Abstract
Although some information is available regarding immune activation in familial Mediterranean fever (FMF), little is known about either peripheral blood T cell activation marker expression or the T cell proliferative response to phytohaemagglutinin (PHA). In the present study, we aimed to investigate the percentages of peripheral blood lymphocyte subsets, T cell expression of cellular activation markers (CD25, CD69, HLA-DR), the T cell response to PHA and serum levels of soluble interleukin-2 receptor (sIL-2R) and interleukin (IL)-10 in patients with FMF. Forty patients with FMF were enrolled into the study. Control groups were sex- and age-matched and consisted of 20 healthy blood donors and 15 patients with inactive Behçet's disease. The patients with FMF in an attack period had higher levels of sIL-2R than those in an attack-free period, and also in comparison with both control groups. The levels of sIL-2R were also found to be higher in patients with FMF in an attack-free period than those in both control groups. The mean levels of IL-10 were found to be lower in patients with FMF in an attack-free period than those in an attack period and were also lower than those in the healthy controls. In an acute attack period, the absolute counts of CD3+HLA-DR+, CD4+CD69+, CD8+CD25+ and CD8+CD69+ T cells in peripheral blood samples were also higher than those in both control groups. Both the percentages and absolute counts of CD4+CD69+ T cells in peripheral blood samples of patients with FMF in an attack-free period were slightly but significantly higher than those in the healthy controls. In conclusion, our study indicates that the T cell system is abnormally activated in patients with FMF in both the attack and attack-free period and that decreased IL-10 levels may create a tendency to perpetuate subclinical immune activation in the attack-free period.
Keywords: familial Mediterranean fever, IL-10, lymphocyte culture, sIL-2R, T cell activity
INTRODUCTION
Familial Mediterranean fever (FMF) is a systemic relapsing autoinflammatory disorder occurring in populations originating from the Mediterranean basin, mainly Turks, Levantine Arabs, Sephardic Jews, Druze and Armenians [1,2]. The disease is characterized by periodic attacks of fever accompanied by serosal membrane inflammation at the affected sites such as peritoneum, pleura or synovium, with a massive influx of polymorphonuclear neutrophils [3]. The attacks are self-limited, lasting 1–3 days, and between episodes the individual is usually free of symptoms. The gene (MEFV) causing the disease maps to the short arm of chromosome 16, encodes a leucocyte- and monocyte-specific inflammatory regulator, and its mutations cause the autoinflammatory phenotype of FMF [4]. Colchicine inhibits leucocyte chemotaxis through a direct effect on cytoplasmic microtubules and is effective in treating or aborting the acute recurrent exacerbations of the disease.
Some immunological abnormalities in FMF, including changes in T and B cells and cytokines, have been reported previously [5–7]. Moreover, impaired tumour necrosis factor-α (TNF-α) production by peripheral mononuclear cells [8,9], while interleukin-1 (IL-1) activity is decreased [10], has been suggested in patients with FMF. In recent studies, IL-6 [11,12] and TNF-α [12] levels were found to be higher in patients with an acute FMF attack compared with those in an attack-free period [11]. Although some information is available regarding immune activation in FMF [12,13], little is known about T cell activation marker expression in peripheral blood and the T cell proliferative response to phytohaemagglutinin (PHA). Therefore, we aimed to investigate the percentages of peripheral blood lymphocyte subsets, T cell expression of cellular activation markers (CD25, CD69, HLA-DR), the T cell response to PHA, and the serum levels of soluble interleukin-2 receptor (sIL-2R) and IL-10 in patients with FMF. Outcomes were compared with a healthy control group and patients with inactive Behçet's disease (BD).
MATERIALS AND METHODS
Patients
Forty patients with FMF (32 male, eight female) and 20 sex- and age-matched healthy volunteers (16 male, four female) were enrolled into the study. Fifteen sex- and age-matched patients (12 men and three women) with BD were also included as a disease control group. None of the patients with FMF had an immunological disorder or another rheumatic disease. The clinical diagnosis of FMF was based on the Tel-Hashomer criteria [14]. Attack-free periods (defined as being free of attacks for at least 3 weeks) and acute phases were determined based on clinical (fever, abdominal pain, arthritis) and laboratory findings (high levels of fibrinogen, white blood cell (WBC) counts and erythrocyte sedimentation rate (ESR)). Nineteen out of 40 patients (14 male, five female) were evaluated only during an attack-free period, 11 patients (10 male, one female) were evaluated only during an attack period. In addition, in order to eliminate inter-individual variability, 10 of the 40 patients (nine male, one female) who donated blood during an attack gave an additional blood sample during an attack-free period, 3–10 weeks after the last attack (double donors). The immunological parameters of patients with active clinical presentations were measured within the first 48 h following the onset of attack.
BD patients were diagnosed using the diagnostic criteria of the International Study Group for Behçet's Disease [15]. They had at least one of the major clinical signs and symptoms (oral ulcer, genital ulcers and cutaneous lesions), and were positive for the pathergy test. All patients with BD, except for six newly diagnosed individuals, were taking colchicine (0·5–2 mg/24 h). None of the BD patients was receiving either steroid or immunosuppressive drugs at the time of the examination. Samples were taken during an inactive period of the disease. All patients and control subjects were informed about the aim and procedures of the study and gave their consent. The study was approved by the Ethical Committee of Gülhane School of Medicine.
