Abstract
Complement activation contributes to inflammation and tissue damage in human demyelinating diseases and in rodent models of demyelination. Inhibitors of complement activation ameliorate disease in the rat model antibody-dependent experimental autoimmune encephalomyelitis and rats unable to generate the membrane attack complex of complement develop inflammation without demyelination. The role of the highly active chemotactic and anaphylactic complement-derived peptide C5a in driving inflammation and pathology in rodent models of demyelination has been little explored. Here we have used a small molecule C5a receptor antagonist, AcF-[OPdChaWR], to examine the effects of C5a receptor blockade in rat models of brain inflammation and demyelination. C5a receptor antagonist therapy completely blocked neutrophil response to C5a in vivo but had no effect on clinical disease or resultant pathology in either inflammatory or demyelinating rat models. We conclude that C5a is not required for disease induction or perpetuation in these strongly complement-dependent disease models.
Keywords: rodent, complement, neuroimmunology, chemokines
INTRODUCTION
The complement (C) system is a central component of innate immunity with key roles for protection against bacterial infection and immune complex deposition [1]. These protective roles are mediated by a number of biologically active products, generated in the course of C activation, that variously possess opsonic, chemotactic and cytolytic properties. The surface-bound fragments C3b and C4b target pathogens and immune complexes for phagocytosis, the fluid-phase fragment C5a is chemotactic for phagocytic cells and activating for many other cell types, and the membrane attack complex (MAC) mediates lytic killing of pathogens. These same products, when generated inappropriately, are responsible for the pathological effects of C in diverse diseases [2].
Multiple sclerosis (MS) is an inflammatory and demyelinating disease of the central nervous system. Although the precise aetiology of MS remains unclear, it is widely accepted that autoimmunity plays a significant role [3]. Evidence from the animal model, experimental autoimmune encephalomyelitis (EAE), implicates an autoimmune T cell response as the initiating factor. However, in unmodified EAE induced in rodents, demyelination and neuronal damage are usually minor, suggesting that additional factors are required to induce pathology more closely resembling the human disease [4,5]. There is now substantial evidence that other components of the immune system are recruited in MS and in EAE to cause myelin damage and neuronal/axonal injury [6–8]. The C system has been implicated as a myelinolytic agent both in vitro and in vivo in man and in experimental animals. Early in vitro models of demyelination using cerebellar explant cultures showed that the demyelinating component of sera from animals with EAE was heat-labile, a classical characteristic of the C system [9]. The classical pathway of C is activated by central nervous system (CNS) myelin in vitro, with MAC-induced demyelination [10,11], and C activation products are deposited in and around areas of demyelination in experimental models and MS [12,13]. In rat EAE, C depletion ameliorates disease [14], and in an antibody-mediated demyelinating form of EAE (ADEAE), induced by injection of antibodies to myelin oligodendrocyte glycoprotein (MOG) at the onset of clinical signs in EAE, inhibition of C using a soluble recombinant form of complement receptor 1 (sCR1) ablated demyelination [15].
C activation generates numerous activities that might contribute to myelin damage, and recent research has focused on the identification of the culprit. Our work has strongly implicated the MAC as a primary cause of myelin loss and axonal damage in rodent models. Rats genetically deficient in C6 activate C normally, generate opsonins and chemotactic fragments of C3 and C5, but cannot generate MAC [16]. When ADEAE was induced in C6 deficient rats, demyelination and axonal injury were absent and clinical disease substantially reduced; restoration of C6 restored pathology [17]. Importantly, the degree of inflammation was similar in C6 deficient and sufficient rats, in contrast to the anti-inflammatory effects of C inhibition with sCR1, leading us to conclude that the C anaphylatoxins drive inflammation in this model.
The C-derived anaphylactic and chemotactic peptide C5a has been shown to play important roles in many C-induced injuries [18,19]. The receptor for C5a (C5aR, CD88) is expressed in human and rodent brain, present on astrocytes, microglia and neurones [20–23]. Expression in naïve brain is low but is markedly up-regulated on brain cells in the context of inflammation, whether induced by infection, focal ischaemia, brain trauma or EAE [24–27]. Recently, a peptide agonist of the C5aR was shown to trigger neuronal apoptosis in vitro and ex vivo, provoking the suggestion that C5a might be involved in signalling neurodegeneration [28].
Agents that block C5a or its receptor have proven effective in many models of C-mediated disease, including ischaemia-reperfusion injuries, arthritis and bowel inflammation [19,29–31]. The peptide antagonist AcF-[OPdChaWR] is a potent blocker of C5aR in man and rat [32,33]. The antagonist blocks C5a-induced leucocyte activation in vitro and inhibits pathology in a number of rat models of ischaemia-reperfusion injury and inflammatory bowel and joint disease [34,35]. We here describe a study of the effects of C5aR blockade using AcF-[OPdChaWR] in the well-documented Lewis rat models of EAE and ADEAE. Disease was monitored clinically and by using multiple pathological parameters. C5aR blockade was confirmed by demonstrating that neutrophils from treated rats were unresponsive to C5a activation in vitro. AcF-[OPdChaWR] treatment had no effect on the clinical course or pathological outcome in either the primarily inflammatory EAE model or the profoundly demyelinating ADEAE model.
MATERIALS AND METHODS
Animals and reagents
Male Lewis rats of mean weight 190 g were obtained from Harlan Olac (Hull, UK) and maintained on a normal feeding regimen throughout the study. All animal studies were performed in accordance with UK Home Office guidelines and under the authorization of a specific Licence for the procedures. The C5aR antagonist (C5aRa) AcF-[OPdChaWR] (supplied by Promics Pty. Ltd, University of Queensland, Australia) was synthesized as previously described [32,36], purified by reverse-phase HPLC and characterized by mass spectrometry and proton nuclear magnetic resonance spectroscopy. The peptide was lyophilized for storage and solubilized at 0·6 mg/ml in a 30% solution of propylene glycol immediately prior to use. Myelin basic protein isolated from guinea pig brain (gpMBP) was purchased from Sigma Chemical Co. (Poole, Dorset, UK). Freund's complete adjuvant (CFA) and Mycobacterium tuberculosis H37 Ra (MtbH37) were from Difco (Epsom, Surrey, UK). The anti-myelin oligodendrocyte protein (MOG) mAb Z12 was produced as previously described [37]. The rabbit anti-mouse C5aR antibody (cross-reactive with rat) was produced in house by immunization with C5a-derived peptide and affinity purified on a column comprising the peptide used as immunogen immobilized on sepharose. The antibody was biotin-labelled using a commercial kit (Perbio Science, Tattenhall, UK). ED1 (mouse anti-rat CD68, Serotec, Oxford, UK; product MCA341R) was used for labelling of macrophages and W3/13 (mouse anti-rat CD43, Serotec; product MCA54R) for labelling of T lymphocytes.
