Abstract
Aspartate can be released from certain hippocampal pathways along with glutamate or GABA. Although aspartate immunoreactivity has been localized to synaptic vesicles and aspartate release is Ca2+-dependent, there has been no clear evidence favoring an exocytotic mechanism. In particular, pretreatment with Clostridial toxins has not consistently inhibited aspartate release, even when release of glutamate from the same tissue samples was markedly inhibited. To address this issue directly, rat hippocampal synaptosomes were permeabilized transiently by electroporation in the presence of active or inactivated Clostridial toxin light chains. Loading rat hippocampal synaptosomes with the active light chain of tetanus toxin or of botulinum neurotoxins A, B or C reduced the K+-evoked release of aspartate at least as much as that of glutamate. These results confirm that aspartate is released by exocytosis in rat hippocampus.
Keywords: Aspartate, Excitatory amino acids, Transmitter release, Hippocampus, Exocytosis, Clostridial toxins
Although hippocampal preparations have long been known to release aspartate along with the recognized transmitters glutamate and GABA [18,19], the mechanism and physiological significance of aspartate release remain unclear. Aspartate immunoreactivity has been localized to synaptic vesicles of certain excitatory [10,11] and inhibitory [12] hippocampal pathways. In those pathways, aspartate immunoreactivity was associated with synaptic vesicles to the same degree as glutamate or GABA immunoreactivity. Aspartate is coreleased with glutamate from the Schaffer collateral-commissural and dentate gyrus associational-commissural pathways in a Ca2+-dependent manner [2,3,19] and could serve as a co-transmitter through its selective activation of NMDA receptors [4,22]. However, the mechanism of aspartate release appears to differ in some respects from that of the recognized amino acid transmitters. In our previous study of rat hippocampal synaptosomes, aspartate release was more sensitive than glutamate release to increases in intracellular [Ca2+] outside the presynaptic active zones, was reduced by KB-R7943, an inhibitor of Na+/Ca2+ exchange, was much less sensitive than glutamate release to block of P/Q-type voltage-dependent Ca2+ channels, and resisted both bafilomycin A1, an inhibitor of vacuolar H+-ATPase, and Clostridial toxins [1]. Importantly, (±) threo-3-methylglutamate, a competitive but non-transportable inhibitor of excitatory amino acid transport, did not reduce aspartate release. This result and the distinct pharmacologies of aspartate and glutamate release processes excluded the possibility that aspartate was released by heteroexchange with released glutamate [20]. Our results suggested that aspartate is released in a neuropeptide-like fashion at sites distinct from those of glutamate release. Previous work by ourselves [28] and others [6,15] also favored independent mechanisms of aspartate and glutamate release.
Despite its Ca2+-dependence, there has been no clear evidence favoring an exocytotic mechanism for aspartate release. Pretreatment of neocortical synaptosomes [17], whole brain synaptosomes [6] or hippocampal slices [10,11] with Clostridial toxins inhibited chemically-evoked aspartate release, as expected for an exocytotic process, but either a high concentration of toxin was used or the possibility of release by heteroexchange with released glutamate was not excluded. In our study of rat hippocampal synaptosomes, pretreatment with Clostridial toxins reduced glutamate release markedly, but did not reduce the simultaneous release of aspartate from the same tissue samples [1]. This result could be viewed as evidence against aspartate exocytosis. If the sites of aspartate release are distinct from the glutamate release sites, however, then internalized toxins may have failed to inhibit aspartate release because they did not reach an inhibitory concentration at the aspartate release sites. Native Clostridial toxins are composed of a heavy chain, which is required for internalization by synaptic terminals, and a light chain, which is endowed with proteolytic activity against one or more of the proteins (SNAREs) required for neuronal exocytosis. Internalization of botulinum neurotoxins (BoNTs) involves binding of the heavy chain to synaptic vesicle proteins expressed on the cell surface, incorporation into the vesicle, and translocation of the toxin light chain into the synaptic terminal cytoplasm as the vesicle interior acidifies [24-26]. The light chain then cleaves its target SNARE protein(s), inhibiting exocytosis. Toxin internalization is expected to take place primarily at and near the presynaptic active zones, where synaptic vesicles are recaptured for reuse. If the aspartate-containing vesicles undergo exocytosis at some distance from the active zones, restricted diffusion and degradation of toxin light chain may limit toxin-induced hydrolysis of SNARE proteins at those sites. We tested this hypothesis with rat hippocampal synaptosomes preloaded with Clostridial toxin light chains by electroporation. Electroporation allows the introduction of exogenous molecules into cells that are otherwise impermeable to those molecules by creating transient pores in the plasma membrane. Because this technique creates these pores at random locations, the concentration of toxin light chain was expected to be about equal at all release sites.
