Abstract
Type I diabetes (TID) is an autoimmune disease characterized in part by the presence of autoantibodies directed against glutamic acid decarboxylase 65 (GAD65), among other pancreatic islet antigens. We investigated the independent epitope specificities of these GAD65 antibodies (GAD65Ab) and their combinations in the sera of new onset TID patients and first-degree relatives positive for GAD65Ab. For our analysis, we used four GAD65-specific recombinant Fabs (rFabs) that recognize different conformational determinants of GAD65 located throughout the molecule, including the N-terminal, the middle and the C-terminal regions. We used these epitope-specific rFabs in competition assays to determine the binding specificity of the autoantibodies found in patient sera. Among the 61 sera from newly diagnosed GAD65Ab-positive TID patients GAD65 binding was competed for 23 sera by all four rFabs, 29 by at least two rFabs, and in nine sera were displaced by one or no rFab. In contrast, none of the 24 sera from GAD65Ab-positive first-degree relatives of TID patients were displaced by all four rFabs. When using all four rFabs simultaneously to compete with GAD65Ab binding, binding of sera from TID patients was reduced by an average of 70%. A significantly weaker competition was observed when evaluating sera of GAD65Ab-positive first-degree relatives (P < 0·0001).
Keywords: autoantibodies autoimmunity, epitope, GAD65, Type I diabetes
INTRODUCTION
Autoantibodies to GAD65 (GAD65Ab) are present in the majority of patients with Type I diabetes (TID) and serve as an important marker of the disease [1–3]. However, the relationship between these autoantibodies and the pathogenesis of TID is not understood. GAD65, one of the primary target antigens of the autoimmune disease process, is thought to play a pathogenic role in the development of TID (reviewed in [4–6]). Moreover, modulation of GAD65 presentation to the T cells by disease-associated GAD65Ab has been implicated as a possible mechanism for the breakdown of tolerance to the pancreatic islets [7–9].
The majority of GAD65Ab in TID patient sera recognize conformation-dependent epitopes located in the middle and the C-terminus of the GAD65 molecule, while few GAD65Ab bind the N-terminus of GAD65 [10–12]. The GAD65Ab epitope pattern of TID sera appears to differ from that of non-diabetic GAD65Ab-positive individuals [12,13]. Studies using human monoclonal antibodies (MoAbs) isolated from patients with TID have confirmed the presence of multiple GAD65Ab epitopes [14–17]. The isolation of MoAbs from patients allows the characterization of epitopes recognized by a specific antibody, however, the relative frequencies of different MoAbs in each patient and in the overall patient population remains unclear due to the polyclonality of the autoimmune response. The aim of this study was to analyse the GAD65Ab present in patients with TID and first-degree relatives positive for GAD65Ab with respect to combinations of GAD65Ab specificities, independence of individual epitope regions and polyclonality.
MATERIALS AND METHODS
Experimental subjects
TID patients
Newly diagnosed GAD65Ab-positive TID patients (n = 61) (mean age 10 years, range 0–16 years; 33 female) were part of a study conducted at the St Görans Children Hospital, Stockholm, Sweden and represented 60% of all children diagnosed in Stockholm during 1993–95. The serum samples were obtained at the clinical diagnosis of diabetes.
First-degree relatives
Healthy GAD65Ab-positive first-degree relatives of TID patients (n = 24) (mean age 46 years, range 8–74 years) were identified by screening individuals from families with at least two siblings with diabetes. These families were identified based on 1170 probands with TID enrolled in the Diabetes Incidence Study in Sweden (DISS) and the Swedish Childhood Diabetes registry.
All subjects or their legal guardians gave informed consent. Local institutional ethics committee approval was obtained prior to collection of all serum samples.