Induction of EAE and ADEAE
The protocol for induction of EAE and ADEAE was essentially as described previously [15,17]. Briefly, rats were immunized in each hind footpad with 50 µl of a 1 : 1 emulsion of gpMBP (1 mg/ml in PBS) and CFA containing 4 mg/ml MtbH37. On day seven, animals were randomly allocated into treatment and control groups; the treatment group received daily doses of C5aRa peptide (1 mg/kg delivered subcutaneously in the flank) and the control group received the same volume of vehicle alone. On day 10, ADEAE animals additionally received an intraperitoneal injection of Z12 mAb (0·8 mg in PBS). Animals were weighed daily and monitored for signs of clinical disease. Disease was scored as: 0, no clinical signs; 0·5, tail weakness; 1, tail atony; 1·5, tail atony and abnormal gait; 2, hind limb weakness; 2·5, complete paralysis of one hind limb; 3, complete paralysis of both hind limbs; 4, moribund. To comply with Home Office Licence conditions, animals that had reached a clinical score of 3 or 4 at the time of assessment were immediately sacrificed and all remaining animals were euthanized at day 14.
Tissue processing, histological and immunocytochemical evaluation
Animals to be euthanized were perfused with 4% paraformaldehyde in PBS via the left ventricle while under terminal anaesthesia. Brains and spinal cords were removed, postfixed in 4% paraformaldehyde in PBS at 4°C overnight, washed in PBS and embedded in paraffin wax or araldite resin using standard techniques.
Paraffin sections were cut as described and stained with H&E to assess inflammation, luxol fast blue/cresyl violet (LFB/CV) to assess myelin loss and Bielschowsky's silver stain to assess axonal loss as described previously [17]. For all parameters, four sections were scored from each animal, taken from cervical, thoracic, lumbar and sacral cord. Scores for the four sections were averaged.
Inflammatory cells in subpial and perivascular areas were separately scored on arbitrary scales of 0–4. For subpial inflammation, 0, no infiltration into the subpial space; 1, minor infiltration; 2, numerous infiltrating cells; 3, most of the pia involved; 4, all of the pia involved. For perivascular inflammation, 0, no perivascular inflammatory cells; 1, one or two cuffed vessels per section; 2, three to five cuffed vessels per section; 3, five to eight cuffed vessels; 4, more than eight cuffed vessels per section.
Neutrophils were identified morphologically in H&E-stained sections under high magnification (40× objective). The total number of neutrophils was counted for the whole cord section, comprising 30–50 high-power fields and recorded as number per field.
Apoptotic cells were identified morphologically in H&E-stained sections and counted as described above. Apoptotic cells were also identified by TUNEL staining. Paraffin wax embedded sections were heated at 58°C for 5 min, dewaxed and dehydrated in xylene and ethanol, washed in PBS, permeabilized and stained as instructed by the manufacturer (NeuroTACS II; cat. No. TA900. R & D Systems, Abingdon, UK).
Demyelination was assessed for each experimental group in LFB/CV stained sections; the area in square millimeters of myelin loss and total white matter area were measured using a graticule and the percentage demyelination reported on a 5-point scale from zero through to 5 for complete loss of myelin.
Axon loss was assessed using Bielschowsky's silver stain; axonal density in different areas of the spinal cord sections was assessed according to a published visual ranking system reported on a scale from 0, no loss, through 5, complete loss of axons [38].
Immunocytochemistry
Dewaxed and rehydrated paraffin wax sections were stained using an overnight, indirect immunoperoxidase method essentially as described [17]. Sections were overlayed with biotinylated primary antibody at an appropriate dilution in PBS (5 µg/ml for anti-C5aR antibody) and incubated overnight at 4°C in a humidity chamber. Sections were washed and sequentially incubated at room temperature with extravidin-HRP (Sigma; 1 : 100 in PBS, 1 h) and substrate (diaminobenzidine/H2O2, 5 min). Sections were washed, counter-stained with haematoxylin and mounted in Xam (BDH, Coventry, UK). The degree of staining in ICC was quantified using the Openlab 3·5 image analysis system (Improvision, Coventry, UK). Density slices were taken from the same region adjacent the central canal in all sections, and the degree of staining measured, with reference to fixed background cut-off values. Multiple fields in three sections were analysed for each animal and a mean value of proportion of total area stained, corrected for secondary control labelling, was generated.
Macrophages were identified by staining with ED1 (1 : 50) following epitope retrieval by proteinase K digestion as suggested by the manufacturer. T lymphocytes were identified by staining with W3/13 (1 : 50) using the manufacturer's protocols. Donkey anti-Mouse HRPO secondary (Sigma) was used at 1 : 200 for each primary mAb. Quantification of ED1 positive cells was performed as for C5aR labelling, while the W3/13 cells were manually counted (because of their low numbers) in multiple fields and sections from each animal and expressed as average cells/section.
Assessment of efficacy of C5aR antagonist in vivo
Animals were sacrificed between 18 and 24 h after their last dose of C5aRa (Day 14 or earlier depending on disease stage). Blood (2–5 ml) was taken directly into a heparinized syringe from the left ventricle of rats under terminal anaesthesia immediately prior to perfusion as described above. Heparinized blood was kept at room temperature and neutrophils isolated within one hour by overlaying on 5 ml NycoPrep 1·077 Animal (Technoclone; product no. 6001455) and centrifugation at room temperature essentially as described (39). Isolated PMN were >90% pure and >90% viable as estimated by microscopy in the presence of trypan blue (0·1% final). PMN were resuspended in Krebs Ringer buffer at 106/ml, loaded with fluo-3 (Molecular Probes, Rijnsbergerweb, Netherlands) by incubation with the dye at a final concentration of 5 µm (from 5 m m stock in DMSO) and 100 µl aliquots placed in wells of a 96-well round-bottomed plate. The plate was placed in a FLUOstar Optima fluorescence plate reader equipped with an automated injector system (BMG Labtech, Offenburg, Germany). Either recombinant C5a (Sigma; 100 ng in 50 µl Krebs buffer) or fMLP (Sigma; 50 ng in 50 µl buffer) was injected into individual wells and the intracellular Ca2+ response monitored essentially as described (http://www.bmg-labtechnologies.com; Application note 2004). The specific response in fluorescence units, corrected for baseline, was calculated for each well.