For each experiment, synaptosomes were prepared from the hippocampi of one female Sprague-Dawley rat (100-125 g; Zivic Laboratories, Pittsburgh, PA, USA) as described by Dunkley et al. [5]. Animal protocols were approved in advance by the Duke University Animal Care and Use Committee according to guidelines of the National Institutes of Health. Every effort was made to minimize the number of rats used, as well as their pain and suffering.
The preparation was suspended in an aerated HEPES-buffered medium that consisted of 122 mM NaCl, 3.1 mM KCl, 1.3 mM CaCl2, 1.2 mM MgSO4, 0.4 mM KH2PO4, 10 mM D-glucose, 25 mM HEPES, 2 mM Na2ATP, and 0.4 mM L-glutamine, pH 7.4. ATP was added to replace ATP lost during permeabilization [23] and glutamine was added as a substrate for resynthesis of any lost glutamate and aspartate. Preliminary studies showed that including glutamine in the electroporation medium maintained the normal K+-evoked release of aspartate and glutamate without changing basal efflux. The preparation was divided into equal 175 μl portions. Clostridial toxin light chain (List Biological Laboratories, Campbell, CA, USA) in a volume of 12 μl was added to one portion and an equal amount of inactivated light chain to the other. Inactivation was achieved by heating the toxin light chain in a boiling water bath for 10-30 min. The tissue protein concentration was 1.5-2.0 μg/ml. Each synaptosome suspension was electroporated with a single pulse from a Gene Pulser Transfection Apparatus (Bio-Rad Laboratories, Hercules, CA, USA). An equal portion of each preparation was transferred to a disk of Whatman GF/A filter paper (Whatman International, Maidstone, UK) in six identical superfusion chambers. For each experiment, three of the tissue samples had been preloaded with active light chain and three with inactivated light chain. Release experiments were carried out and amino acids in the superfusate were quantitated by HPLC as previously described [1]. Briefly, the tissue samples were superfused dropwise from above at 32°C and a flow rate of 1 ml/min. The superfusion medium was the same as the electroporation medium, except that HEPES was replaced by an equal concentration of NaHCO3 (pH 7.4 by gassing with water-saturated 95% O2/5% CO2), the glucose concentration was reduced to 1 mM, and ATP and glutamine were omitted. The medium was supplemented with 16 μM fatty acid-free bovine serum albumin (BSA) for the first 56 min to reduce oxidative damage and then BSA was removed. After collecting a sample of effluent for determination of basal amino acid efflux, synaptosomes were challenged with a 2 min exposure to 25 mM KCl (with commensurate reduction of NaCl). Basal and evoked releases of aspartate and glutamate from the same tissue samples were quantitated simultaneously. K+-evoked release was the difference between values obtained during and before exposure to 25 mM KCl normalized to the amount of tissue protein in the sample. Results are expressed as means ± S.E.M.
