vassarstats program.
RESULTS
Patients and treatment
Tables 1 and 2 demonstrate the characteristics of patients in both the slow progressor and control groups. All patients were from the same treatment centre and so had access to the same standard of medical intervention, surgery and supportive care during the course of their illness. Of note, at enrolment five of eight slow progressors, but none of the controls, had survived for 2 years or more following a diagnosis of visceral metastases (Table 2).
Table 1.
Clinical characteristics of patients: comparison of slow progressors with controls
| Slow progressors | Controls | |
|---|---|---|
| n | 8 | 8 |
| Median age (range) | 52 (30–69) | 72 (36–91) |
| Median survival (range) since diagnosis of primary melanoma, in months | 102 (52–156) | 37 (16–228) |
| Median survival (range) since diagnosis of first metastatic disease, in months | 72 (24–120) | 15 (0–48) |
Table 2.
Clinical characteristics of patients: survival and sites of metastatic disease
| Survival (months) since diagnosis of: | ||||
|---|---|---|---|---|
| Patient | Primary melanoma | Cutaneous recurrence or unresectable regional lymph node metastasis | Visceral or distant nodal metastasis | |
| Slow progressors | 1 | 79 | 71 | |
| 2 | 52 | 24 | ||
| 3 | 156 | 81 | 47 | |
| 4 | 156 | 120 | 94 | |
| 5 | 82 | 58 | 47 | |
| 6 | 98 | 74 | ||
| 7 | 107 | 107 | ||
| 8 | 126 | 67 | 63 | |
| Controls | 9 | 41 | 41 | |
| 10 | 228 | 48 | ||
| 11 | 34 | 4 | ||
| 12 | 22 | 5 | ||
| 13 | 16 | 12 | ||
| 14 | 17 | 17 | ||
| 15 | 46 | 18 | ||
| 16* | 40 | |||
This elderly patient was receiving medical therapy for an unresected lentigo maligna melanoma of the face.
Cytokines
Results were confounded by heterogeneity in cytokine levels (Table 3), a situation observed previously in other studies [12]. There were no significant differences between the slow progressors and controls in levels of TNF-α, IFN-γ, MCP-1, IL-2, IL-4, IL-6 or IL-12. One slow progressor (patient 2) had levels at the upper limit of detection for IL-6 and MCP-1 (IL-6 measured 2000 pg/ml, MCP-1 was 20 000 pg/ml), while IL-4 levels were measurable in two of eight controls but were undetectable (<1 pg/ml) in plasma from all slow progressors.
Table 3.
Plasma cytokine values (pg/ml) in slow progressors and controls
| Cytokine measured | Slow progressors | Controls | P-value (Mann–Whitney) |
|---|---|---|---|
| TNF-α | 93·15 ± 106 | 25 ± 89 | 0·23 |
| IFN-γ | <3·125 | <3·125 | 1 |
| MCP-1 | 481·2 ± 6868 | 467 ± 162 | 0·79 |
| IL-2 | <31·25 | <31·25 | 1 |
| IL-4 | <1 | 1 ± 1·46 | 0·40 |
| IL-6 | 39·1 ± 691 | 6·25 ± 463 | 0·34 |
| IL-12 | <6·25 | <6·25 | 1 |
Surface staining
There were no statistically significant differences in innate and adaptive absolute numbers or percentages of lymphocyte subsets including CD3 (all lymphocytes), CD4 (T helper cells), CD8 (T suppressor cells), CD19 (B cells) and CD16/56 (natural killer cells) (Table 4).
Table 4.
Immune cell subsets in slow progressors and controls with melanoma (median ± standard deviation)
| Subset measured (normal range) | Slow progressors | Controls | P-value (Mann–Whitney) |
|---|---|---|---|
| CD3% (65–90) | 73·2 ± 7·3 | 67·3 ± 4·7 | 0·17 |
| CD3 (800–2660) | 1576 ± 344 | 1191 ± 276 | 0·17 |
| CD19% (5–25) | 11·8 ± 3·7 | 10·9 ± 1·7 | 0·56 |
| CD19 (100–600) | 231 ± 106 | 204 ± 30 | 0·10 |
| CD4% (30–65) | 40 ± 8·8 | 41 ± 7 | 0·75 |
| CD4 (450–1660) | 805 ± 281 | 720 ± 82 | 0·09 |
| CD8% (15–50) | 27·8 ± 5·4 | 25·4 ± 1·7 | 0·29 |
| CD8 (190–1210) | 551 ± 155 | 441 ± 275 | 0·24 |
| CD16/56% (3–30) | 11·6 ± 9·1 | 16·6 ± 2·5 | 0·09 |
| CD16/56 (50–620) | 264 ± 249 | 214 ± 220 | 0·91 |
| ISUM (950–3880) | 2230 ± 488 | 1651 ± 536 | 0·34 |
| CD4 : CD8 ratio | 1·3 ± 0·7 | 1·6 ± 0·6 | 0·75 |
The percentage of each cell type as well as absolute values (cell counts are measured in cells/mm3) were recorded. ISUM = immunological sum of all subsets.