Statistical analysis
For comparison between groups, Mann–Whitney U nonparametric test was used; the exact two-tailed P-value corrected for ties is quoted throughout. For correlation of apoptosis with clinical score, nonparametric Spearman rank correlation was used.
RESULTS
C5aR antagonist therapy specifically blocks neutrophil response to C5a
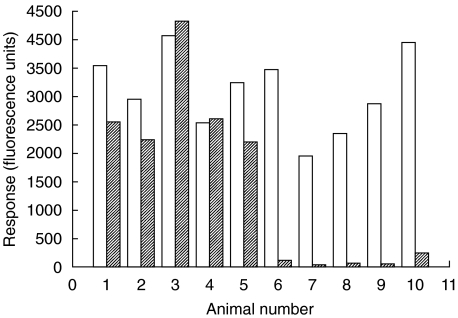
Neutrophils were isolated from rats in which ADEAE had been induced with or without C5aRa therapy and loaded with fluo-3 dye. The time elapsed between last dose of C5aRa and neutrophil isolation was between 18 and 24 h. Pharmokinetic studies of C5aRa delivered subcutaneously in rats have demonstrated a slow, sustained absorption phase with maximum plasma levels attained after 3–4 h [40]. Response to C5a and fMLP was measured by monitoring Ca2+response spectrophotometrically. As shown in Fig. 1, neutrophils isolated from each of 5 untreated ADEAE animals responded to either C5a or fMLP with Ca2+ transients of similar magnitude. In contrast, cells isolated from C5aR antagonist treated animals responded to fMLP with a transient of similar magnitude but in every case failed to respond to C5a, demonstrating that the C5aR had been specifically blocked in vivo and remained blocked 18–24 h after administration of agent.
Fig. 1.
C5aR antagonist blocks response of rat neutrophils to C5a in vivo. Isolated neutrophils were loaded with fluo-3 dye and the Ca2+ response to fMLP (□) and C5a ( ) measured spectrofluorimetrically. Sets 1–5 represent untreated ADEAE rats and sets 6–10 represent ADEAE rats treated with C5aRa. Response to C5a is lost in the latter set.
) measured spectrofluorimetrically. Sets 1–5 represent untreated ADEAE rats and sets 6–10 represent ADEAE rats treated with C5aRa. Response to C5a is lost in the latter set.
C5aR antagonist therapy does not modulate clinical disease in EAE or ADEAE
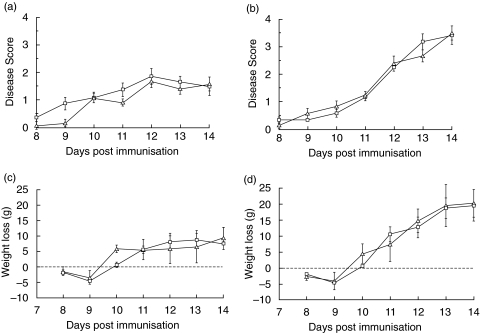
For EAE, animals were randomly allocated into control and therapy groups (seven in each group) and treated as detailed in methods. Clinical score and weight were assessed daily. Clinical disease was first apparent on day 8 and the incidence was 100% by day 10. The mean scores (± sem) for the two groups on each day after disease onset are shown in Fig. 2a. In previous studies we have shown that onset of clinical disease correlates closely with the first signs of CNS inflammation and inflammatory cell infiltration [12,14,15]. No inflammatory infiltration occurs prior to disease onset and the initiation of treatment on day 7 therefore ensures that cells that will migrate into the CNS were exposed to the C5aR antagonist in the periphery. Disease severity was very similar in each group at each time point and statistical analysis confirmed that there were no significant differences between the groups. In both groups, the majority of animals lost weight during the course of the disease. The mean weight loss (expressed with reference to day 7 weight as zero) was similar in the two groups and no significant difference was obtained at any time-point in the course of the disease (Fig. 2c).
Fig. 2.
C5aR antagonist does not modulate clinical disease in EAE or ADEAE. EAE (a,c) and ADEAE (b,d) were induced in groups of Lewis rats as described in methods. In each model, one group was treated daily from day 7 with the C5aRa (□) while a second group was sham treated (Δ). Clinical score (a,b) and weight (c,d) were measured daily. For measurement of weight loss, weight at day 7 was taken as zero. In the ADEAE groups, Z12 mAb was given on day 10. Each group comprised 6 or 7 animals and results shown are means ± standard errors of the mean for the groups. No statistical differences were found between the groups for either clinical score or weight loss in either EAE or ADEAE.
ADEAE was induced in a separate experiment. Induction of EAE and allocation into control and therapy groups (six in each group) was performed as described above. All animals received Z12 mAb on day 10 to trigger demyelination. Clinical disease was first apparent on day 8 and the incidence was 75% on day 10. Addition of Z12 mAb markedly exacerbated disease, with all animals showing moderate to severe signs (scores of 2–3) by day 12. The mean scores (± sem) on each day after disease onset are shown in Fig. 2b. There were no significant differences in disease score between the two groups at any time-point in the course of disease. All animals lost weight during the course of the disease. The mean weight loss (expressed with reference to day 7 as zero) was similar in the two groups and no significant difference was obtained at any time-point in the course of the disease (Fig. 2d).