Ten millilitres of venous blood was drawn and centrifuged at 3000 r.p.m. for 30 min for the measurement of IL-10 and sIL-2R levels. The specimens were stored at −20°C until analysis. For the determination of lymphocyte subsets, subgroups and cell cultures, peripheral blood samples (4 and 10 ml, respectively) were drawn into tubes with acid citrate dextrose (ACD) and heparin, and analysed on the same day.
Analyses
WBC count.
WBC numbers were counted by using an automated whole blood counter (Cell-Dyn 1700, Abbot, Santa Clara, CA, USA) and the reference range was between 3600 and 10 000/mm3.
ESR.
The Westergreen method was used. Normal levels were 0–15 mm/h for males and 0–20 mm/h for females.
Fibrinogen.
Fibrinogen levels were measured by a photo-optic method using a fibrinogen reagent (Sigma–Aldrich Chemie GmbH, Taufkirchen, Germany) and an Amelung AMAX 190 Plus analyser.
Serum IL-10 and sIL-2R levels
The levels of serum IL-10 (Cytimmune Sciences Inc., College Park, MD, USA) and sIL-2R (Bender MedSystems Diagnostics GmbH, Vienna, Austria) were measured by enzyme immunoassay (EIA). Absorbance readings were carried out on EL 312e Biokinetics Reader. Concentrations of unknown samples were determined from a curve obtained with the standards. According to the kit information, the sensitivities were 1·6 pg/ml and 0·036 ng/ml; intra-assay variations ±8·4% and ±1·3%; interassay variations ±10·2% and ± 1·9% for IL-10 and sIL-2R, respectively.
Determination of lymphocyte subgroups in peripheral blood samples
Flow cytometry (FACSCalibur, Becton Dickinson Co., San Jose, CA, USA) was used to analyse cells labelled with monoclonal antibodies conjugated with a flourochrome, which had bound to specific cell surface molecules. The panel of monoclonal antibodies used for the procedure is given in Table 1.
Table 1.
The panel of monoclonal antibodies used for the determination of lymphocyte subgroups in peripheral blood samples
| Panel | Kit | Clone | Isotype | Flourochrome |
|---|---|---|---|---|
| CD45/CD14 | Simultest™ LeucoGATE™ | CD45/CD14 | CD45; IgG1 | CD45; FITC |
| CD45; 2D1 | CD14; MΦP9 | CD14; IgG2 | CD14; PE | |
| IgG1/IgG2 (control) | Simultest™ control γ1γ2a(IgG1/IgG2) | γ1(IgG1); X40 | X40; IgG1 | – |
| γ2(IgG2); X39 | X39; IgG2 | – | ||
| CD4/CD8 | Simultest™ CD4/CD8 | CD4; SK3 | CD4; IgG1 | CD4; FITC |
| CD8; SK1 | CD8; IgG1 | CD8; PE | ||
| CD4/CD69 | CD4 (Leu™-3a) | CD4; SK3 | CD4; IgG1 | CD4; FITC |
| CD69 (Leu™-23) | CD69; L78 | CD69; IgG1 | CD69; PE | |
| CD8/CD69 | CD8 (Leu™-3a) | CD8; SK3 | CD8; IgG1 | CD8; FITC |
| CD69 (Leu™-23) | CD69; L78 | CD69; IgG1 | CD69; PE | |
| CD4/CD25 | CD4 (Leu™-3a) | CD4; SK3 | CD4; IgG1 | CD4; FITC |
| CD25 (anti-IL-2R) | CD25; 2A3 | CD25; IgG1 | CD25; PE | |
| CD8/CD25 | CD8 (Leu™-2a) | CD8; SK3 | CD8; IgG1 | CD8; FITC |
| CD25 (anti-IL-2R) | CD25; 2A3 | CD25; IgG1 | CD25; PE | |
| CD3/CD19 | Simultest™ CD3/CD19 | CD3; SK7 | CD3; IgG1 | CD8; FITC |
| CD19; 4G7 | CD19; IgG1 | CD25; PE | ||
| CD3/CD16CD56 | Simultest™ CD3/CD16+CD56 | CD3; SK7 | CD3; IgG1 | CD3; FITC |
| CD16; B73·1 | CD16; IgG1 | CD16+ CD56; PE | ||
| CD56; MY31 | CD56; IgG1 | |||
| CD3/HLA-DR | Simultest™ CD3/anti-HLA-DR | CD3; SK7 | CD3; IgG1 | CD3; FITC |
| Anti-HLA-DR; L243 | Anti-HLA-DR; IgG2 | Anti-HLA-DR; PE |
FitC: fluorescein isotyocyanate; PE: phycoerythrin.
For the determination of lymphocyte subgroups, 20 µl of monoclonal antibodies and 100 µl of blood samples drawn previously into tubes with ACD were collected into a single tube, and the cells were labelled via incubation for 20 min in the dark and at the temperature recommended by the manufacturer (Becton Dickinson Co., San Jose, CA, USA). Erythrocytes were lysed using FACS lysing solution (Becton Dickinson Co.) and removed by washing with phosphate-buffered saline (PBS). The tubes were then prepared for analysis by the addition of PBS containing 1% paraformaldehyde to the cells. Analyses were carried out with the cellquest software program (Becton Dickinson).