Recombinant Fabs
The four recombinant Fabs (rFabs) used in this study have been described previously in detail [18]. Briefly, rFabs DP-A, DP-D, and DP-C were isolated from a TID patient [17] and recognize epitopes at amino acid residues 483–585, 96–173 and 195–412, respectively [15]. b96.11 was isolated from a patient with Autoimmune Polyendocrine Syndrome Type I (APS-I) and recognizes an epitope at amino acid residues 308–365 [15,19].
Competition of rFabs with TID sera
Recombinant [35S]-GAD65 was produced in an in vitro coupled transcription/translation system with Sp6 RNA polymerase and nuclease treated rabbit reticulocyte lysate (Promega, Madison, WI, USA) as described previously [20]. The in vitro translated [35S]-GAD65 was stored at −70°C and used within 2 weeks. The capacity of the rFab to inhibit GAD65 binding by GAD65Ab in human sera was evaluated in a competitive radioimmunoassay (RIA), using Protein A Sepharose (Zymed Laboratories, Carlton Court, CA, USA) as the precipitating agent as described previously [18].
Statistical analysis
Binding of GAD65Ab to GAD65 in the presence of rFab was expressed as follows: (cpm of [35S]-GAD65 bound in the presence of rFab/cpm of [35S]-GAD65 bound in the absence of rFab) × 100.
The background competition for each rFab was established in competition experiments with normal control sera. The background was subtracted prior to calculation of percentage inhibition. The cut-off for specific competition was determined as >10% by using rFab NQ22/61·1 as a negative control [21] (a kind gift from Dr J. Foote, Fred Hutchinson Research Center, Seattle), specific to an irrelevant target, phenyl oxazolone, at 5 µg/ml. All samples were analysed in triplicate and the average intra-assay coefficient of variation was 4·5% (range 0·016–15%). The significance of differences in competition was tested with the non-parametric Mann–Whitney U-test. Competition levels within each group were analysed using the non-parametric Wilcoxon matched pair test. A P-value <0·05 was considered significant.
RESULTS
Combinations of GAD65Ab with different epitope specificities
We analysed TID patient sera for the presence of combinations of GAD65Ab with different specificities (Table 1). Of the 61 samples, 23 (38%) were displaced by all four rFabs, 29 (47%) by at least two rFabs, six (10%) were displaced by only one rFab and three were not displaced by any of the rFabs. The most common combination of GAD65Ab specificities found in patient sera (60%) reacted with epitopes recognized by rFab DP-A (C-terminus), b96.11 (M-terminus) and DP-D (N-terminus) (data not shown). When evaluating the sera of first-degree relatives for combinations of GAD65Ab specificities, we could not detect any obvious grouping. Most of the sera (12/24 = 50%) were competed by only one rFab, while 5/24 (21%) were not competed by any of the rFabs (Table 1). No correlation between the number of recognized epitopes or between any GAD65Ab epitope specificity and age at onset was observed when analysing the TID samples (data not shown).
Table 1.
Combination of GAD65Ab specificities in sera of TID patients and first-degree relatives
| TID | First-degree relatives | |
|---|---|---|
| No displacement | 3 | 5 |
| One rFabs | 6 | 12 |
| Two rFabs | 13 | 5 |
| Three rFabs | 16 | 2 |
| Four rFabs | 23 | 0 |
Binding of radiolabelled GAD65 with serum was competed with rFabs. The numbers of serum samples competed with different combinations of rFabs are shown.