C5aR antagonist therapy does not influence pathology in EAE/ADEAE
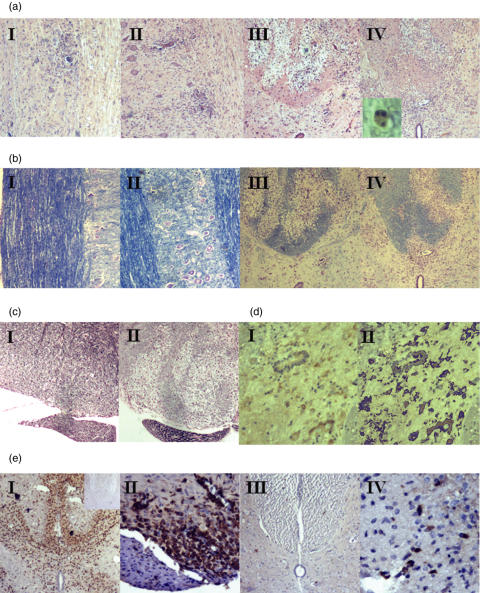
All animals were sacrificed on day 14 unless previously euthanized because of the severity of disease. Fixed spinal cord was taken from all rats and sectioned for staining. The degree of inflammatory infiltrate was assessed in H&E stained cervical cord sections by observers blinded to the sample identity. In EAE, inflammatory cell infiltration was predominantly subpial and perivascular and comprised both mononuclear and polymorphonuclear (PMN) cells (Fig. 3a.I.II). In ADEAE, the infiltrate was more diffusely distributed through the parenchyma and contained a higher proportion of mononuclear cells (Fig. 3a.III.IV). In both models, the degree and composition of the inflammatory infiltrate, scored as described in methods, was not significantly different in C5aR antagonist treated animals when compared with controls in the same model (Table 1). Sub-pial and perivascular infiltration, assessed separately, also did not differ between groups (data not included). Macrophage infiltration, assessed by staining with the specific mAb ED1, was unaffected by treatment with C5aR antagonist (Fig. 3e.I.II; Table 2). Staining with the T cell specific mAb W3/13 demonstrated small numbers of infiltrating T cells in sections from all ADEAE animals, unaffected by treatment with C5aR antagonist (Fig. 3e.III.IV; Table 2). Sections from naïve animals showed no inflammatory infiltrate and were negative when stained with ED1 (inset,in 3E,I) or W3/13 (not shown).
Fig. 3.
C5aR antagonist does not alter pathology in EAE or ADEAE. (a, b) Spinal cord sections were stained with H&E to reveal the amount of inflammatory infiltrate (a) or LFB to identify areas of demyelination (b). AI, BI, untreated EAE, clinical score 2; AII, BII, C5aRa-treated EAE, clinical score 2; AIII, BIII, untreated ADEAE, clinical score 3; AIV, BIV, C5aRa-treated ADEAE, clinical score 3. AI, AII, BI and BII are taken in the longitudinal plane to demonstrate lack of myelin loss. All other panels are transverse sections. Extensive myelin loss and inflammatory infiltrate are apparent in ADEAE sections whereas myelin staining is intact in EAE sections. TUNEL labelling revealed apoptotic cells in all ADEAE rats, whether treated with C5aRa or untreated (AIV, inset). (c) Axonal loss was assessed by the Bielschowsky stain. EAE animals showed no axonal loss (CI) whilst ADEAE animals showed regions of marked axonal loss regardless of treatment regimen (CII). Axon sparing in peripheral nerve fibre is apparent. (d) Distribution of C5a receptor in ADEAE spinal cord, stained section (DI) and image generated from analysis software to calculate area of staining (DII). (e) staining for macrophage marker ED1 (EI, EII) and T cell marker W3/13 (EIII, EIV) in ADEAE spinal cord (clinical score 3). Inset in EI confirms the lack of staining in spinal cord sections from naïve rats.All figures are at ×40 magnification, except EII (×100) and EIV (×200).
Table 1.
Pathological indicies in EAE/ADEAE – effect of C5aR antagonist
| PMN/hpf | Apop/hpf | Axon loss | Demyelination | |
|---|---|---|---|---|
| ADEAE (C) | 1·467 ± 0·162 | 2·03 ± 0·294 | 1·5 ± 0·22 | 2·167 ± 0·167 |
| ADEAE (T) | 1·367 ± 0·118 | 1·73 ± 0·339 | 1·3 ± 0·21 | 1·833 ± 0·477 |
| EAE (C) | 0·195 ± 0·086 | 1·525 ± 0·547 | nd | nd |
| EAE (T) | 0·292 ± 0·147 | 1·167 ± 0·400 | nd | nd |
All values are mean ± SEM; nd denotes not detected. C = control, T = treated with C5aR antagonist. Neutrophils (PMN) and apoptotic cells were counted and are expressed as number per high power field (hpf). Axon loss and demyelination were scored as described in Methods. Statistical analysis revealed no significant differences between control and treated groups for any of the listed parameters.
Table 2.
Immunocytochemical parameters in ADEAE – effect of C5aR antagonist
| TUNEL stain (cells/section) | W3/13 staining (cells/section) | ED1 staining (cells/section) | C5aR staining (area stained) | |
|---|---|---|---|---|
| ADEAE (C) | 43·4 ± 9·51 | 16·3 ± 1·8 | 176·6 ± 40·4 | 86·2 ± 19·8 |
| ADEAE (T) | 29·1 ± 7·57 | 14·5 ± 3·3 | 254·2 ± 19·5 | 96·8 ± 12·8 |
All values are mean ± SEM. Statistical analysis revealed no significant differences between control and treated groups for any of the listed parameters.
Apoptotic cells, identified morphologically by the characteristic condensed nucleus, were quantified in all sections and numbers compared between groups. No significant difference in apoptosis was seen between treated and untreated groups in the same model (Table 1). In the ADEAE group, an independent measure of apoptosis was used, TUNEL staining of sections. Positive cells were counted in comparable cord areas and numbers compared between treated and control groups. No significant difference was seen between the groups (Table 2). Comparison of the frequency of apoptotic cells (TUNEL-positive) in individual animals and clinical score at the end of the experiment revealed a highly significant positive correlation (F1,10 = 13·89; P = 0·04).
Myelin loss was assessed by staining with LFB/CV. Myelin was grossly intact in EAE animals, whether C5aR antagonist-treated or untreated, with strong and homogeneous staining throughout the white matter (Fig. 3b.I.II). In contrast, large perivascular plaques of myelin loss were present in all ADEAE animals, whether C5aR antagonist-treated or untreated (Fig. 3b.III.IV). Myelin loss in ADEAE was scored as described in methods by an observer blinded to the sample identity. The degree of demyelination did not differ between the treated and control groups (Table 1).
Our previous work has demonstrated that axonal loss correlates closely with myelin loss in ADEAE and is not detected in EAE (as confirmed in Fig. 3c.I). Axonal loss was assessed in ADEAE using Bielschowsky's silver stain by an observer ignorant of sample identity. Loss of axons was evident in areas of myelin loss in all ADEAE animals (Fig. 3c.II) and the degree of axonal loss was not significantly different in treated and control groups (Table 1).
