Abstract
The lectin pathway of complement activation is used by a collectin, mannan-binding lectin (MBL), and two ficolins, L-ficolin and H-ficolin, to opsonize microorganisms for phagocytosis. We published evidence recently that MBL insufficiency is associated with recurrent respiratory infections in childhood. We have now measured serum L-ficolin in 313 respiratory infection patients and 74 healthy control children. L-ficolin concentrations below the lower limit of the control group were found in 6% of the patients (P < 0·02) and were associated most strongly with children having co-existing atopic disorders (11%; P = 0·002). We suggest that L-ficolin may have a role in protection from microorganisms complicating allergic disease.
Keywords: allergy, innate immunity, L-ficolin, mannan-binding lectin (MBL), respiratory infections
INTRODUCTION
A miscellany of humoral factors are believed to be of importance in the innate immune system [1]. One such factor, mannan-binding lectin (MBL), is a member of the collectin subfamily of C-type lectins that possess a collagen-like region in addition to a C-type lectin domain [2]. MBL deficiency or insufficiency is associated with susceptibility to infections generally, and is thought to influence the course of many diseases [3,4]. MBL functions as an opsonin, and although it can opsonize microorganisms independently of complement [5], its main influence in vivo is thought to arise from activating MBL-associated serine protease-2 (MASP-2) and thus initiating the lectin pathway of complement activation [6]. Biologically relevant MBL [usually detected by enzyme-linked immunosorbent assay (ELISA)] is actually functional MBL, consisting of higher oligomers of the basic triplet subunit; only those multimeric forms can bind to carbohydrate patterns with high avidity and are capable of complement activation [7,8].
MBL is the only collectin known to activate the lectin pathway of complement activation, but two other plasma proteins, L-ficolin (P35) and H-ficolin (Hakata antigen), share this property [9]. Ficolins, like collectins, possess a collagen-like region, but it is combined with a fibrinogen-type carbohydrate recognition domain, not a C-type lectin domain [9]. Ficolins also form a basic subunit in which a triple collagen-like helix forms a tail, with the fibrinogen-like lectin domains forming a cluster of globular heads; these subunits then oligomerize to form the functional protein [9,10]. L-ficolin has been shown to bind several species of bacteria, opsonizing them and activating complement in a manner similar to that of MBL [11,12].
Recently, MBL insufficiency, with or without a co-existing immune abnormality, was found to be associated with respiratory infections in childhood, and this relationship was particularly strong in subjects with concomitant impairments of humoral immunity [13]. We have now extended our investigations to assess the possible influence of L-ficolin in a similar cohort of children.
PATIENTS AND METHODS
Patients
The patients were 313 children with recurrent respiratory tract infections who attended the Polish Mothers’ Memorial Hospital in Lodz for investigation of possible immune deficiency. Ages ranged from 1 to 16 years (mean 8 years), and were included in this study on the basis of having had either two serious respiratory infections (e.g. pneumonias) in 1 year, or a minimum of eight upper respiratory infections within the same year.
All patients were investigated for possible immune abnormalities [total blood count and differential; immunoglobulins; natural killer (NK) cells, B cells and T cell subsets; CH50 and complement components, etc.] as described previously [13]. On that basis, the patients were divided into five categories for analysis: group I, patients with no detectable laboratory immune abnormalities (n = 95); group II, patients with atopic disorders (mainly asthma or allergic rhinitis) and a few with elevated IgE only (n = 90); group III, patients with abnormalities of humoral immunity (mainly hypogammaglobulinaemia or selective IgA deficiency) (n = 65); group IV, patients with cellular abnormalities (mainly T cell lymphopenias) (n = 46); and group X, patients with both humoral and cellular defects (n = 17). (Further patient details are provided in [13], but an important difference is that in this study patients in group X are unique to group X. In our previous analysis concerning MBL, the patients in group X were also included in groups III and IV). Blood samples for serum preparation were obtained from patients when no signs or symptoms of infection were evident to the attending physicians. Control sera were obtained from 74 age-matched children attending the same hospital for reasons unconnected with infections or respiratory disease.
Approval of the local ethical commission was obtained, as was informed parental consent.
Laboratory methods
L-ficolin was assayed by ELISA, as described elsewhere [14]. Briefly, antigen was captured using one monoclonal antibody (GN4) to L-ficolin on the solid phase and detected using another biotinylated monoclonal antibody (GN5). The interassay coefficient of variation during the duration of these measurements was 12%; tests were repeated at least once on all sera giving relatively low values.