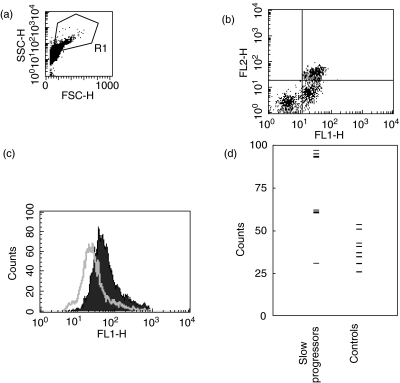
The percentage of cells staining for CD91, including monocytes, showed no statistical differences. The fluorescence intensity of CD91 staining was, however, significantly higher on the monocytes of slow progressors versus controls (median ± 95% confidence interval = 70·3 ± 22·9 versus 39 ± 9·4; P = 0·006; Fig. 1). Although there was considerable variation in the CD91 median fluorescence intensity on the monocytes of slow progressors, all but one had a CD91 median fluorescence intensity of greater than 60, whereas all controls had an intensity of less than 55 (Fig. 1d). CD91 levels did not correlate with cytokines or lymphocyte subset values.
Fig. 1.
CD91 levels are increased on monocytes of melanoma slow progressors. Cells in the live gate (a) were analysed for CD91 (FL1 x-axis) and CD14 staining (b, FL2 y axis). The CD14+ cells were gated and the CD91 median fluorescence intensity on these was plotted on a histogram (c). CD91 fluorescence intensity is shown for a representative slow progressor (solid area) and control (open area). Fluorescence intensity for each patient is plotted in (d); the median CD91 fluorescence intensity was significantly higher in slow progressors than controls (see text). Isotype control staining was less than 2·5%.
DISCUSSION
A number of tumour and host factors have been implicated in the pathogenesis and progression of melanoma. Host factors include two major susceptibility genes for melanoma, CDKN2A and CDK4; GST and MC1R contribute to only a minority of cases [13–15]. Studies have not, however, previously examined host factors that may protect against rapid progression. Here we demonstrate that CD91 expression is up-regulated on CD14+ monocytes of melanoma slow progressors, a finding with parallels in HIV disease. Increased CD91 levels may not prevent tumours (or viral infections) occurring, but may modulate the course of the disease once it has been established by maintaining cytotoxic adaptive immunity.
Pathways regulating expression of CD91 are poorly understood, although correlative evidence exists for down-regulation of CD91-mediated immune activation as a mechanism of immune escape. Thus levels of a CD91 ligand, α2-macroglobulin, are elevated in animal models of cancer [16], and during recovery of animals from autoimmune encephalitis [17]. Indeed, infusion of animals with α2-macroglobulin prevents experimental autoimmune pathology. It is conceivable that antimelanoma therapy in the slow progressor group (particularly immunotherapies) led to higher CD91 levels on monocytes, which these data suggest may be protective against rapid progression. However, no immunotherapy or other treatment modality has yet been associated with survival benefit in metastatic melanoma.
The binding of CD91 either to antigenic peptides and HSPs, or to T cell-derived α-defensins, could represent a potential mechanism of slow progression in melanoma. CD91 is the only independently confirmed HSP receptor [4,5,18]; previous work has shown that HSP–peptide complexes are taken up by antigen-presenting cells, and that the peptides are re-presented by the major histocompatibility complex (MHC) class I on these cells to CD8+ cytotoxic T cells [3,19–21]. The CD91-mediated internalization of HSPs represents an exogenous portal by which chaperoned antigen may enter the classical proteosome/TAP-dependent MHC class I pathway, so-called cross-presentation [22,23]. Thus relatively efficient cross-presentation of HSP-chaperoned antigen, via CD91, may represent a mechanism explaining the association between CD91 expression and outcome in both melanoma and HIV disease.
This small study demonstrates no differences in lymphocyte, natural killer subsets and levels of plasma cytokines between slow progressors and controls. It is of interest that TNF-α levels were detectable in all the patients with slow progression and the role of TNF-α in the disruption of tumour endothelium is becoming established [24,25]. Intriguingly, TNF-α is thought to lead to the production of both IL-6 and MCP-1 and hence may contribute to the regulation of tumour progression [26].