C5aR antagonist therapy does not influence C5aR expression in spinal cord
Cord sections from all ADEAE animals were subjected to standard antigen retrieval methods then stained with a biotinylated rabbit anti-mouse C5aR antibody and developed as described in methods. The degree of staining was quantified using an image analysis system (Fig. 3d.I.II). There was no significant difference in C5aR expression between the control and treated groups. There was no staining for C5aR in naïve animals (data not shown).
DISCUSSION
C5aR is abundantly expressed on neutrophils and C5a is a powerful neutrophil chemoattractant, guiding neutrophils to the inflammatory site [41]. C5aR is also present and functional on other leucocytes, including monocytes and T lymphocytes and here too C5a acts as a chemoattractant [42]. However, C5a is not the only leucocyte chemoattractant; a large battery of chemokines are produced by endogenous and infiltrating cells at inflammatory sites that attract leucocyte subsets through membrane receptors [43,44]. An understanding of the relative importance of these various chemoattractants is essential for rational therapy of inflammation. The availability of specific C5aR antagonists and C5aR knockout mice has enabled the roles of C5a to be addressed thoroughly in many rodent models of inflammatory diseases. In the large majority of cases, C5a has been found to be an important inflammatory mediator and removal of the capacity to generate or respond to C5a has markedly reduced inflammation, infiltration of neutrophils and other leucocytes and resultant pathology [34,35,45]. It might therefore be anticipated that inflammation in EAE, ADEAE and other models of CNS pathology would be modulated in the absence of C5a responses. Although C5aR expression in noninflamed CNS tissue is absent or negligible, expression is markedly up-regulated by inflammation. In the Lewis rat EAE model, C5aR is expressed concomitant with onset of clinical disease on infiltrating inflammatory cells and on resident microglia, astrocytes and neurones [24]. A similarly striking induction of expression occurs in mice.
The inflammation-induced up-regulation of C5aR in EAE suggests a role in pathology. However, absence of C5aR in knockout mice did not influence disease severity, the extent or composition of the inflammatory cell infiltrate, or local cytokine production in the MOG peptide-induced murine EAE model [46]. The MOG-induced murine EAE model is characterized by a mononuclear cell dominant inflammatory infiltrate with no myelin loss or axonal destruction, implying a mild injury. Importantly, the role of C in this model is uncertain in that different studies using C3 knockout mice have shown disease modulation or no effect on disease [47,48]. We have shown that mice deficient in CD59a develop a much more severe disease in this model, with substantial demyelination, axonal damage and a mixed infiltrate that included neutrophils [49]. Taken together, these data suggest that the model is, at least in part, C dependent but raise the possibility that the failure to observe an effect of C5aR deficiency was an artefact of the model rather than a true indication of the role of C5a in CNS inflammation. The Lewis rat EAE/ADEAE models are known from a large number of studies using C depletion or anti-C agents to be strongly dependent on C activation [14,15]. In the ADEAE model, the extent of demyelination correlates with the efficiency of C activation by the anti-MOG mAb used to trigger disease, and demyelination is completely dependent on the capacity to generate MAC [17,50]. The extent of C activation in the ADEAE model can be demonstrated by staining for MAC, present in abundance in all areas of myelin loss. This massive generation of MAC will inevitably be accompanied by massive generation of C5a. Lewis rat EAE/ADEAE therefore offers a better model to explore the role of C5a in driving CNS inflammation and for this reason was chosen for the current study.
The peptide C5aR antagonist AcF-[OPdChaWR] was administered subcutaneously, daily through the course of the disease. Although access to the CNS parenchyma was not formally demonstrated, the blood–brain barrier is severely compromised in these models and it would be expected that this small peptide antagonist would readily access the CNS. Conclusive proof that C5aR blockade had occurred requires direct installation of agent into the CNS, a procedure that is not permitted under our Home Office licence. Of note, in MOG-induced EAE/ADEAE in the Lewis rat, onset of clinical disease is between days 8 and 10 and CNS inflammatory cell infiltration is not detected prior to disease onset [12,14,15]. The decision to begin therapy on day 7 was based upon these observations and ensured that cells migrating into the CNS has been exposed to agent in the periphery.
Treatment with C5aR antagonist had no effect on clinical disease, weight loss or any of the multiple pathological parameters measured in EAE and ADEAE. In ADEAE, demyelination and axonal loss were equally severe in treated and untreated animals. Of particular relevance, the degree and composition of the inflammatory infiltrate was unaffected. Neutrophils, a well-described feature of the inflammatory infiltrate in these models, were present in similar numbers in treated and untreated animals, despite the clear demonstration of effective blockade of C5aR in blood neutrophils from animals at the end-point of disease. Neutrophil C5aR blockade was demonstrated in cells isolated from animals between 18 and 24 h after the last subcutaneous dose of antagonist, suggesting that this dosing regimen effectively blocked C5aR throughout the course of the experiment. These findings are supported by recent pharmacokinetic studies of AcF-[OPdChaWR] in rats [40]. Neutrophils are short-lived cells recruited acutely to the site of pathology. Neutrophils observed in the CNS at sacrifice would therefore have been recruited from the periphery several days after the initiation of therapy.
Leucocyte infiltration into the CNS is known to be a highly regulated process with multiple routes of entry and different entry requirements [51]. The ‘traditional’ route, direct extravasation from blood in capillary beds into the parenchyma, requires breach of the blood–brain barrier; an additional restriction, absent in other tissues, which profoundly influences entry. In the rat EAE model, blood–brain barrier breakdown occurs concomitant with the onset of clinical disease [37]. In the present study, C5aR antagonist administration preceded this event. Alternative routes via either the cerebrospinal fluid or the subarachnoid space may also be important in some situations and likely have yet other sets of entry requirements. Numerous leucocyte-expressed adhesion molecules and CNS-expressed chemokines have now been implicated as players in this complex game. An evolving concept here is that of redundancy – deletion of an important receptor/ligand interaction is often without effect because other systems exist to fill that role. For example, individual deletion of the chemokine receptor CCR-5, the receptor for the important macrophage chemoattractant MIP-1α, was without effect in EAE [52], whereas deletions of the receptor for the monocyte chemoattractant MCP-1 have in different studies been shown to inhibit EAE or have no effect other than to alter the neutrophil/monocyte balance in the infiltrate [53,54]. The findings in C5aR knockout mice [46], implying that C5a/C5aR are redundant for leucocyte migration into the CNS, are compromised by uncertainty over the role of C in this model. This implication is, however, much strengthened by the demonstration here that blockade of the C5aR is without effect in the C-dependent Lewis rat EAE/ADEAE models.