Preliminary experiments determined the optimal electroporation voltage. Capacitance was maintained at 960 μF and the voltage was varied. Voltages of 300 V or above essentially abolished the K+-evoked release of aspartate and glutamate, suggesting irreversible tissue damage. Reducing the voltage to 250 V inhibited the release of glutamate by 46 ± 14% (P <0.02, Student's t-test, compared with non-electroporated synaptosomes from the same preparations, n = 10), with variable effects on aspartate release. The highest voltage that did not reduce K+-evoked release was 200 V (aspartate release, 112 ± 27% of control, glutamate release, 95 ± 19% of control, n = 9). Thus the Gene Pulser was set at 200 V and 960 μF (0.19 coulomb) for these experiments.
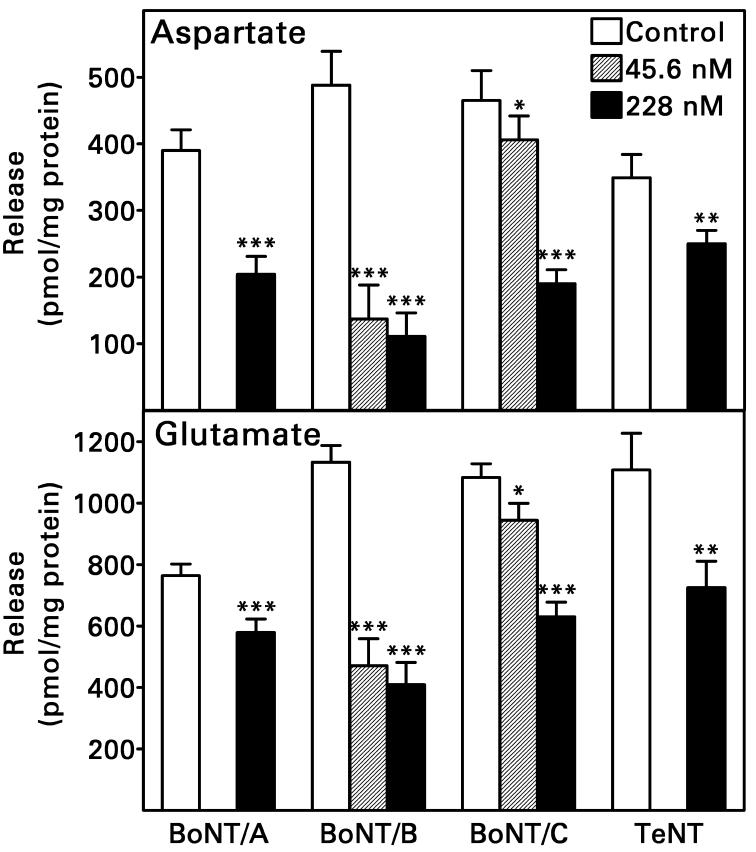
Loading rat hippocampal synaptosomes with the active light chain of tetanus toxin (TeNT) or of BoNT/A, B or C reduced the K+-evoked releases of both aspartate and glutamate (Fig. 1). Analysis of variance revealed no significant difference in the effects of Clostridial toxin light chains on release of the two amino acids; if anything, aspartate release was inhibited to a greater degree. When synaptosomes were permeabilized in the presence of 228 nM light chain, BoNT/B light chain was the most efficacious (68 ± 10% and 56 ± 8% reductions in aspartate and glutamate release, respectively, n = 6) and TeNT light chain was the least efficacious (28 ± 9% and 35 ± 10% reductions in aspartate and glutamate release, respectively, n = 8). For BoNT/C light chain, the inhibitory effect on amino acid release was clearly concentration-dependent. BoNT/B light chain was more potent; a concentration of 45.6 nM reduced both aspartate and glutamate release as effectively as a concentration of 228 nM.
Fig. 1.
Transient permeabilization of rat hippocampal synaptosomes by electroporation in the presence of active Clostridial toxin light chain reduced the subsequent K+-evoked release of aspartate and glutamate. Controls were permeabilized in the presence of heat-inactivated toxin light chain. Values are means ± S.E.M. for n = 6-15 tissue samples from 2-5 rats. Two-way analysis of variance (pretreatment X amino acid) was followed by a Newman-Keuls test when more than one toxin light chain concentration was studied. *P = 0.05, **P <0.005, ***P <0.001, compared to control samples studied in the same experiments. Increasing the concentration of BoNT/C light chain from 45.6 to 228 nM increased its inhibition of aspartate/glutamate release (P <0.001), but increasing the BoNT/B concentration similarly did not (P >0.5). There were no significant interactions between pretreatment and amino acid (P >0.05).