Serum MBL concentration and MBL genotype data used in analyses were obtained previously [13]. The routine laboratory tests used to assess immune status and the local reference ranges used to define abnormality have been published elsewhere [15].
Statistics
The statistical analyses were performed using Prism for Windows software from Graph Pad (San Diego, CA, USA). Median values were compared by the Mann–Whitney U-test, and proportions of low : normal by Fisher's exact test (two-sided).
RESULTS
L-ficolin levels in patients and controls
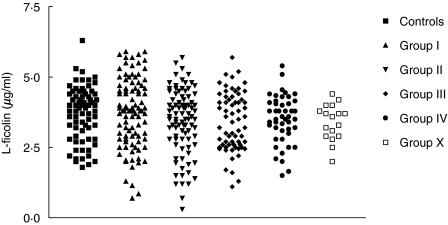
The median L-ficolin concentration in the 313 patients as a single group did not differ significantly from that of the 74 controls (3·7 µg/ml versus 3·95 µg/ml; P = 0·19). However, low values were obviously more common in patients (Fig. 1) and that is reflected in the statistics for each patient subgroup, as summarized in Table 1.
Fig. 1.
L-ficolin concentrations according to patient classification. The patients and groups were classified as described in the Patients and Methods section and compared to the values from the group of control children without respiratory diseases.
Table 1.
L-ficolin concentrations in patients and controls
| L-ficolin (µg/ml) | ||||
|---|---|---|---|---|
| Range | Mean | Median | Proportion < 1·8 µg/ml* | |
| Control group | 1·8–6·3 | 3·72 | 3·95 | |
| Patient group I | 0·7–5·9 | 3·75 | 3·8 | 4·2% (P = 0·135) |
| Patient group II | 0·3–5·7 | 3·45 | 3·6 | 11·1% (P = 0·002) |
| Patient group III | 1.1–5.7 | 3.41 | 3.4 | 6.1% (P = 0.046) |
| Patient group IV | 1.5–5.4 | 3.51 | 3.55 | 4.3% (P = 0.145) |
| Patient group X | 2.0–4.4 | 3.4 | 3.6 | 0% |
Significance values refer to comparisons with the control group using Fisher's exact test (two-sided).
The distribution of values in the control group was Gaussian, with the lowest concentration at 1.8 µg/ml. If <1.8 µg/ml is taken as the definition of relative deficiency for the purpose of analysis, a significant proportion of patients (6.4%) had abnormally low levels (P = 0.019; Fisher's exact test). Moreover, L-ficolin deficiency so defined was associated most strongly with patient group II, children with atopic disorders (11%; P = 0.002). Indeed, of the other patient groups, only group III came close to achieving statistical significance. The value of 1.8 µg/ml also corresponds to the mean minus 2 s.d., and if the data are re-analysed at the slightly different level of ≤1.8 µg/ml, the values in patient group II still achieve a statistically significant difference from those in the control group (P = 0.01).
Alternatively, the 5th centile (2.0 µg/ml) could be used as a cut-off value for analysis, as any cut-off is to some extent arbitrary and the 5th centile undeniably represents relatively low values. At that level, only patient group II (14%; P = 0.07) exhibited a difference of borderline statistical significance.
The proportions of values at or above the 95th centile (≥5.0 µg/ml) in the various patient groups were also compared to the controls. Patient group I was found to have a significantly higher proportion (18.9%; P = 0.01), but not even the slightest trend in that direction was apparent in the other groups. This relative abundance of high values in group I accounts for its having similar mean and median values to the control group.
Of the patients in group II with L-ficolin <1.8 µg/ml, five (50%) had asthma and four (40%) had allergic rhinitis. The corresponding proportions (46% and 38%, respectively) were similar when ≤2.0 µg/ml was used as a cut-off value. In patient group II as a whole, 39% had asthma and 43% had allergic rhinitis.
Relationship between L-ficolin and MBL
There was no correlation whatsoever between L-ficolin and MBL in the patients’ sera (Spearman's r = 0.07) or in the control sera (r = 0.06).
DISCUSSION
Because MBL functions as an opsonin via the lectin pathway of complement activation, it is widely assumed that the ficolins, P35 ( L-ficolin) and the Hakata antigen (H-ficolin) function in a similar or complementary manner. This assumption is supported by the ability of H-ficolin to inhibit the growth of Aerococcus viridans [16], but in contrast to MBL, there have been few disease association studies involving ficolins. H-ficolin concentrations correlated inversely with severity of hepatic cirrhosis [17] and correlated positively with disease activity in SLE [18], but neither association can be linked to infection. L-ficolin concentrations were excessively low in a few patients with recurrent miscarriage [14], and were decreased markedly in haematology patients who had received chemotherapy (in contrast to MBL), but there was no relationship evident with susceptibility to infection (again in contrast to MBL) in these chemotherapy patients [19].