GAD65Ab in TID are polyclonal
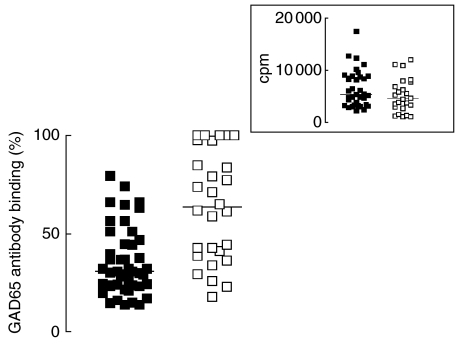
We tested the prevalence of GAD65Ab with specificities similar or identical to the four rFabs by incubating sera of patients with TID with all four rFabs simultaneously (Fig. 1, left panel). GAD65Ab binding was reduced significantly to a median of 30% (range 13–79%) (P < 0·0001). Incubation of the sera of GAD65Ab-positive first-degree relatives with all four rFabs reduced the median binding to 68% (range 17–100%) (Fig. 1, right panel). Compared to the results of the TID patients’ sera, this inhibition was significantly weaker (P < 0·0001). The GAD65Ab titre and degree of inhibition were significantly correlated both for TID sera (P = 0·005) and first-degree relative sera (P = 0·037), demonstrating that the degree of inhibition was highest in the high titre samples (data not shown). When comparing the GAD65Ab titre of both groups, no significant differences were observed (Fig. 1, insert).
Fig. 1.
Inhibition of GAD65 binding by GAD65Ab positive sera in the presence of four rFabs. Sera of TID patients (black squares) and first-degree relatives (white squares) were incubated with four rFabs simultaneously to compete binding of GAD65Ab in these sera. Remaining GAD65Ab binding following the competition is shown. Insert: GAD65Ab titre of TID patients (black squares) and first-degree relatives (white squares) are compared. Median binding is indicated by a horizontal line.
Cross-competition between rFab and MoAbs of different epitope specificities
The epitope specificity of the rFabs was determined by competitive RIA. Binding to GAD65 by each intact IgG MoAb was competed with each rFab. The data in Table 2 summarize the degree of displacement of the MoAbs by the rFabs. All rFabs competed with the GAD65 binding of the respective originating MoAb in a concentration-dependent manner. rFabs b96.11 and DP-C inhibited GAD65 binding of MoAb DP-C and b96.11, respectively, to a low extent (10%). rFab DP-C inhibited GAD65 binding of MoAb DP-D (20%), but rFab DP-D did not inhibit MoAb DP-C binding to GAD65.
Table 2.
Cross-competition of rFabs with IgG MoAbs
| DP-D IgG | DP-C IgG | DP-A IgG | b96.11 IgG | |
|---|---|---|---|---|
| rFab DP-D | + + + | – | – | – |
| rFab DP-C | + + | + + + | – | + |
| rFab DP-A | – | – | + + + | – |
| rFab b96.11 | – | + | – | + + + |
Binding of radiolabelled GAD65 with IgG MoAbs was competed with rFabs. Degrees of inhibition of MoAbs binding are indicated (+ + +: inhibition between 80 and 50%, ++: inhibition of 20–50%, +: inhibition of 10–20%, –: inhibition <10%).
Independent binding to GAD65Ab epitopes
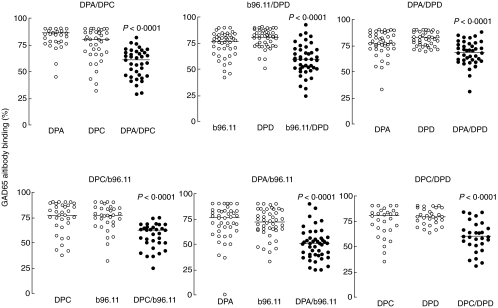
We next analysed whether GAD65Ab in sera of TID patients bound independently to the four epitopes defined by the rFabs. To this end, we performed cross-competition of the 61 TID patients’ sera, using each rFab alone and in combination with every other rFab. Competition achieved by one rFab was compared with that observed in the presence of the other rFab and both rFabs (Fig. 2). In each combination, we found that the competition achieved by both rFabs was significantly stronger than by either of the rFabs alone. This indicated that each of the rFabs competed with the binding of TID-associated GAD65Ab independently. The rFabs to middle epitopes (DP-C, b96.11) or the N-terminus (DP-D) did not affect the competition of binding by rFab to C-terminal epitopes (DP-A). Reciprocally, blocking the epitopes in the C-terminal part of the protein (bound by rFab DP-A) did not affect the competition by rFabs DP-C, DP-D or b96.11. The middle region of GAD65 (bound by DP-C and b96.11) did not interact significantly with the N-terminal region (bound by DP-D) or the C-terminal end of the protein (bound by DP-A).