Absence of MAC in C6-deficient rats inhibited disease, myelin loss and axonal injury in ADEAE but had little effect on leucocyte infiltration; in contrast, inhibition of C activation with sCR1 inhibited all disease parameters, including leucocyte infiltration [15,17]. These data imply that C activation products other than MAC drive inflammation and provoked us to examine further the role of C5a. The elimination of C5a leaves few other candidates. Therapy with sCR1 inhibits the C3- and C5-convertases and thus inhibits also the production of C3a. Receptors for C3a (C3aR) are expressed on leucocytes, although usually at low copy number, and C3a is at best a weak chemoattractant [55,56]. However, resident cells (astrocytes, microglia and neurones) and infiltrating cells (T lymphocytes and macrophages) all express C3aR in the inflamed CNS [56]. C3a has been shown to trigger the production of chemokines from epithelial cells [57]. It is thus possible that C3a acts locally on resident CNS cells to trigger production of chemokines from these cells that then drive further leucocyte infiltration. The role of C3a in EAE models should now be interrogated using recently described C3aR knockout mice and specific C3aR antagonists.
Acknowledgments
This work was funded by The Wellcome Trust (Programme Grant 0685900) and the UK Multiple Sclerosis Society (Project Grant 577/01).
REFERENCES
- 1.Walport MJ. Complement. First of two parts. N Engl J Med. 2001;344:1058–66. doi: 10.1056/NEJM200104053441406. [DOI] [PubMed] [Google Scholar]
- 2.Morgan BP, Gasque P, Singhrao SK, Piddlesden SJ. Role of complement in inflammation and injury in the nervous system. Exp Clin Immunogenet. 1997;14:19–23. [PubMed] [Google Scholar]
- 3.Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. N Engl J Med. 2000;343:938–52. doi: 10.1056/NEJM200009283431307. [DOI] [PubMed] [Google Scholar]
- 4.Storch MK, Piddlesden S, Haltia M, Iivanainen M, Morgan BP, Lassmann H. Multiple sclerosis. in situ evidence for antibody- and complement-mediated demyelination. Ann Neurol. 1998;43:465–71. doi: 10.1002/ana.410430409. [DOI] [PubMed] [Google Scholar]
- 5.Kornek B, Storch MK, Weissert R, et al. Multiple sclerosis and chronic autoimmune encephalomyelitis. a comparative quantitative study of axonal injury in active, inactive, and remyelinated lesions. Am J Pathol. 2000;157:267–76. doi: 10.1016/S0002-9440(10)64537-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davie CA, Barker GJ, Webb S, Tofts PS, Thompson AJ, Harding AE, McDonald WI, Miller DH. Persistent functional deficit in multiple sclerosis and autosomal dominant cerebellar ataxia is associated with axon loss. Brain. 1995;118:1583–92. doi: 10.1093/brain/118.6.1583. [DOI] [PubMed] [Google Scholar]
- 7.Waxman SG. Demyelinating diseases – new pathological insights, new therapeutic targets. N Engl J Med. 1998;338:323–5. doi: 10.1056/NEJM199801293380610. [DOI] [PubMed] [Google Scholar]
- 8.Lucchinetti C, Bruck W, Parisi J, Scheithauer B, Rodriguez M, Lassmann H. Heterogeneity of multiple sclerosis lesions. implications for the pathogenesis of demyelination. Ann Neurol. 2000;47:707–17. doi: 10.1002/1531-8249(200006)47:6<707::aid-ana3>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 9.Bornstein MB, Appel H. Tissue culture studies of demyelination. Ann NY Acad Sci. 1965;122:280–6. doi: 10.1111/j.1749-6632.1965.tb20212.x. [DOI] [PubMed] [Google Scholar]
- 10.Liu WT, Vanguri P, Shin ML. Studies on demyelination in vitro: the requirement of membrane attack components of the complement system. J Immunol. 1983;131:778–82. [PubMed] [Google Scholar]
- 11.Scolding NJ, Morgan BP, Houston A, Campbell AK, Linington C. Compston DA. Normal rat serum cytotoxicity against syngeneic oligodendrocytes. Complement activation and attack in the absence of anti-myelin antibodies. J Neurol Sci. 1989;89:289–300. doi: 10.1016/0022-510x(89)90030-0. [DOI] [PubMed] [Google Scholar]
- 12.Linington C, Lassmann H, Morgan BP, Compston DA. Immunohistochemical localisation of terminal complement component C9 in experimental allergic encephalomyelitis. Acta Neuropathol. 1989;79:78–85. doi: 10.1007/BF00308961. [DOI] [PubMed] [Google Scholar]
- 13.Compston DA, Morgan BP, Campbell AK, Wilkins P, Cole G, Thomas ND, Jasani B. Immunocytochemical localization of the terminal complement complex in multiple sclerosis. Neuropathol Appl Neurobiol. 1989;15:307–16. doi: 10.1111/j.1365-2990.1989.tb01231.x. [DOI] [PubMed] [Google Scholar]
- 14.Linington C, Morgan BP, Scolding NJ, Wilkins P, Piddlesden SJ. Compston DA. The role of complement in the pathogenesis of experimental allergic encephalomyelitis. Brain. 1989;112:895–911. doi: 10.1093/brain/112.4.895. [DOI] [PubMed] [Google Scholar]
- 15.Piddlesden SJ, Storch MK, Hibbs M, Freeman AM, Lassmann H, Morgan BP. Soluble recombinant complement receptor 1 inhibits inflammation and demyelination in antibody-mediated demyelinating experimental allergic encephalomyelitis. J Immunol. 1994;152:5477–84. [PubMed] [Google Scholar]
- 16.Leenaerts PL, Stad RK, Hall BM, Van Damme BJ, Vanrenterghem Y, Daha MR. Hereditary C6 deficiency in a strain of PVG/c rats. Clin Exp Immunol. 1994;97:478–82. doi: 10.1111/j.1365-2249.1994.tb06113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mead RJ, Singhrao SK, Neal JW, Lassmann H, Morgan BP. The membrane attack complex of complement causes severe demyelination associated with acute axonal injury. J Immunol. 2002;168:458–65. doi: 10.4049/jimmunol.168.1.458. [DOI] [PubMed] [Google Scholar]