There is some evidence for single nucleotide polymorphisms in the human L-ficolin gene [20] and its promoter region [21], giving rise to allelic variation that could influence serum concentration. This work is preliminary, however, and has been published only in abstract form. Nevertheless, L-ficolin concentration varies only about fivefold in most healthy adults, in contrast to the corresponding 1000-fold concentration range for MBL. It could therefore be argued that L-ficolin deficiency does not exist, that everyone has an adequate circulating level of the protein and that abnormally low concentrations, such as are found in some chemotherapy patients, arise simply as a result of disease or its treatment and are not the cause of disease or disease complications. Indeed, it is reasonable to assume that chemotherapy results in a lowering of circulating L-ficolin. However, in the present investigation, blood was sampled when the patients were well, so it seems more likely that low L-ficolin concentration influences disease rather than being caused by disease.
The investigation described here is by far the largest disease association study concerning L-ficolin, and the first to show a significant association with human infectious disease. The data pattern illustrated in Fig. 1 is superficially similar to that published recently for MBL with a similar series of patients [13], with a minority of low values found in the various patient subgroups. The disease association is weaker with L-ficolin than with MBL, but what is more striking is the relative strengths of association within the patient subgroups. The strongest and most significant association with childhood respiratory infections was in the allergy/atopic group. This contrasts starkly with the MBL association, which was linked most strongly with patients having a concomitant defect of humoral immunity and which was in no way enhanced by the co-existence of allergic signs or symptoms.
This special relationship with atopic patients was not a prior hypothesis and therefore should be regarded with caution until independently confirmed. Nevertheless, it is plausible that L-ficolin may be particularly important in protection from microorganisms complicating allergic diseases affecting the lungs. It may be relevant that only L-ficolin has been shown to activate complement after binding to lipoteichoic acid, a common cell-wall component of Gram-positive bacteria [12]. Although MBL can bind some Gram-positive bacteria, most notably Staphylococcus aureus [5,22,23], there was no detectable interaction between MBL and Streptococcus pneumoniae [20], a bacterium L-ficolin might be expected to bind. We suggest that L-ficolin may recognize and opsonize a different spectrum of microorganisms from MBL, and so exhibit functional complementarity rather than redundancy.
Acknowledgments
The Polish authors co-operate within the Polish Research Network, ‘The molecular basis of immunity’.
References
- 1.Beutler B. Innate immunity: an overview. Mol Immunol. 2004;40:845–59. doi: 10.1016/j.molimm.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 2.Van De Wetering JK, Van Golde LMG, Batenburg JJ. Collectins. Players of the innate immune system. Eur J Biochem. 2004;271:1229–49. doi: 10.1111/j.1432-1033.2004.04040.x. [DOI] [PubMed] [Google Scholar]
- 3.Kilpatrick DC. Mannan-binding lectin. clinical significance and applications. Biochim Biophys Acta. 2002;1572:401–13. doi: 10.1016/s0304-4165(02)00321-5. [DOI] [PubMed] [Google Scholar]
- 4.Kilpatrick DC. Mannan-binding lectin and its role in innate immunity. Transfus Med. 2002;12:335–51. doi: 10.1046/j.1365-3148.2002.00408.x. [DOI] [PubMed] [Google Scholar]
- 5.Neth O, Jack DL, Johnson M, Klein NJ, Turner MW. Enhancement of complement activation and opsonophagocytosis by complexes of mannose-binding lectin with mannose-binding lectin-associated serine protease after binding to Staphylococcus aureus. J Immunol. 2002;169:4430–6. doi: 10.4049/jimmunol.169.8.4430. [DOI] [PubMed] [Google Scholar]