Fig. 2.
Competition of GAD65-binding of patients’ sera using various rFab combinations. The competition observed in the presence of a single rFab (white circles) was compared with the competition seen in the presence of two rFabs (black circles). P-values for the comparisons are indicated.
DISCUSSION
While it is understood that the humoral response to GAD65 in TID patients’ sera is polyclonal, little is known about the prevalence of individual GAD65Ab specificities and the interaction between different epitopes. Analysis of competitive binding to GAD65 with four GAD65-specific rFabs was used to address these questions. Our results demonstrate a heterogeneous humoral response to GAD65 with the recognition of multiple independent GAD65Ab epitopes in 61 patients with TID.
Schwartz and colleagues identified 13 non-overlapping GAD65Ab epitopes using a panel of 16 MoAbs (including the four antibodies studied in this report) [15]. While the MoAbs were isolated from patients with TID or APS-1, it remained to be shown whether GAD65Ab present in a wider selection of TID patients’ sera showed the same independent binding. We studied the independence of epitope recognition using cross-competition experiments and confirmed that the GAD65Ab epitopes were bound independently from each other. The modest competition of rFab DP-C with the binding of MoAb DP-D was not confirmed by the combined competition assay on human sera. It should be noted that rFab DP-C competed with the binding of MoAb DP-D, but not vice versa. This discrepancy in competition pattern may be due to the lower affinity of DP-D for GAD65 [7,9,18]. Interestingly, no competition was observed between purified IgG of DP-D and DP-C when evaluated on human islets by immunohistochemistry [17]. The different readout may be due to the distinct nature and sensitivities of the assays.
Simultaneous competition using all four rFabs reduced the binding of GAD65Ab in TID sera to a median of 30%. A significantly weaker competition was observed using all four rFab to compete with sera from GAD65Ab-positive first-degree relatives. This confirms our previous studies demonstrating that the epitope specificities in the TID differ from those in non diabetic control subjects [18]. In both groups, a widely variable degree of competition was observed. The reason for this diversity is unclear; one could speculate that those first-degree relatives who demonstrated greater degrees of competition by the rFabs may eventually progress to TID. An increased frequency of GAD65Ab (6–13%) has been reported in first-degree relatives [22–25]. In combination with autoantibodies to insulin and IA-2, GAD65Ab are highly predictive for the future development of diabetes in these individuals [23,26,27], in that among GAD65Ab-positive first-degree relatives, ∼40% will go on to develop diabetes [25]. The first-degree relatives evaluated in this study were part of a cross-sectional population, and no follow-up is available to test this speculation. As we observed a positive correlation between the degree of competition with rFab and GAD65Ab titre, it cannot be excluded that the antibody titre also influences the multiplicity of the GAD65Ab response, even when the difference in GAD65Ab titre of the TID patients and the first-degree relatives was not found to be statistically significant. It is obvious from these results that our panel of rFab does not cover the entire range of GAD65Ab specificities and invites further isolation of GAD65Ab from both TID patients and control subjects.
Our analysis of the TID patients’ sera using rFabs suggested that the majority of these patients have GAD65Ab reacting with at least four different epitopes. An alternative explanation would be that the four rFabs block binding to more than one epitope and thus block GAD65Ab of other epitope specificities. While we cannot exclude this possibility, our data indicate that rFabs whose epitopes are located in close vicinity do not interfere with each other's binding. The simultaneous binding of two or more rFabs to GAD65 suggests that the antibodies do not induce conformational changes to the molecule.