Our results provide the clearest evidence to date that aspartate can be released from hippocampal synaptic terminals by an exocytotic, presumably vesicular, mechanism. Neurotransmitter release requires the formation of a core complex by three SNARE proteins [16]. The Clostridial toxin light chains used in the present study cleave and inactivate one or more of these proteins – SNAP-25 (BoNT/A, BoNT/C), syntaxin 1 (BoNT/C), and synaptobrevin 2 (BoNT/B, TeNT). None completely inhibited aspartate or glutamate release. Incomplete inhibition by the toxin light chains was expected, however, because SNARE proteins already complexed are inaccessible to them [14,21]. The quantitatively different effects of toxin light chains that hydrolyze the same SNARE protein can be explained by differences in affinity and kinetics. For example, the light chains of BoNT/B and TeNT cleave the same peptide bond in synaptobrevin 2, yet BoNT/B light chain inhibited aspartate/glutamate release to a greater degree. This difference is consistent with the greater binding affinity of BoNT/B light chain and its more rapid catalytic rate [8]. Thus aspartate release, at least in hippocampus, depends as much on SNARE protein integrity as glutamate release.
Release of aspartate by exocytosis implies the existence of a mechanism for loading synaptic vesicles with the amino acid. Previous studies indicated that both exogenous and recently-synthesized aspartate gain access to the releasable pool [1,7]. These findings imply that aspartate is transported, but no vesicular transporter for aspartate has been identified and aspartate appears to be neither a substrate for nor an inhibitor of any of the vesicular glutamate transporters [9]. The resistance of aspartate release to bafilomycin A1 [1] suggests that vesicular transport of aspartate may not be proton-dependent. Thus a vesicular aspartate transporter may have escaped detection because the studies assumed that such transport must require the activity of vacuolar H+-ATPase.
Our results are consistent with the hypothesis that the inability of relatively low concentrations of extracellularly-applied Clostridial toxins to inhibit aspartate release [1] reflected limited access of the enzymatically-active, cytoplasmically-released light chain to the aspartate release sites. Due to methodological differences between the two studies, however, further investigations are needed to substantiate the implied physical separation of aspartate and glutamate release sites. The colocalization of vesicular aspartate with vesicular glutamate in synaptic terminals of certain excitatory hippocampal pathways [10,11,13] and its colocalization with vesicular GABA in others [12] suggests a physiological role for aspartate different from that of other transmitter amino acids. We suggested that aspartate release at a distance from the presynaptic active zones should activate extrasynaptic NMDA receptors and that aspartate may indeed be a major endogenous agonist for these receptors [1]. Preliminary electrophysiological data are consistent with this hypothesis [27].