We and others [27] have been unable to detect significant quantities of CD91 on peripheral blood myeloid or plasmacytoid dendritic cells, and the limited CD91 expression that we are able to detect disappears after a few days in culture. The lack of CD91 on blood dendritic cells may reflect the fact that they are immature cells en route to the tissues where they become fully competent in antigen capture. We have found no evidence of CD91 expression on CD3+ T cells but consistently observe expression on CD14+ monocytes, the majority of which stain for CD91. There are also high levels of expression on monocyte-derived dendritic cells [2,28]; unlike peripheral blood dendritic cells these can be obtained in significant quantities and have been shown to uptake, process and present antigen efficiently [29]. Pulsing of monocyte-derived DCs represents a potential avenue for use in therapy.
Up-regulation of CD91-mediated processes, possibly including endocytosis of HSP–peptide complexes and cross-presentation of tumour antigens, may represent a useful antitumour therapeutic strategy. Alternatively, CD91 up-regulation may serve as a surrogate marker for response to therapy.
REFERENCES
- 1.Whelan M, Whelan J, Russell N, et al. Cancer immunotherapy: an embarrassment of riches? Drug Discov Today. 2003;8:253–8. doi: 10.1016/s1359-6446(03)02633-3. [DOI] [PubMed] [Google Scholar]
- 2.Stebbing J, Gazzard B, Kim L, et al. The heat shock protein receptor CD91 is upregulated in monocytes of HIV-1-infected ‘true’ long-term non-progressors. Blood. 2003;101:4000–4. doi: 10.1182/blood-2002-11-3353. [DOI] [PubMed] [Google Scholar]
- 3.Stebbing J, Gazzard B, Portsmouth S, et al. Disease-associated dendritic cells respond to disease-specific antigens through the common heat shock protein receptor. Blood. 2003;102:1806–14. doi: 10.1182/blood-2003-03-0891. [DOI] [PubMed] [Google Scholar]
- 4.Binder RJ, Han DK, Srivastava PK. CD91: a receptor for heat shock protein gp96. Nat Immunol. 2000;1:151–5. doi: 10.1038/77835. [DOI] [PubMed] [Google Scholar]
- 5.Basu S, Binder RJ, Ramalingam T, et al. CD91 is a common receptor for heat shock proteins gp96, hsp90, hsp70, and calreticulin. Immunity. 2001;14:303–13. doi: 10.1016/s1074-7613(01)00111-x. [DOI] [PubMed] [Google Scholar]
- 6.Nassar T, Akkawi S, Bar-Shavit R, et al. Human alpha-defensin regulates smooth muscle cell contraction: a role for low-density lipoprotein receptor-related protein/alpha 2-macroglobulin receptor. Blood. 2002;100:4026–32. doi: 10.1182/blood-2002-04-1080. [DOI] [PubMed] [Google Scholar]
- 7.Zhang L, Yu W, He T, et al. Contribution of human alpha-defensin-1, -2 and -3 to the anti-HIV-1 activity of CD8 antiviral factor. Science. 2002;298:995–1000. doi: 10.1126/science.1076185. [DOI] [PubMed] [Google Scholar]
- 8.Musey L, Hughes J, Schacker T, et al. Cytotoxic-T-cell responses, viral load, and disease progression in early human immunodeficiency virus type 1 infection. N Engl J Med. 1997;337:1267–74. doi: 10.1056/NEJM199710303371803. [DOI] [PubMed] [Google Scholar]
- 9.Migueles SA, Laborico AC, Shupert WL, et al. HIV-specific CD8(+) T cell proliferation is coupled to perforin expression and is maintained in nonprogressors. Nat Immunol. 2002;3:1061–8. doi: 10.1038/ni845. [DOI] [PubMed] [Google Scholar]
- 10.Spicer J, Gore ME. Malignant melanoma. In: Price P, Sikora K, editors. Treatment of cancer. London: Arnold; 2002. pp. 831–50. [Google Scholar]
- 11.Stebbing J, Bower M. What can oncologists learn from HIV? Lancet Oncol. 2003;4:438–45. doi: 10.1016/s1470-2045(03)01142-2. [DOI] [PubMed] [Google Scholar]
- 12.Stebbing J, Benson C, Eisen T, et al. The treatment of advanced renal cell cancer with high-dose oral thalidomide. Br J Cancer. 2001;85:953–8. doi: 10.1054/bjoc.2001.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanetsky PA, Holmes R, Walker A, et al. Interaction of glutathione S-transferase M1 and T1 genotypes and malignant melanoma. Cancer Epidemiol Biomarkers Prev. 2001;10:509–13. [PubMed] [Google Scholar]