- 18.Heideman M, Hugli TE. Anaphylatoxin generation in multisystem organ failure. J Trauma. 1984;24:1038–43. doi: 10.1097/00005373-198412000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Mulligan MS, Schmid E, Beck-Schimmer B, et al. Requirement and role of C5a in acute lung inflammatory injury in rats. J Clin Invest. 1996;98:503–12. doi: 10.1172/JCI118818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gasque P, Singhrao SK, Neal JW, Gotze O, Morgan BP. Expression of the receptor for complement C5a (CD88) is up-regulated on reactive astrocytes, microglia, and endothelial cells in the inflamed human central nervous system. Am J Pathol. 1997;150:31–41. [PMC free article] [PubMed] [Google Scholar]
- 21.Sayah S, Patte C, Gasque P, Chan P, Ischenko A, Vaudry H, Fontaine M. Characterization of rat C5a anaphylatoxin receptor (C5aR): cloning of rat C5aR cDNA and study of C5aR expression by rat astrocytes. Brain Res Mol Brain Res. 1997;48:215–22. doi: 10.1016/s0169-328x(97)00094-6. [DOI] [PubMed] [Google Scholar]
- 22.Van Beek J, Bernaudin M, Petit E, Gasque P, Nouvelot A, MacKenzie ET, Fontaine M. Expression of receptors for complement anaphylatoxins C3a and C5a following permanent focal cerebral ischemia in the mouse. Exp Neurol. 2000;161:373. doi: 10.1006/exnr.1999.7273. [DOI] [PubMed] [Google Scholar]
- 23.Akatsu H, Abe M, Miwa T, Tateyama H, Maeda S, Okada N, Kojima K, Okada H. Distribution of rat C5a anaphylatoxin receptor. Microbiol Immunol. 2002;46:863–74. doi: 10.1111/j.1348-0421.2002.tb02774.x. [DOI] [PubMed] [Google Scholar]
- 24.Nataf S, Davoust N, Barnum SR. Kinetics of anaphylatoxin C5a receptor expression during experimental allergic encephalomyelitis. J Neuroimmunol. 1998;91:147–55. doi: 10.1016/s0165-5728(98)00169-6. [DOI] [PubMed] [Google Scholar]
- 25.Stahel PF, Kossmann T, Morganti-Kossmann MC, Hans VHJ, Barnum SR. Experimental diffuse axonal injury induces enhanced neuronal C5a receptor mRNA expression in rats. Mol Brain Res. 1997;50:205–12. doi: 10.1016/s0169-328x(97)00189-7. [DOI] [PubMed] [Google Scholar]
- 26.Muller-Ladner U, Jones JL, Wetsel RA, Gay S, Raine CS, Barnum SR. Enhanced expression of chemotactic receptors in multiple sclerosis lesions. J Neurol Sci. 1996;144:135–41. doi: 10.1016/s0022-510x(96)00217-1. [DOI] [PubMed] [Google Scholar]
- 27.Stahel PF, Kariya K, Shohami E, Barnum SR, Eugster H, Trentz O, Kossmann T, Morganti-Kossmann MC. Intracerebral complement C5a receptor (CD88) expression is regulated by TNF and lymphotoxin-alpha following closed head injury in mice. J Neuroimmunol. 2000;109:164–72. doi: 10.1016/s0165-5728(00)00304-0. [DOI] [PubMed] [Google Scholar]
- 28.Farkas I, Takahashi M, Fukuda A, et al. Complement C5a receptor-mediated signaling may be involved in neurodegeneration in Alzheimer's disease. J Immunol. 2003;170:5764–71. doi: 10.4049/jimmunol.170.11.5764. [DOI] [PubMed] [Google Scholar]
- 29.Mulligan MS, Schmid E, Till GO, et al. C5a-dependent up-regulation in vivo of lung vascular P-selectin. J Immunol. 1997;158:1857–61. [PubMed] [Google Scholar]
- 30.Kroshus TJ, Rollins SA, Dalmasso AP, et al. Complement inhibition with an anti-C5 monoclonal antibody prevents acute cardiac tissue injury in an ex vivo model of pig-to-human xenotransplantation. Transplantation. 1995;60:1194–202. [PubMed] [Google Scholar]
- 31.Wang Y, Hu Q, Madri JA, Rollins SA, Chodera A, Matis LA. Amelioration of lupus-like autoimmune disease in NZB/WF1 mice after treatment with a blocking monoclonal antibody specific for complement component C5. Proc Natl Acad Sci USA. 1996;93:8563–8. doi: 10.1073/pnas.93.16.8563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Finch AM, Wong AK, Paczkowski NJ, Wadi SK, Craik DJ, Fairlie DP, Taylor SM. Low-molecular-weight peptidic and cyclic antagonists of the receptor for the complement factor C5a. J Med Chem. 1995;42:1965–74. doi: 10.1021/jm9806594. [DOI] [PubMed] [Google Scholar]
- 33.Short A, Wong AK, Finch AM, Haaima G, Shiels IA, Fairlie DP, Taylor SM. Effects of a new C5a receptor antagonist on C5a- and endotoxin-induced neutropenia in the rat. Br J Pharmacol. 1999;126:551–4. doi: 10.1038/sj.bjp.0702338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Woodruff TM, Strachan AJ, Dryburgh N, Shiels IA, Reid RC, Fairlie DP, Taylor SM. Antiarthritic activity of an orally active C5a receptor antagonist against antigen-induced monarticular arthritis in the rat. Arthritis Rheum. 2002;46:2476–85. doi: 10.1002/art.10449. [DOI] [PubMed] [Google Scholar]
- 35.Woodruff TM, Arumugam TV, Shiels IA, Reid RC, Fairlie DP, Taylor SM. A potent human C5a receptor antagonist protects against disease pathology in a rat model of inflammatory bowel disease. J Immunol. 2003;171:5514–20. doi: 10.4049/jimmunol.171.10.5514. [DOI] [PubMed] [Google Scholar]
- 36.Wong AK, Finch AM, Pierens GK, Craik DJ, Taylor SM, Fairlie DP. Small molecular probes for G-protein-coupled C5a receptors: Conformationally constrained antagonists derived from the C terminus of the human plasma protein C5a. J Med Chem. 1998;41:3417–25. doi: 10.1021/jm9800651. [DOI] [PubMed] [Google Scholar]