Cell culture and T cell activation marker analysis
Cells were cultured from 26 FMF patients in both an attack and an attack-free period, 20 healthy controls and 15 patients with BD. Peripheral heparinized blood was diluted with serum saline (at a ratio of 1 : 1), and mononuclear cells were obtained by standard Ficoll-Hypaque separating solution (Seromed®, Biochrom KG Berlin, Germany) gradient centrifugation. Mononuclear cells were harvested from the interface using a sterile Pasteur pipette. The cells were then washed twice with RPMI-1640 medium (Sigma Chemical Co., St Louis, MO, USA) and resuspended in the same medium containing 100 U/ml penicillin, 100 µg/ml streptomycin (Sigma Chemical Co.) and 10% heat-inactivated fetal calf serum (FCS) (Biological Industries, Kibbutz, Beit, Haemek, Israel). For depletion of monocytes, the cells were seeded in a culture flask and incubated at 37°C in a 5% CO2 humidified atmosphere for 1 h to allow the monocytes to attach to the flask bed. The medium containing non-adherent cells was transferred to another culture flask and incubated for an additional hour to further deplete the monocytes. At the end of this time, the non-adherent cell suspension was collected and the cells washed once with RPMI-1640 medium. The viability of the cells was found to be 98% by staining with acridine-orange. The cells were then analysed by flow cytometry and both lymphocyte and monocyte cell populations were gated in side–forward scattering (SSC–FSC) and CD45/CD14 plots. In this way, the percentage of lymphocytes was found to be 97% following monocyte depletion.
Cells were cultured at a concentration of 106 cell/ml in 24-well plates (Costar, Cambridge, MA, USA) with RPMI-1640 medium containing 100 U/ml penicillin, 100 µg/ml streptomycin and 10% FCS, and incubated for 24 h in a humidified atmosphere with 5% CO2. Cultures were either left unstimulated or stimulated with PHA (15 µg/ml) (PHA-M: Bacto®, Difco Laboratories, Detroit, MI, ISA) for evaluation of CD25, CD69 and HLA-DR expression on CD3+ T cells. At the end of 24 h, cells were centrifuged, washed and resuspended in PBS. For the analysis of activation markers expressed on T cells, cells were incubated with the appropriate monoclonal antibodies for 20 min, washed and fixed for flow cytometry analysis. The panel of monoclonal antibodies used is given in Table 2. T cells were gated in a SSC–FSC plot and analysed for CD25, CD69 and HLA-DR expression on CD3+ T cells (Fig. 1).
Table 2.
The panel of monoclonal antibodies used for T cell activation marker analysis
| Panel | Kit | Clone | Isotype | Flourochrome |
|---|---|---|---|---|
| CD45/CD14 | Simultest™ LeucoGATE™ | CD45; 2D1 | CD45; IgG1 | CD45; FITC |
| CD45/CD14 | CD14; MΦP9 | CD14; IgG2 | CD14; PE | |
| IgG1/IgG2(control) | Simultest™ control γ1γ2a | γ1(IgG1); X40 | X40; IgG1 | – |
| (IgG1/IgG2) | γ2(IgG2); X39 | X39; IgG2 | – | |
| CD3/CD25 | CD3 (Leu™-4) | CD3; SK7 | CD3; IgG1 | CD3; FITC |
| CD25 (anti-IL-2R) | CD25; 2A3 | CD25; IgG1 | CD25; PE | |
| CD3/CD69 | CD3 (Leu™-4) | CD3; SK7 | CD3; IgG1 | CD3; FITC |
| CD69 (Leu™-23) | CD69; L78 | CD69; IgG1 | CD69; PE | |
| CD3/HLA-DR | Simultest™ | CD3; SK7 | CD3; IgG1 | CD3; FITC |
| CD3/anti-HLA-DR | Anti-HLA-DR; L243 | Anti-HLA-DR; IgG2 | Anti-HLA-DR; PE |
FitC: fluorescein isotyocyanate; PE: phycoerythrin.
Fig. 1.
The percentages of CD3+CD25+, CD3+CD69+ and CD3+HLA-DR+ T cells in unstimulated (a) and stimulated (b) cultures. The T cell population was gated in an SSC–FSC plot and analysed for CD25, CD69 and HLA-DR expression in the CD3+ T cell population. The percentages represent the net percentage (%) of positive cells in the appropriate quadrant. The dot-plots are from a single experiment representative of the results obtained with patients in an attack-free period of FMF.
Statistical analysis
All the statistical analyses were performed using the spss (SPSS 10·0, SPSS Inc., Chicago, IL, USA) statistical package. Descriptive statistics were presented as arithmetic mean ±standard deviation. For the tests of normality, we used the Kolmogorov–Smirnov test. Multiple comparisons were performed using the Kruskal–Wallis test. The differences between the two groups were evaluated by the Mann–Whitney U-test. For repeated measurements we used the paired-samples test or the Wilcoxon's signed-rank test. To investigate the relations among the variables, we used the Spearman's rank correlation test. P-values less than or equal to 0·05 were evaluated as statistically significant [16].
RESULTS
Twenty-one patients with FMF in an attack period had abdominal pain (100%), eight (38%) had pleuritic chest pain and three (14%) had arthritis/arthralgia. No significant differences were found between the FMF patients and both control groups with respect to mean age (data not shown). Sixteen of the 40 FMF patients (40%) had a family history of the disease. Thirty-one patients with FMF were on a continuous colchicine treatment (1–2 mg/day). The nine newly diagnosed FMF patients were not receiving treatment.