An increase in the number of recognized epitopes during the progression to diabetes in prediabetic children has been reported previously [28]. The presence of multiple GAD65Ab epitopes in newly diagnosed patients with TID has been hypothesized to occur by intramolecular spreading of an autoimmune response from a single epitope located in the middle region to multiple epitopes located in the middle and at the C-terminus [29]. In our cross-sectional analysis of combinations of GAD65Ab specificity in newly diagnosed TID patients, we found that 65% of the samples reacted with epitopes at the C-terminus and the middle region. The most common combination of GAD65Ab specificities in TID patient sera was defined by competition with rFabs b96.11 and DP-A. This epitope combination could reflect the early GAD65Ab response to the middle epitope that spreads to the C-terminus. The presence of GAD65Ab binding to four independent epitopes in very young children (1 year of age) supports the hypothesis that events associated with epitope speading of the autoimmune response occur very early during the autoimmune insult. In addition, our finding that the inhibition of GAD65 binding by the four rFabs was correlated directly with the GAD65Ab titre agrees with earlier findings that GAD65Ab specific to conformational epitopes at the middle and C-terminal of GAD65 correlate with high GAD65Ab titres [29].
We and others reported that GAD65Ab in TID differ from GAD65Ab in other endocrine autoimmune phenotypes [12,13] and have recently described two TID-specific GAD65Ab epitopes [18]. A better understanding of the nature of the epitopes and their prevalence in different individuals may help to clarify the development of disease-specific GAD65Ab. TID specific GAD65Ab epitopes occur together with GAD65Ab epitopes observed also in other phenotypes [18]. A longitudinal study using samples from prediabetic individuals will be necessary to study the appearance of GAD65Ab specific to disease-associated epitopes. The mechanism of the development and possible implications on the disease progression of these disease-specific epitopes warrants further investigation.
Acknowledgments
This study has been supported by the Juvenile Diabetes Foundation International and the National Institute of Health (grants DK26190 and DK53004 to Åke Lernmark and DK 17047 to the DERC), and a Career Development Award from the American Diabetes Association for CSH. We gratefully acknowledge Dr Åke Lernmark for helpful advice and critical reading of the manuscript.
REFERENCES
- 1.Atkinson MA, Maclaren NK, Scharp DW, Lacy PE, Riley WJ. 64,000 Mr autoantibodies as predictors of insulin-dependent diabetes. Lancet. 1990;335:1357–60. doi: 10.1016/0140-6736(90)91241-2. [DOI] [PubMed] [Google Scholar]
- 2.Sabbah E, Kulmala P, Veijola R, et al. Childhood Diabetes in Finland Study Group. Glutamic acid decarboxylase antibodies in relation to other autoantibodies and genetic risk markers in children with newly diagnosed insulin-dependent diabetes. J Clin Endocrinol Metab. 1996;81:2455–9. doi: 10.1210/jcem.81.7.8675560. [DOI] [PubMed] [Google Scholar]
- 3.Vandewalle CL, Falorni A, Svanholm S, Lernmark A, Pipeleers DG, Gorus FK. High diagnostic sensitivity of glutamate decarboxylase autoantibodies in insulin-dependent diabetes mellitus with clinical onset between age 20 and 40 years. The. J Clin Endocrinol Metab. 1995;80:846–51. doi: 10.1210/jcem.80.3.7883841. Belgian Diabetes Registry. [DOI] [PubMed] [Google Scholar]