- 37.Linington C, Bradl M, Lassmann H, Brunner C, Vass K. Augmentation of demyelination in rat acute allergic encephalomyelitis by circulating mouse monoclonal antibodies directed against a myelin/oligodendrocyte glycoprotein. Am J Pathol. 1988;130:443–54. [PMC free article] [PubMed] [Google Scholar]
- 38.van Waesberghe JH, Kamphorst W, De Groot CJ, et al. Axonal loss in mutiple sclerosis lesions: magnetic resonance imaging insights into substrates of disability. Ann Neurol. 1999;46:747–54. doi: 10.1002/1531-8249(199911)46:5<747::aid-ana10>3.3.co;2-w. [DOI] [PubMed] [Google Scholar]
- 39.Freeman GE, Dalton CA, Brooks PM. A Nycodenz gradient method for the purification of neutrophils from the peripheral blood of rats. J Immunol Meth. 1991;139:241–9. doi: 10.1016/0022-1759(91)90194-k. [DOI] [PubMed] [Google Scholar]
- 40.Arumugam TV, Woodruff TM, Stocks SZ, et al. Protective effect of a human C5a receptor antagonist against hepatic ischaemia-reperfusion injury in rats. J Hepatol. 2004;40:934–41. doi: 10.1016/j.jhep.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 41.Hugli TE. The structural basis for anaphylatoxin and chemotactic functions of C3a, C4a, and C5a. Crit Rev Immunol. 1981;1:321–66. [PubMed] [Google Scholar]
- 42.Nataf S, Davoust N, Ames RS, Barnum SR. Human T cells express the C5a receptor and are chemoattracted to C5a. J Immunol. 1999;162:4018–23. [PubMed] [Google Scholar]
- 43.Mackay CR. Chemokines: immunology's high impact factors. Nat Immunol. 2001;2:95–101. doi: 10.1038/84298. [DOI] [PubMed] [Google Scholar]
- 44.Minami M, Satoh M. Chemokines and their receptors in the brain: pathophysiological roles in ischemic brain injury. Life Sci. 2003;74:321–7. doi: 10.1016/j.lfs.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 45.Welch TR, Frenzke M, Witte D, Davis AE. C5a is important in the tubulointerstitial component of experimental immune complex glomerulonephritis. Clin Exp Immunol. 2002;130:43–8. doi: 10.1046/j.1365-2249.2002.01957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reiman R, Gerard C, Campbell IL, Barnum SR. Disruption of the C5a receptor gene fails to protect against experimental allergic encephalomyelitis. Eur J Immunol. 2002;32:1157–63. doi: 10.1002/1521-4141(200204)32:4<1157::AID-IMMU1157>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 47.Nataf S, Carroll SL, Wetsel RA, Szalai AJ, Barnum SR. Attenuation of experimental autoimmune demyelination in complement-deficient mice. J Immunol. 2000;165:5867–73. doi: 10.4049/jimmunol.165.10.5867. [DOI] [PubMed] [Google Scholar]
- 48.Calida DM, Constantinescu C, Purev E, Zhang GX, Ventura ES, Lavi E, Rostami A. Cutting edge. C3, a key component of complement activation, is not required for the development of myelin oligodendrocyte glycoprotein peptide-induced experimental autoimmune encephalomyelitis in mice. J Immunol. 2001;166:723–6. doi: 10.4049/jimmunol.166.2.723. [DOI] [PubMed] [Google Scholar]
- 49.Mead RJ, Neal JW, Griffiths MR, Linington C, Botto M, Lassmann H, Morgan BP. Deficiency of the complement regulator CD59a enhances disease severity, demyelination and axonal injury in murine acute experimental allergic encephalomyelitis. Laboratory Invest. 2004;84:21–8. doi: 10.1038/labinvest.3700015. [DOI] [PubMed] [Google Scholar]
- 50.Piddlesden SJ, Lassmann H, Zimprich F, Morgan BP, Linington C. The demyelinating potential of antibodies to myelin oligodendrocyte glycoprotein is related to their ability to fix complement. Am J Pathol. 1993;143:555–64. [PMC free article] [PubMed] [Google Scholar]
- 51.Ransohoff RM, Kivisakk P, Kidd G. Three or more routes for leukocyte migration into the central nervous system. Nat Rev Immunol. 2003;3:569–81. doi: 10.1038/nri1130. [DOI] [PubMed] [Google Scholar]
- 52.Tran EH, Kuziel WA, Owens T. Induction of experimental autoimmune encephalomyelitis in C57BL/6 mice deficient in either the chemokine macrophage inflammatory protein-1alpha or its CCR5 receptor. Eur J Immunol. 2000;30:1410–5. doi: 10.1002/(SICI)1521-4141(200005)30:5<1410::AID-IMMU1410>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 53.Izikson L, Klein RS, Charo IF, Weiner HL, Luster AD. Resistance to experimental autoimmune encephalomyelitis in mice lacking the CC chemokine receptor (CCR) 2. J Exp Med. 2000;192:1075–80. doi: 10.1084/jem.192.7.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fife BT, Huffnagle GB, Kuziel WA, Karpus WJ. CC chemokine receptor 2 is critical for induction of experimental autoimmune encephalomyelitis. J Exp Med. 2000;192:899–905. doi: 10.1084/jem.192.6.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zwirner J, Gotze O, Begemann G, Kapp A, Kirchhoff K, Werfel T. Evaluation of C3a receptor expression on human leucocytes by the use of novel monoclonal antibodies. Immunology. 1999;97:166–72. doi: 10.1046/j.1365-2567.1999.00764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gasque P, Singhrao SK, Neal JW, Wang P, Sayah S, Fontaine M, Morgan BP. The receptor for complement anaphylatoxin C3a is expressed by myeloid cells and non-myeloid cells in inflamed human CNS. Analysis in multiple sclerosis and bacterial meningitis. J Immunol. 1998;160:3543–54. [PubMed] [Google Scholar]
- 57.Monsinjon T, Gasque P, Ischenko A, Fontaine M. C3a binds to the seven transmembrane anaphylatoxin receptor expressed by epithelial cells and triggers the production of IL-8. FEBS Lett. 2001;487:339–46. doi: 10.1016/s0014-5793(00)02320-6. [DOI] [PubMed] [Google Scholar]