Comparisons of the acute phase reactants, serum sIL-2R and IL-10 levels in all groups are given in Table 3 and Fig. 2. The mean levels of ESR, fibrinogen and WBC counts were higher in patients with FMF in an attack period than those in an attack-free period, and higher than both control groups. Similarly, serum sIL-2R levels (Fig. 2a) were higher in patients with FMF in an attack period than those in an attack-free period (Z = 5·651, P < 0·001), and also higher than both healthy and disease control groups (Z = 5·430, P < 0·001; Z = 5·066, P < 0·001, respectively). The levels of sIL-2R were also found to be higher in patients with FMF in an attack-free period than those of both the healthy and disease control groups (Z = 2·011, P = 0·044; Z = 2·342, P = 0·019, respectively). In addition, the mean levels of IL-10 (Fig. 2b) were lower in patients with FMF in an attack-free period than those in an attack period (Z = 3·582, P < 0·001) and the healthy controls (Z = 2·069, P = 0·039). A positive correlation was observed between ESR and sIL-2R both in patients with FMF in an attack period (r = 0·716, P < 0·001) and those in an attack-free period (r = 0·435, P = 0·018). In patients with FMF in an attack period there was a positive correlation between the mean levels of ESR and IL-10 (r = 0·457, P = 0·037).
Table 3.
Comparisons of activation markers, serum sIL-2R and IL-10 levels in patients with FMF and controls [median (range)]
| Parameters | FMF (attack-free) (n = 29*) | FMF (attack) (n = 21*) | Healthy controls (n = 20) | Behçet's disease (n = 15) | χ2 | P** |
|---|---|---|---|---|---|---|
| ESR (mm/h) | 14 (20)a | 38 (45)b | 15 (21)c | 12 (17)d | 47·276 | <0·001 |
| WBC counts (/mm3) | 5800 (3600)e | 10000 (6600)f | 6900 (3600)g | 6500 (3700)h | 34·154 | <0·001 |
| Fibrinogen (mg/dl) | 322 (260)i | 400 (215)j | 325 (176)k | 330 (174)l | 20·695 | <0·001 |
| sIL-2R (ng/ml) | 1·0 (1·7) | 2·2 (3·5) | 0·8 (1·7) | 0·5 (1·1) | 48·715 | <0·001 |
| IL-10 (pg/ml) | 3·3 (8·4) | 7·0 (11·9) | 5·2 (9·6) | 5·2 (9·3) | 13·192 | 0·004 |
ESR: erythrocyte sedimentation rate; WBC: white blood cell; sIL-2R: soluble interleukin 2 receptor; IL-10: interleukin 10; FMF: familial Mediterranean fever.
With double donors;
multiple comparisons were performed using the Kruskall–Wallis test. P < 0.001 for
versusb,
versus
,b versus
,
versus
,f versus
,f versus
,
versus k; P = 0.001 for
versusj and j versusl.
Fig. 2.
Comparison of data from peripheral blood samples of patients with FMF and controls. The presented results are based on the analysis of 29 patients with FMF in an attack-free period, 21 patients with FMF in an attack period, 20 healthy and 15 disease controls. Boxes show the ranges of 1st and 3rd quartiles and extreme values, with the thick horizontal bars representing median values. The differences between two groups were evaluated by the Mann–Whitney U-test. P-values are indicated above the boxes when a level of significance <0·05 was reached in comparisons of the study groups. ○* represent extreme values, sIL-2R: soluble interleukin 2 receptor, IL-10: interleukin-10, CD: cluster of differentiation, FMF: familial Mediterranean fever, HC: healthy controls, BD: Behçet's disease.
The percentages and absolute counts of T cells expressing activation markers (HLA-DR, CD69 and CD25) on the cell surface that were found to be statistically significant among the groups are given in Table 4. The absolute count of CD3+HLA-DR+ T cells (Fig. 2c) was higher in patients with FMF in an attack period than those in an attack-free period (Z = 2·860, P = 0·004), and higher than both healthy and disease control groups (Z = 2·243, P = 0·025; Z = 1·973, P = 0·048, respectively). The percentage of CD4+CD69+ T cells (Fig. 2d) both in patients with FMF in an attack and in an attack-free period was higher than that in healthy controls (Z = 3·034, P = 0·002; Z = 2·530, P = 0·011, respectively). While the absolute counts of CD4+CD69+ T cells (Fig. 2e) in patients with FMF in an attack period were higher than both the healthy and disease control groups (Z = 3·286, P = 0·001; Z = 2·519, P = 0·012, respectively), they were higher in patients with FMF in an attack-free period only in comparison with the healthy controls (Z = 2·014, P = 0·044). The absolute counts of CD4+CD69+, CD8+CD25+ and CD8+CD69+ T cells (Fig. 2e,f,g) in patients with FMF in an attack period were higher than those in an attack-free period (Z = 2·526, P = 0·012; Z = 2·742, P = 0·006; Z = 3·450, P = 0·001, respectively). The absolute counts of CD8+CD25+ and CD8+CD69+ T cells in patients with FMF in an attack period were higher than those in the healthy controls (Z = 3·052, P = 0·002; Z = 2·791, P = 0·005, respectively). The percentages of CD4+, CD8+, CD8+CD25+, CD8+CD69+, CD19+, CD3–CD16+CD56+, the CD4+/CD8+ ratio and the absolute counts of CD4+, CD8+, CD19+, CD3–CD16+CD56+ did not differ significantly among the groups (data not shown). Patients taking colchicine and non-colchicine users, and double donors, displayed similar net effects, which did not differ at all, with respect to changes in these immunological parameters (data not shown).