- 4.Yoon JW, Sherwin RS, Kwon H, Jun HS. Has GAD a central role in Type I diabetes? J Autoimmun. 2000;15:273–8. doi: 10.1006/jaut.2000.0442. [DOI] [PubMed] [Google Scholar]
- 5.Nepom G, Quinn A, Sercarz E, Wilson DB. How important is GAD in the etiology of spontaneous disease in human and murine Type I diabetes? J Autoimmun. 2003;20:193–4. doi: 10.1016/s0896-8411(03)00027-1. [DOI] [PubMed] [Google Scholar]
- 6.Baekkeskov S, Kanaani J, Jaume JC, Kash S. Does GAD have a unique role in triggering IDDM? J Autoimmun. 2000;15:279–86. doi: 10.1006/jaut.2000.0443. [DOI] [PubMed] [Google Scholar]
- 7.Jaume JC, Parry SL, Madec AM, Sonderstrup G, Baekkeskov S. Suppressive effect of glutamic acid decarboxylase 65-specific autoimmune B lymphocytes on processing of T cell determinants located within the antibody epitope. J Immunol. 2002;169:665–72. doi: 10.4049/jimmunol.169.2.665. [DOI] [PubMed] [Google Scholar]
- 8.Reijonen H, Daniels TL, Lernmark A, Nepom GT. GAD65-specific autoantibodies enhance the presentation of an immunodominant T-cell epitope from GAD65. Diabetes. 2000;49:1621–6. doi: 10.2337/diabetes.49.10.1621. [DOI] [PubMed] [Google Scholar]
- 9.Banga JP, Moore JK, Duhindan N, et al. Modulation of antigen presentation by autoreactive B cell clones specific for GAD65 from a type I diabetic patient. Clin Exp Immunol. 2004;135:74–84. doi: 10.1111/j.1365-2249.2004.02343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daw K, Powers AC. Two distinct glutamic acid decarboxylase auto-antibody specificities in IDDM target difference epitopes. Diabetes. 1995;44:216–20. doi: 10.2337/diab.44.2.216. [DOI] [PubMed] [Google Scholar]
- 11.Falorni A, Ackefors M, Carlberg C, et al. Diagnostic sensitivity of immunodominant epitopes of glutamic acid decarboxylase (GAD65) autoantibodies epitopes in childhood IDDM. Diabetologia. 1996;39:1091–8. doi: 10.1007/BF00400659. [DOI] [PubMed] [Google Scholar]
- 12.Hampe CS, Hammerle LP, Bekris L, et al. Recognition of glutamic acid decarboxylase (GAD) by autoantibodies from different GAD antibody-positive phenotypes. J Clin Endocrinol Metab. 2000;85:4671–9. doi: 10.1210/jcem.85.12.7070. [DOI] [PubMed] [Google Scholar]
- 13.Powers AC, Bavik K, Tremble J, Daw K, Scherbaum WA, Banga JP. Comparative analysis of epitope recognition of glutamic acid decarboxylase (GAD) by autoantibodies from different autoimmune disorders. Clin Exp Immunol. 1999;118:349–56. doi: 10.1046/j.1365-2249.1999.01030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Syren K, Lindsay L, Stoehrer B, et al. Immune reactivity of diabetes-associated human monoclonal autoantibodies defines multiple epitopes and detects two domain boundaries in glutamate decarboxylase. J Immunol. 1996;157:5208–14. [PubMed] [Google Scholar]
- 15.Schwartz HL, Chandonia JM, Kash SF, et al. High-resolution autoreactive epitope mapping and structural modeling of the 65 kDa form of human glutamic acid decarboxylase. J Mol Biol. 1999;287:983–99. doi: 10.1006/jmbi.1999.2655. [DOI] [PubMed] [Google Scholar]
- 16.Richter W, Northemann W, Müller M, Böhm BO. Mapping of an autoreactive epitope with glutamate decarboxylase using a diabetes-associated monoclonal antibody and an epitope cDNA library. Hybridoma. 1996;15:103–8. doi: 10.1089/hyb.1996.15.103. [DOI] [PubMed] [Google Scholar]