Acknowledgment
This study was supported by NIH grant NS 47096.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bradford SE, Nadler JV. Aspartate release from rat hippocampal synaptosomes. Neuroscience. 2004;128:751–765. doi: 10.1016/j.neuroscience.2004.06.065. [DOI] [PubMed] [Google Scholar]
- 2.Burke SP, Nadler JV. Regulation of glutamate and aspartate release from slices of the hippocampal CA1 area: effects of adenosine and baclofen. J. Neurochem. 1988;51:1541–1551. doi: 10.1111/j.1471-4159.1988.tb01123.x. [DOI] [PubMed] [Google Scholar]
- 3.Burke SP, Nadler JV. Effects of glucose deficiency on glutamate/aspartate release and excitatory synaptic responses in the hippocampal CA1 area in vitro. Brain Res. 1989;500:333–342. doi: 10.1016/0006-8993(89)90329-6. [DOI] [PubMed] [Google Scholar]
- 4.Curras MC, Dingledine R. Selectivity of amino acid transmitters acting at N-methyl-Daspartate and amino-3-hydroxy-5-methyl-4-isoxazolepropionate receptors. Mol. Pharmacol. 1992;41:520–526. [PubMed] [Google Scholar]
- 5.Dunkley PR, Heath JW, Harrison SM, Jarvie PE, Glenfield PJ, Rostas JAP. A rapid Percoll gradient procedure for isolation of synaptosomes directly from an S1 fraction: homogeneity and morphology of subcellular fractions. Brain Res. 1988;441:59–71. doi: 10.1016/0006-8993(88)91383-2. [DOI] [PubMed] [Google Scholar]
- 6.Fleck MW, Barrionuevo G, Palmer AM. Release of D,L-threo-β-hydroxyaspartate as a false transmitter from excitatory amino acid-releasing nerve terminals. Neurochem. Int. 2001;39:75–81. doi: 10.1016/s0197-0186(00)00111-x. [DOI] [PubMed] [Google Scholar]
- 7.Fleck MW, Barrionuevo G, Palmer AM. Synaptosomal and vesicular accumulation of L-glutamate, L-aspartate and D-aspartate. Neurochem. Int. 2001;39:217–225. doi: 10.1016/s0197-0186(01)00018-3. [DOI] [PubMed] [Google Scholar]
- 8.Foran P, Shone CC, Dolly JO. Differences in the protease activities of tetanus and botulinum B toxins revealed by the cleavage of vesicle-associated membrane protein and various sized fragments. Biochemistry. 1994;33:15365–15374. doi: 10.1021/bi00255a017. [DOI] [PubMed] [Google Scholar]
- 9.Fremeau RT, Voglmaier S, Seal RP, Edwards RH. VGLUTs define subsets of excitatory neurons and suggest novel roles for glutamate. Trends Neurosci. 2004;27:98–103. doi: 10.1016/j.tins.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 10.Gundersen V, Chaudry FA, Bjaalie JG, Fonnum F, Ottersen OP, Storm-Mathisen J. Synaptic vesicular localization and exocytosis of L-aspartate in excitatory nerve terminals: a quantitative immunogold analysis in rat hippocampus. J. Neurosci. 1998;18:6059–6070. doi: 10.1523/JNEUROSCI.18-16-06059.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gundersen V, Fonnum F, Ottersen OP, Storm-Mathisen J. Redistribution of neuroactive amino acids in hippocampus and striatum during hypoglycemia: a quantitative immunogold study. J. Cereb. Blood Flow Metab. 2001;21:41–51. doi: 10.1097/00004647-200101000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Gundersen V, Holten AT, Storm-Mathisen J. GABAergic synapses in hippocampus exocytose aspartate on to NMDA receptors: quantitative immunogold evidence for co-transmission. Mol. Cell. Neurosci. 2004;26:156–165. doi: 10.1016/j.mcn.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 13.Gundersen V, Ottersen OP, Storm-Mathisen J. Aspartate- and glutamate-like immunoreactivities in rat hippocampal slices: depolarization-induced redistribution and effects of precursors. Eur. J. Neurosci. 1991;3:1281–1299. doi: 10.1111/j.1460-9568.1991.tb00061.x. [DOI] [PubMed] [Google Scholar]