Table 4.
Comparisons of the percentages and absolute counts of T cells expressing activation markers on the cell surface in patients with FMF and controls [median (range)]
| Parameters | FMF (attack-free) (n = 29*) | FMF (attack) (n = 21*) | Healthy controls (n = 20) | Behçet's disease (n = 15) | χ2 | P** |
|---|---|---|---|---|---|---|
| CD3+HLA-DR+ (/mm3) | 42 (236) | 90 (145) | 33 (157) | 56 (150) | 9·296 | 0·026 |
| CD4+CD69+ (%) | 1·7 (2·8) | 2·0 (2·8) | 0·8 (2·8) | 1·3 (2·8) | 10·658 | 0·014 |
| CD4+CD69+ (/mm3) | 23 (52) | 36 (63) | 13 (44) | 16 (43) | 14·825 | 0·002 |
| CD8+CD25+ (/mm3) | 12 (38) | 20 (48) | 12 (29) | 11 (43) | 10·419 | 0·015 |
| CD8+CD69+ (/mm3) | 15 (34) | 26 (46) | 17 (52) | 18 (59) | 12·554 | 0·006 |
CD: cluster of differentiation; FMF: familial Mediterranean fever.
With double donors;
multiple comparisons were performed using the Kruskall–Wallis test.
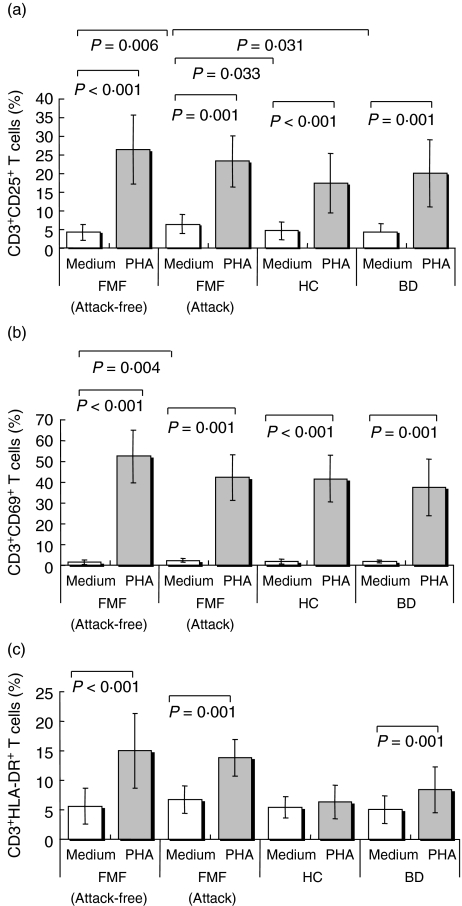
Comparisons of the percentages of CD3+CD25+, CD3+CD69+, CD3+HLA-DR+ T cells in unstimulated and stimulated cell cultures from patients with FMF and both control groups are shown in Table 5 and Fig. 3. Unexpectedly, we observed the early appearance (within 24 h) of CD3+HLA-DR+ T cells in stimulated cultures from patients with FMF in an attack-free period. The percentages of CD3+CD25+ (Fig. 3a), CD3+CD69+ (Fig. 3b) and CD3+HLA-DR+ (Fig. 3c) T cells were higher in stimulated, compared with unstimulated, cell cultures from patients both with FMF in an attack period (Z = 3·410, P = 0·001; Z = 3·408, P = 0·001; Z = 3·408, P = 0·001, respectively) and in an attack-free period (t = 11·903, P < 0·001; t = 20·474, P < 0·001; t = 7·414, P < 0·001, respectively), and also from patients with BD (Z = 3·298, P = 0·001; Z = 3·409, P = 0·001; Z = 3·296, P = 0·001, respectively). These parameters, except for the percentage of CD3+HLA-DR+ T cells (Fig. 3c), were also higher in stimulated cultures of healthy controls compared to the unstimulated cultures (t = 5·799, P < 0·001; t = 15·306, P < 0·001, respectively).
Table 5.
Comparisons of the percentages of CD3+CD25+, CD3+CD69+, and CD3+HLA-DR+ T cells in unstimulated (medium alone) and stimulated (medium + PHA) cell cultures from patients with FMF and controls
| FMF (attack-free) (n = 26*) | FMF (attack) (n = 15*) | Healthy controls (n = 20) | Behçet's disease (n = 15) | |||||
|---|---|---|---|---|---|---|---|---|
| Parameters (%) | Medium | PHA | Medium | PHA | Medium | PHA | Medium | PHA |
| CD3+CD25+ | 4·2 ± 2·2 | 26·4 ± 9·2 | 6·4 ± 2·5 | 23·2 ± 6·9 | 4·6 ± 2·4 | 17·3 ± 8·0 | 4·2 ± 2·3 | 20·0 ± 9·1 |
| CD3+CD69+ | 1·5 ± 1·0 | 52·3 ± 12·7 | 2·3 ± 0·9 | 42·1 ± 11·1 | 1·9 ± 1·2 | 41·5 ± 11·2 | 1·8 ± 0·8 | 37·4 ± 13·7 |
| CD3+HLA-DR+ | 5·6 ± 2·9 | 14·9 ± 6·3 | 6·7 ± 2·3 | 13·8 ± 3·1 | 5·4 ± 1·8 | 6·3 ± 2·8 | 5·0 ± 2·3 | 8·4 ± 3·9 |
CD: cluster of differentiation; FMF: familial Mediterranean fever.