- 17.Madec AM, Rousset F, Ho S, Robert F, Thivolet C, Orgiazzi J, Lebecque S. Four IgG anti-islet human monoclonal antibodies isolated from a Type I diabetes patient recognize distinct epitopes of glutamic acid decarboxylase 65 and are somatically mutated. J Immunol. 1996;156:3541–9. [PubMed] [Google Scholar]
- 18.Padoa CJ, Banga JP, Madec AM, et al. Recombinant Fabs of human monoclonal antibodies specific to the middle epitope of GAD65 inhibit Type I diabetes-specific GAD65Abs. Diabetes. 2003;52:2689–95. doi: 10.2337/diabetes.52.11.2689. [DOI] [PubMed] [Google Scholar]
- 19.Tremble J, Morgenthaler NG, Vlug A, et al. Human B cells secreting immunoglobulin G to glutamic acid decarboxylase-65 from a nondiabetic patient with multiple autoantibodies and Graves’ disease: a comparison with those present in Type I diabetes. J Clin Endocrinol Metab. 1997;82:2664–70. doi: 10.1210/jcem.82.8.4171. [DOI] [PubMed] [Google Scholar]
- 20.Falorni A, Örtqvist E, Persson B, Lernmark Å. Radioimmunoassays for glutamic acid decarboxylase (GAD65) and GAD65 autoantibodies using 35S or 3H recombinant human ligands. J Immunol Meth. 1995;186:89–99. doi: 10.1016/0022-1759(95)00139-2. [DOI] [PubMed] [Google Scholar]
- 21.Berek C, Jarvis JM, Milstein C. Activation of memory and virgin B cell clones in hyperimmune animals. Eur J Immunol. 1987;17:1121–9. doi: 10.1002/eji.1830170808. [DOI] [PubMed] [Google Scholar]
- 22.Yu L, Cuthbertson DD, Eisenbarth GS, Krischer JP. Diabetes Prevention Trial 1. prevalence of GAD and ICA512 (IA-2) autoantibodies by relationship to proband. Ann NY Acad Sci. 2002;958:254–8. [PubMed] [Google Scholar]
- 23.Verge CF, Gianani R, Kawasaki E, et al. Prediction of type I diabetes in first-degree relatives using a combination of insulin, GAD, and ICA512bdc/IA-2 autoantibodies. Diabetes. 1996;45:926–33. doi: 10.2337/diab.45.7.926. [DOI] [PubMed] [Google Scholar]
- 24.Aanstoot H-J, Sigurdsson E, Jaffe M, et al. Value of antibodies to GAD65 combined with islet cell cytoplasmic antibodies for predicting IDDM in a childhood population. Diabetologia. 1994;37:917–24. doi: 10.1007/BF00400948. [DOI] [PubMed] [Google Scholar]
- 25.Kulmala P, Savola K, Petersen JS, et al. Prediction of insulin-dependent diabetes mellitus in siblings of children with diabetes − a population-based study. J Clin Invest. 1998;101:327–36. doi: 10.1172/JCI119879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bingley PJ, Christie MR, Bonifacio E, et al. Combined analysis of autoantibodies improves prediction of IDDM in islet cell antibody-positive relatives. Diabetes. 1994;43:1304–10. doi: 10.2337/diab.43.11.1304. [DOI] [PubMed] [Google Scholar]
- 27.Christie MR, Roll U, Payton MA, Hatfield EC, Ziegler AG. Validity of screening for individuals at risk for type I diabetes by combined analysis of antibodies to recombinant proteins. Diabetes Care. 1997;20:965–70. doi: 10.2337/diacare.20.6.965. [DOI] [PubMed] [Google Scholar]
- 28.Bonifacio E, Lampasona V, Bernasoni L, Ziegler AG. Maturation of the humoral autoimmune response to epitopes of GAD in preclinical childhood Type I diabetes. Diabetes. 2000;49:202–8. doi: 10.2337/diabetes.49.2.202. [DOI] [PubMed] [Google Scholar]
- 29.Sohnlein P, Muller M, Syren K, et al. The Childhood Diabetes Finland Study Group. Epitope spreading and a varying but not disease-specific GAD65 antibody response in Type I diabetes. Diabetologia. 2000;43:210–7. doi: 10.1007/s001250050031. [DOI] [PubMed] [Google Scholar]