- 6.Fujita T, Matsushita M, Endo Y. The lectin-complement pathway − its role in innate immunity and evolution. Immunol Rev. 2004;198:185–202. doi: 10.1111/j.0105-2896.2004.0123.x. [DOI] [PubMed] [Google Scholar]
- 7.Garred P, Larsen F, Madsen HO, Koch C. Mannose-binding lectin deficiency − revisited. Mol Immunol. 2003;40:73–84. doi: 10.1016/s0161-5890(03)00104-4. [DOI] [PubMed] [Google Scholar]
- 8.Terai T, Kobayashi K, Matsushita M, Miyakawa H, Mafune N, Kikuta H. Relationship between gene polymorphisms of mannose-binding lectin (MBL) and two molecular forms of MBL. Eur J Immunol. 2003;33:2755–63. doi: 10.1002/eji.200323955. [DOI] [PubMed] [Google Scholar]
- 9.Lu J, Teh C, Kishore U, Reid KBM. Collectins and ficolins: sugar pattern recognition molecules of the mammalian immune system. Biochim Biophys Acta. 2002;1572:387–400. doi: 10.1016/s0304-4165(02)00320-3. [DOI] [PubMed] [Google Scholar]
- 10.Ohashi T, Erickson HP. The disulphide bonding pattern in ficolin multimers. J Biol Chem. 2004;279:6534–9. doi: 10.1074/jbc.M310555200. [DOI] [PubMed] [Google Scholar]
- 11.Matsushita M, Endo Y, Taira S, et al. A novel human serum lectin with collagen- and fibrinogen-like domains that functions as an opsonin. J Biol Chem. 1996;271:2448–54. doi: 10.1074/jbc.271.5.2448. [DOI] [PubMed] [Google Scholar]
- 12.Lynch NJ, Roscher S, Hartung T, et al. L-ficolin specifically binds to lipoteichoic acid, a cell wall constituent of Gram-positive bacteria, and activates the lectin pathway of complement. J Immunol. 2004;172:1198–202. doi: 10.4049/jimmunol.172.2.1198. [DOI] [PubMed] [Google Scholar]
- 13.Cedzynski M, Szemraj J, Swierzko AS, et al. Mannan-binding lectin insufficiency in children with recurrent infections of the respiratory system. Clin Exp Immunol. 2004;136:304–11. doi: 10.1111/j.1365-2249.2004.02453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kilpatrick DC, Fujita T, Matsushita M. P35, an opsonic lectin of the ficolin family, in human blood from neonates, normal adults, and recurrent miscarriage patients. Immunol Lett. 1999;67:109–12. doi: 10.1016/s0165-2478(98)00147-3. [DOI] [PubMed] [Google Scholar]
- 15.Zeman K, editor. Immunodeficiency diseases in children. 2. Warsaw: PZWL; 2003. [in Polish]. [Google Scholar]
- 16.Tsujimura M, Miyazaki T, Kojima E, et al. Serum concentration of Hakata antigen, a member of the ficolins, is linked with inhibition of Aerococcus viridans growth. Clin Chim Acta. 2002;325:139–46. doi: 10.1016/s0009-8981(02)00274-7. [DOI] [PubMed] [Google Scholar]
- 17.Fukutomi T, Ando B, Sakamoto S, Sakai H, Nawata H. Thermolabile β-2 macroglobulin (Hakata antigen) in liver disease: biochemical and immunohistochemical study. Clin Chim Acta. 1996;255:93–106. doi: 10.1016/0009-8981(96)06393-0. [DOI] [PubMed] [Google Scholar]
- 18.Yoshizawa S, Nagasawa K, Yae Y, Niho Y, Okochi K. A thermolabile β2-macroglobulin (TMG) and the antibody against TMG in patients with systemic lupus erythematosus. Clin Chim Acta. 1997;264:219–25. doi: 10.1016/s0009-8981(97)00078-8. [DOI] [PubMed] [Google Scholar]
- 19.Kilpatrick DC, McLintock LA, Allan EK, et al. No strong relationship between mannan-binding lectin or plasma ficolins and chemotherapy-related infections. Clin Exp Immunol. 2003;134:279–84. doi: 10.1046/j.1365-2249.2003.02284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hummelshoj T, Madsen HO, Fujita T, Matsushita M, Garred P. Identification and functional analysis of two amino acid substituting polymorphisms in the ficolin-2 gene (FCN2) Mol Immunol. 2004;41 :Abstract 98. [Google Scholar]
- 21.Fog LM, Hummelshoj T, Madsen HO, Fujita T, Matsushita M, Garred P. Promoter polymorphisms in the FCN2 gene. Mol Immunol. 2004;41 :Abstract 59. [Google Scholar]
- 22.Neth O, Jack DL, Dodds AW, Holzel H, Klein NJ, Turner MW. Mannose-binding lectin binds to a range of clinically relevant microorganisms and promotes complement activation. Infect Immun. 2000;68:688–93. doi: 10.1128/iai.68.2.688-693.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi L, Takahashi K, Dundee J, et al. Mannose-binding deficient mice are susceptible to infection with Staphylcoccus aureus. J Exp Med. 2004;199:1379–90. doi: 10.1084/jem.20032207. [DOI] [PMC free article] [PubMed] [Google Scholar]