With double donors;
multiple comparisons were performed using the Kruskall–Wallis test.
Fig. 3.
Comparisons of the percentages of CD3+CD25+(a), CD3+CD69+(b), and CD3+HLA-DR+(c) T lymphocytes in unstimulated (medium alone) and stimulated (naeduimt + PHA) cell cultures from patients with familial Mediterranean fever (FMF) and controls. Results presented are based on analysis of 26 patients with FMF in an attack-free period, 15 patients with FMF in an attack period, 20 healthy and 15 disease controls. Bars represent arithmetic mean ± standard deviation. The differences between two groups were evaluated by Mann–Whitney U-test. P-values are indicated above the bars when a level of significance < 0·05 was reached in comparisons of study groups. CD: cluster of differentiation, HC: healthy controls, BD: Behçet's disease.
The only significant changes in the percentages of CD3+CD25+ T cells between unstimulated and stimulated cultures were observed when comparing patients with FMF in an attack-free period and with healthy controls: the percentage change was significantly higher in the FMF attack-free patients (Z = 3·064, P = 0·002). In comparison, changes in the percentages of CD3+CD69+ T cells between unstimulated and stimulated cultures were significantly higher in patients with FMF in an attack-free period when compared with either patients in an attack period (Z = 2·519, P = 0·012), healthy controls (Z = 2·817, P = 0·005) or patients with BD (Z = 3·236, P = 0·001). Changes in the percentages of CD3+HLA-DR+ T cells between unstimulated and stimulated cultures were higher in patients with FMF in an attack-free period compared with either healthy controls (Z = 4·723, P < 0·001) or patients with BD (Z = 3·080, P = 0·002). Similarly, the changes were also higher in patients with FMF in an attack period compared with healthy controls (Z = 4·819, P < 0·001) and patients with BD (Z = 3·178, P = 0·001). In unstimulated cultures, the percentages of CD3+CD25+ T cells in patients with FMF in an attack period were higher than those in patients in an attack-free period (Z = 2·752, P = 0·006), healthy controls (Z = 2·134, P = 0·033) and patients with BD (Z = 2·155, P = 0·031). The percentages of CD3+CD69+ T cells in unstimulated cultures in patients with FMF in an attack period were only significantly higher than those in an attack-free period (Z = 2·915, P = 0·004).
DISCUSSION
In the current study, as in others [12,13], the patients with FMF in an attack period had higher levels of sIL-2R, an in vivo marker of T cell activation, than FMF patients in an attack-free period and both control groups. The higher levels of sIL-2R might favour the development of a Th1 response in an attack period of FMF. Centola et al. have suggested that MEFV expression is increased by proinflammatory activators, including the Th1 cytokine interferon (IFN)-γ, tumour necrosis factor (TNF) and lipopolysaccharide, and that it may have a place in the Th1-mediated response [4]. Their study [4] also demonstrated that MEFV mediates a Th1-responsive, negative feedback loop during proinflammatory activation of myeloid cells and that the pathophysiological features of FMF result from defects in this inhibitory activity. Similarly, it has been proposed recently that Th1 polarization is a key feature in the pathogenesis of FMF [17].
In contrast to a recent report [12], IL-10 levels were significantly lower in patients with FMF in an attack-free period in comparison with healthy controls and patients undergoing an acute attack. This could suggest that the attack-free period is associated with not only the overactivation of the Th1 subset in the peripheral blood but also the decreased function of Th2 cells. However, it has been proposed that IL-10 is associated not only with the Th2 response but also with the function of CD4+CD25+ T regulatory cells which inhibit immune responses at a very early stage [18]. The effects of IL-10 on immune responses are mostly inhibitory on T cells, mainly by affecting monocytes and macrophage function [19–21]. IL-10 down-regulates the production of inflammatory cytokine synthesis of Th1 cells and inhibits macrophage functioning, natural killer cells, peripheral blood mononuclear cells and Th1 cells [22,23]. In IL-10 gene-deficient mice, overproduction of inflammatory cytokines and the development of chronic inflammatory diseases have been shown [24]. The predominant effect of IL-10 is to reduce inflammation [25]. Thus, the significant decrease of IL-10 observed in our study in an attack-free period of FMF cannot limit effectively the T cell activity during this time and may contribute to the perpetuation of subclinical immune activation.