- 14.Bishop DT, Demenais F, Goldstein AM, et al. Geographical variation in the penetrance of CDKN2A mutations for melanoma. J Natl Cancer Inst. 2002;94:894–903. doi: 10.1093/jnci/94.12.894. [DOI] [PubMed] [Google Scholar]
- 15.Tucker MA, Goldstein AM. Melanoma etiology: where are we? Oncogene. 2003;22:3042–52. doi: 10.1038/sj.onc.1206444. [DOI] [PubMed] [Google Scholar]
- 16.Maltseva N, Zorina R, Mingaljev N, et al. Tissue distributiion of rat macroglobulins in tumour-bearing rats. Int J Exp Pathol. 1999;80:105–8. doi: 10.1046/j.1365-2613.1999.00103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hunter N, Weston K, Bowern N. Suppression of experimental allergic encephalomyelitis by alpha 2-macroglobulin. Immunol. 1991;73:58–63. [PMC free article] [PubMed] [Google Scholar]
- 18.Banerjee PP, Vinay DS, Mathew A, et al. Evidence that glycoprotein 96 (B2), a stress protein, functions as a Th2-specific costimulatory molecule. J Immunol. 2002;169:3507–18. doi: 10.4049/jimmunol.169.7.3507. [DOI] [PubMed] [Google Scholar]
- 19.Moroi Y, Mayhew M, Trcka J, et al. Induction of cellular immunity by immunization with novel hybrid peptides complexed to heat shock protein 70. Proc Natl Acad Sci USA. 2000;97:3485–90. doi: 10.1073/pnas.070550797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ishii T, Udono H, Yamano T, et al. Isolation of MHC class I-restricted tumor antigen peptide and its precursors associated with heat shock proteins hsp70, hsp90, and gp96. J Immunol. 1999;162:1303–9. [PubMed] [Google Scholar]
- 21.Castellino F, Boucher PE, Eichelberg K, et al. Receptor-mediated uptake of antigen/heat shock protein complexes results in major histocompatibility complex class I antigen presentation via two distinct processing pathways. J Exp Med. 2000;191:1957–64. doi: 10.1084/jem.191.11.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Srivastava P. Roles of heat-shock proteins in innate and adaptive immunity. Nat Rev Immunol. 2002;2:185–94. doi: 10.1038/nri749. [DOI] [PubMed] [Google Scholar]
- 23.Srivastava P. Interaction of heat shock proteins with peptides and antigen presenting cells: chaperoning of the innate and adaptive immune responses. Annu Rev Immunol. 2002;20:395–425. doi: 10.1146/annurev.immunol.20.100301.064801. [DOI] [PubMed] [Google Scholar]
- 24.Mocellin S, Provenzano M, Lise M, et al. Increased TIA-1 gene expression in the tumor microenvironment after locoregional administration of tumor necrosis factor-alpha to patients with soft tissue limb sarcoma. Int J Cancer. 2003;107:317–22. doi: 10.1002/ijc.11369. [DOI] [PubMed] [Google Scholar]
- 25.Eggermont AM, de Wilt JH, ten Hagen TL. Current uses of isolated limb perfusion in the clinic and a model system for new strategies. Lancet Oncol. 2003;4:429–37. doi: 10.1016/s1470-2045(03)01141-0. [DOI] [PubMed] [Google Scholar]
- 26.Neumark E, Sagi-Assif O, Shalmon B, et al. Progression of mouse mammary tumors: MCP-1-TNFalpha cross-regulatory pathway and clonal expression of promalignancy and antimalignancy factors. Int J Cancer. 2003;106:879–86. doi: 10.1002/ijc.11337. [DOI] [PubMed] [Google Scholar]
- 27.Delneste Y, Magistrelli G, Gauchat J, et al. Involvement of LOX-1 in dendritic cell-mediated antigen cross-presentation. Immunity. 2002;17:353–62. doi: 10.1016/s1074-7613(02)00388-6. [DOI] [PubMed] [Google Scholar]
- 28.Lipsker D, Ziylan U, Spehner D, et al. Heat shock proteins 70 and 60 share common receptors which are expressed on human monocyte-derived but not epidermal dendritic cells. Eur J Immunol. 2002;32:322–32. doi: 10.1002/1521-4141(200202)32:2<322::AID-IMMU322>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 29.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994;179:1109–18. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]