- 14.Hayashi T, McMahon H, Yamasaki S, Binz T, Hata Y, Südhof TC, Niemann H. Synaptic vesicle membrane fusion complex: action of Clostridial neurotoxins on assembly. EMBO J. 1994;13:5051–5061. doi: 10.1002/j.1460-2075.1994.tb06834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herrero MT, Oset-Gasque MJ, Cañadas S, Vicente S, González MP. Effect of various depolarizing agents on endogenous amino acid neurotransmitter release in rat cortical neurons in culture. Neurochem. Int. 1998;32:257–264. doi: 10.1016/s0197-0186(97)00088-0. [DOI] [PubMed] [Google Scholar]
- 16.Jahn R, Südhof TC. Membrane fusion and exocytosis. Annu. Rev. Biochem. 1999;68:863–911. doi: 10.1146/annurev.biochem.68.1.863. [DOI] [PubMed] [Google Scholar]
- 17.McMahon HT, Foran P, Dolly JO, Verhage M, Wiegant VM, Nicholls DG. Tetanus toxin and botulinum toxins type A and B inhibit glutamate, γ-aminobutyric acid, aspartate, and met-enkephalin release from synaptosomes. J. Biol. Chem. 1992;267:21338–21343. [PubMed] [Google Scholar]
- 18.Nadler JV, Martin D, Bustos GA, Burke SP, Bowe MA. Regulation of glutamate and aspartate release from the Schaffer collaterals and other projections of CA3 hippocampal pyramidal cells. Prog. Brain Res. 1990;83:115–130. doi: 10.1016/s0079-6123(08)61245-5. [DOI] [PubMed] [Google Scholar]
- 19.Nadler JV, Vaca KW, White WF, Lynch GS, Cotman CW. Aspartate and glutamate as possible transmitters of excitatory hippocampal afferents. Nature. 1976;260:538–540. doi: 10.1038/260538a0. [DOI] [PubMed] [Google Scholar]
- 20.Nicholls D, Attwell D. The release and uptake of excitatory amino acids. Trends Pharmacol. Sci. 1990;11:462–468. doi: 10.1016/0165-6147(90)90129-v. [DOI] [PubMed] [Google Scholar]
- 21.Otto H, Hanson PI, Jahn R. Assembly and disassembly of a ternary complex of synaptobrevin, syntaxin, and SNAP-25 in the membrane of synaptic vesicles. Proc. Natl. Acad. Sci. USA. 1997;94:6197–6201. doi: 10.1073/pnas.94.12.6197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patneau DK, Mayer ML. Structure-activity relationships for amino acid transmitter candidates acting at N-methyl-D-aspartate and qusiqualate receptors. J. Neurosci. 1990;10:2385–2399. doi: 10.1523/JNEUROSCI.10-07-02385.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramanathan M, Kuo H-R, Lambert CW, Ingoglia NA. Introduction of macromolecules into synaptosomes using electroporation. J. Neurosci. Meth. 2000;96:19–23. doi: 10.1016/s0165-0270(99)00182-x. [DOI] [PubMed] [Google Scholar]
- 24.Rossetto O, Morbiato L, Caccin P, Rigoni M, Montecucco C. Presynaptic enzymatic neurotoxins. J. Neurochem. 2006;97:1534–1545. doi: 10.1111/j.1471-4159.2006.03965.x. [DOI] [PubMed] [Google Scholar]
- 25.Schiavo G, Matteoli M, Montecucco C. Neurotoxins affecting neuroexocytosis. Physiol. Rev. 2000;80:717–766. doi: 10.1152/physrev.2000.80.2.717. [DOI] [PubMed] [Google Scholar]
- 26.Simpson LL. Identification of the major steps in botulinum toxin action. Annu. Rev. Pharmacol. Toxicol. 2004;44:167–193. doi: 10.1146/annurev.pharmtox.44.101802.121554. [DOI] [PubMed] [Google Scholar]
- 27.Zhang X-Y, Wang L, Nadler JV. Exocytotic release of aspartate and its possible function in rat hippocampus studied with Clostridial toxins. Soc. Neurosci. Abstr. 2006;36 #36.14. [Google Scholar]
- 28.Zhou M, Peterson CL, Lu Y-B, Nadler JV. Release of glutamate and aspartate from CA1 synaptosomes: selective modulation of aspartate release by ionotropic glutamate receptors ligands. J. Neurochem. 1995;64:1556–1566. doi: 10.1046/j.1471-4159.1995.64041556.x. [DOI] [PubMed] [Google Scholar]