The cell-surface activation markers used in this study were CD69, CD25 and HLA-DR. CD69 is an early activation marker, and can be detected within 1 h of stimulation of T cells with mitogens and purified antigens [26,27]. The expression of CD25, the α-chain of the IL-2 receptor, can be detected on T cells after 18 h, while a rise in the expression of HLA-DR can be detected from 48 h of stimulation with PHA [26]. Although HLA-DR expression is a relatively late event during T cell activation, in contrast to healthy controls, we observed early expression of HLA-DR in stimulated cell cultures from FMF patients in an attack-free period and in patients with BD. These findings may suggest that CD3+ T cells of patients in an attack-free period are more sensitive to polyclonal activators. In addition, it is highly probable that patients with FMF have a lower threshold for T cell activation than normal controls. In the acute attack period, the absolute counts of CD3+HLA-DR+, CD4+CD69+ T cells in peripheral blood samples were higher not only in comparison with FMF patients in an attack-free period but also in comparison with both control groups, while the absolute counts of CD8+CD25+ and CD8+CD69+ T cells were found to be higher in patients with FMF in an attack period than those of attack-free FMF patients and healthy controls. Moreover, in patients with FMF in an attack period, changes in the percentages of CD3+HLA-DR+ T cells between unstimulated and stimulated cell cultures were higher than the changes in both healthy and disease controls.
The levels of sIL-2R were found to be higher in patients with FMF in an attack-free period than in the healthy controls. It is interesting to note that sIL-2R levels were also higher in patients with FMF in an attack-free period than in patients with BD. This may suggest indirectly that the attack-free period of FMF has a higher T cell activity than the remission period of BD. Similarly, in some studies, proinflammatory cytokines were shown to rise above the normal limits in an attack-free period of FMF [8,28]. In addition, both the percentages and absolute counts of CD4+CD69+ T cells in peripheral blood samples from patients with FMF in an attack-free period were slightly but significantly higher than those in the healthy controls. Moreover, in patients with FMF in an attack-free period, the changes in the percentages of CD3+CD25+, CD3+CD69+ and CD3+HLA-DR+ T cells between unstimulated and stimulated cultures were higher than those in the healthy controls, while the changes in the percentages of CD3+CD69+ and CD3+HLA-DR+ T cells between unstimulated and stimulated cultures were higher in patients with FMF in an attack-free period than in patients with BD. These findings suggest that immune activation may still be present, even though the disease is considered inactive according to the clinical criteria. In this context, the possibility of continuing subclinical inflammation in about two-thirds of the patients during an attack-free period has been reported recently [3]. Direskeneli et al. [29] and Kiraz et al. [30] also suggested that the disease sustained its activity in an attack-free period.
In conclusion, our study indicates that the T cell system is activated abnormally in patients with FMF, either when symptomatic or in remission. We also conclude from the present data that the decreased IL-10, along with increased sIL-2R, levels and increased CD4+CD69+ counts in the attack-free period, seem to be important factors for the continuing subclinical immune activation. Although decreased IL-10 levels may create a tendency to perpetuate subclinical immune activation, the trigger that starts the inflammatory attacks is still unknown.
REFERENCES
- 1.Ben-Chetrit E, Levy M. Familial Mediterranean fever. Lancet. 1998;351:659–64. doi: 10.1016/S0140-6736(97)09408-7. [DOI] [PubMed] [Google Scholar]
- 2.Orbach H, Ben-Chetrit E. Familial Mediterranean fever. Minerva Med. 2001;92:421–30. [PubMed] [Google Scholar]
- 3.Korkmaz C, Ozdogan H, Kasapcopur O, Yazici H. Acute phase response in familial Mediterranean fever. Ann Rheum Dis. 2002;61:79–81. doi: 10.1136/ard.61.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centola M, Wood G, Frucht DM, et al. The gene for familial Mediterranean fever, MEFV, is expressed in early leukocyte development and is regulated in response to inflammatory mediators. Blood. 2000;95:3223–31. [PubMed] [Google Scholar]
- 5.Gang N, Drenth JP, Langevitz P, et al. Activation of the cytokine network in familial Mediterranean fever. J Rheumatol. 1999;26:890–7. [PubMed] [Google Scholar]
- 6.Karagezian KG, Nazaretian EE, Zavgorodniaia AM, Ovnanian KO. Immune disorders in periodic disease. Klin Med. 2000;78:24–5. [PubMed] [Google Scholar]
- 7.Melamed A, Cabili S, Zakuth V, Spirer Z. The immune regulation in familial Mediterranean fever. J Clin Lab Immunol. 1988;26:125–8. [PubMed] [Google Scholar]
- 8.Schattner A, Lachmi M, Livneh A, Pras M, Hahn T. Tumor necrosis factor in familial Mediterranean fever. Am J Med. 1991;90:434–8. [PubMed] [Google Scholar]
- 9.Ozyilkan E, Simsek H, Telatar H. Tumor necrosis factor in familial Mediterranean fever. Am J Med. 1992;92:579–80. doi: 10.1016/0002-9343(92)90762-z. [DOI] [PubMed] [Google Scholar]
- 10.Rozenbaum M, Katz R, Rozner I, Pollack S. Decreased interleukin-1 activity released from circulating monocytes of patients with familial Mediterranean fever during in vitro stimulation by lipopolysaccharide. J Rheumatol. 1992;19:416–8. [PubMed] [Google Scholar]
- 11.Akcan Y, Bayraktar Y, Arslan S, Van Thiel DH, Zerrin BC, Yildiz O. The importance of serial measurements of cytokine levels for the evaluation of their role in pathogenesis in familial Mediterraean fever. Eur J Med Res. 2003;8:304–6. [PubMed] [Google Scholar]
- 12.Baykal Y, Saglam K, Yilmaz MI, Taslipinar A, Akinci SB, Inal A. Serum sIL-2r, IL-6, IL-10 and TNF-alpha level in familial Mediterranean fever patients. Clin Rheumatol. 2003;22:99–101. doi: 10.1007/s10067-002-0682-1. [DOI] [PubMed] [Google Scholar]
- 13.Erken E, Gunesacar R, Ozbek S, Konca K. Serum soluble interleukin-2 receptor levels in familial Mediterranean fever. Ann Rheum Dis. 1996;55:852–5. doi: 10.1136/ard.55.11.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Livneh A, Langevitz P, Zemer D, Zaks N, Kees S, Lidar T. Criteria for the diagnosis of familial Mediterranean fever. Arthritis Rheum. 1997;40:1879–85. doi: 10.1002/art.1780401023. [DOI] [PubMed] [Google Scholar]
- 15.International Study Group for Behçet's Disease. Criteria for diagnosis of Behçet's disease. Lancet. 1990;335:1078–80. [PubMed] [Google Scholar]
- 16.Dawson B, Trapp RG. Basic and clinical biostatistics. Singapore: Mc-Graw Hill; 2001. [Google Scholar]
- 17.Aypar E, Ozen S, Okur H, Kutluk T, Besbas N, Bakkaloglu A. Th1 polarization in familial Mediterranean fever. J Rheumatol. 2003;30:2011–3. [PubMed] [Google Scholar]
- 18.Shevach EM. Regulatory T cells in autoimmunity. Annu Rev Immunol. 2000;18:423–49. doi: 10.1146/annurev.immunol.18.1.423. [DOI] [PubMed] [Google Scholar]
- 19.Saito I. Structure of IL-10 and its role in autoimmune exocrinopathy. Crit Rev Immunol. 2000;20:153–65. [PubMed] [Google Scholar]
- 20.Conti P, Kempuraj D, Kandere K, et al. IL-10, an inflammatory/inhibitory cytokine, but not always. Immunol Lett. 2003;86:123–9. doi: 10.1016/s0165-2478(03)00002-6. [DOI] [PubMed] [Google Scholar]
- 21.Asadullah K, Sterry W, Volk HD. Interleukin-10 therapy-review of a new approach. Pharmacol Rev. 2003;55:241–69. doi: 10.1124/pr.55.2.4. [DOI] [PubMed] [Google Scholar]
- 22.Corinti S, Albanesi C, Sala A, Pastore S, Girolomoni G. Regulatory activity of autocrine IL-10 on dendritic cell functions. J Immunol. 2001;166:4312–8. doi: 10.4049/jimmunol.166.7.4312. [DOI] [PubMed] [Google Scholar]
- 23.Prakken BJ, Wendling U, van der Zee R, Rutten VPMG, Kuis W, van Eden W. Induction of IL-10 and inhibition of experimental arthritis are specific features of microbial heat shock proteins that are absent for other evolutionarily conserved immunodominant proteins. J Immunol. 2001;167:4147–53. doi: 10.4049/jimmunol.167.8.4147. [DOI] [PubMed] [Google Scholar]
- 24.Lang R, Rutschman RL, Greaves DR, Murray PJ. Autocrine deactivation of macrophages in transgenic mice constitutively overexpressing IL-10 under control of the human CD68 promoter. J Immunol. 2002;168:3402–11. doi: 10.4049/jimmunol.168.7.3402. [DOI] [PubMed] [Google Scholar]
- 25.Mocellin S, Panelli MC, Wang E, Nagorsen D, Marincola FM. The dual role of IL-10. Trends Immunol. 2003;24:36–43. doi: 10.1016/s1471-4906(02)00009-1. [DOI] [PubMed] [Google Scholar]
- 26.Hara T, Jung LKL, Bjorndahl JM, Fu SM. Human T cell activation. III. Rapid induction of a phosphorylated 28 kD/32 kD disulfide-linked early activation antigen (EA-1) by 12-o-tetraconoyl phorbol-13-acetate, mitogens, and antigens. J Exp Med. 1986;164:1988–2005. doi: 10.1084/jem.164.6.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Testi R, Phillips JH, Lanier LL. Leu 23 induction as an early marker of functional CD3/T cell antigen receptor triggering. Requirement for receptor cross-linking, prolonged elevation of intracellular (Ca++) and stimulation of protein kinase C. J Immunol. 1989;142:1854–60. [PubMed] [Google Scholar]
- 28.Schattner A, Gurevitz A, Zemer D, Hahn T. Induced TNF production in vitro as a test for familial Mediterranean fever. Q J Med. 1996;89:205–10. doi: 10.1093/qjmed/89.3.205. [DOI] [PubMed] [Google Scholar]
- 29.Direskeneli H, Ozdogan H, Korkmaz C, Akoglu T, Yazici H. Serum soluble intercellular adhesion molecule-1 and interleukin-8 levels in familial Mediterranean fever. J Rheumatol. 1999;26:1983–6. [PubMed] [Google Scholar]
- 30.Kiraz S, Ertenli I, Arici M, et al. Effects of colchicine on inflammatory cytokines and selectins in familial Mediterranean fever. Clin Exp Rheumatol. 1998;16:721–4. [PubMed] [Google Scholar]