Abstract
Intranasal vaccination may offer practical benefits and better protection against respiratory infections, including tuberculosis. In this paper, we investigated the persistence of the Mycobacterium bovis-strain bacille Calmette–Guérin (BCG) Pasteur, lung granuloma formation and protection against pathogenic tuberculous challenge in mice. A pronounced BCG dose-dependent granulomatous infiltration of the lungs was observed following intranasal, but not after subcutaneous, vaccination. Corresponding doses of BCG, over a 100-fold range, imparted similar protection against H37Rv challenge when comparing the intranasal and subcutaneous vaccination routes. Interestingly, a BCG dose-dependent reduction of the H37Rv challenge infection was observed in the lungs, but not in the spleens, following both intranasal and subcutaneous vaccination. In the light of the observed concurrence between the extent of granuloma formation and the level of protection of the lungs, we conclude that intranasal vaccination leading to best protective efficacy needs to be balanced with an acceptable safety margin avoiding undue pathology in the lungs.
Keywords: BCG, intranasal vaccination, lung granuloma, tuberculosis, vaccine dosage
INTRODUCTION
Vaccination of humans by the nasal route could offer practical advantages for vaccine administration, particularly in developing countries, and a potentially better efficacy against respiratory diseases by engaging local immune responses in the lung. Recent studies have demonstrated that intranasal vaccination with bacille Calmette–Guérin (BCG) led to better protection of mice against challenge by Mycobacterium tuberculosis[1] or M. bovis[2] which was attributed to an enhanced and more rapid production of interferon (IFN)-gamma by T cells [3]. Better protection was also observed in the majority of studies using aerosol delivery of BCG [4]. However, intranasal and pulmonary vaccination also carries a potential risk of inducing adverse immunopathological reactions in the lungs [5], as demonstrated in a recent study using aerosol-delivered BCG, which resulted in more peribronchiolar granulomatous lesions than subcutaneous vaccination [4].
In this paper, we have reassessed the protective efficacy of intranasal vaccination of BCG against low-dose aerosol challenge by M. tuberculosis, and compared the extent of pulmonary granuloma formation in intranasally and subcutaneously immunized mice. A range of BCG doses was compared to determine how this might impact on protection. The latter aspect is pertinent in view of the controversy on whether activation of Th2 cells by high BCG doses does [6], or does not [7] influence protection and also because large BCG doses have shown protection when used for intragastric, intrarectal [8,9] and intranasal [1] delivery.
MATERIALS AND METHODS
Bacteria
M. bovis, BCG Pasteur strain (Statens Serum Institut, Copenhagen, Denmark) was propagated using batch culture in a chemically defined culture medium (CMM) [10]. M. tuberculosis H37Rv (National Collection of Type Cultures 7416) was grown on Middlebrook 7H10 agar (Difco, Detroit, USA) containing 0·2% (v/v) glycerol and 10% (v/v) OADC enrichment. Seed lots of bacteria were stored at −70°C. For enumeration, samples were plated on Middlebrook 7H10 agar containing 0·2% (v/v) glycerol and 10% (v/v) OADC enrichment and incubated for 3 weeks.
Vaccination
BALB/cJCit, C57BL/6 J and C3H/HeN strain female mice, 6–8 weeks old, were purchased from Charles River, UK. Intranasal inoculations with 30 µl BCG suspensions onto external nares, in fractions spread over a period of 30–45 s, alternating between nostrils, were performed under mild halothane anaesthesia. Subcutaneous inoculations were performed with 50 µl BCG suspensions at the back of the neck.
Challenge with M. tuberculosis
Mice were challenged with H37Rv bacilli by aerosol, using a contained Henderson apparatus [11]. Briefly, aerosols of mean particle diameter of 2 µm were generated by a Collison three-jet nebulizer from a water suspension of M. tuberculosis H37Rv containing approximately 5 × 107 colony-forming units (CFU)/ml. The aerosol was delivered for 5 min directly to the snouts of animals at 55 l/min air flow rate, resulting in an estimated inhaled dose at time 0 of approximately 500 CFU/lung. At 4 weeks post-challenge mice were killed and the lungs and spleens were removed and tested for number of viable mycobacteria as described below.
CFU assay
Lungs and spleens of mice following BCG vaccination or M. tuberculosis challenge were homogenized in 1 ml of sterile distilled water and decimal dilutions were plated on Middlebrook 7H11 agar (Difco) containing 0·2% (v/v) glycerol and 10% (v/v) OADC. Samples of the lungs from the intranasal groups and samples of spleens from the subcutaneous groups were also plated on Thiophene 2 carboxylic acid hydrazide (TCH) agar (Sigma, UK), which was selective for M. tuberculosis but not BCG. Plates were incubated for 3 weeks at 37°C. Log-transformed CFU counts per ml of lung or spleen homogenate, with a detection limit of 5 CFU per organ, were compared using the unpaired Student's t-test, P < 0·05 being considered as significant. Comparison of CFU counts on 7H11 and selective TCH agar plates revealed no significant differences (P > 0·05), indicating that the CFU could be attributed solely to M. tuberculosis without any contribution from persisting BCG.
Histopathology of lungs: morphometric analysis
Lungs were removed at 12 weeks after BCG vaccination and were fixed in 10% neutral buffered formalin, processed on a Tissue-Tek VIP 150 and embedded into wax. Sections of 5 µm were cut at the widest organ diameter using a Leica RM2035 and were stained on Varistain 24–3 with haematoxylin and eosin and mounted using the DPX mountant. Lung cross-sections were scanned on an Olympus BX51 microscope, linked with a ColourView camera and digitized images were analysed using the AnalySIS software (version 3·2) of the Soft Imaging System. The values of square pixels for each granulomatous lesion were totalled for the entire section area examined. After subtracting the areas occupied by bronchiolar spaces, the percentage of the granulomatously infiltrated area was calculated.
Statistical analyses
The data from this study were analysed by using two-sample t-tests, linear regression analyses, F-tests and Mann–Whitney tests with minitab version 13·32. Graphs were obtained using Excel.
RESULTS
Persistence of BCG in organs
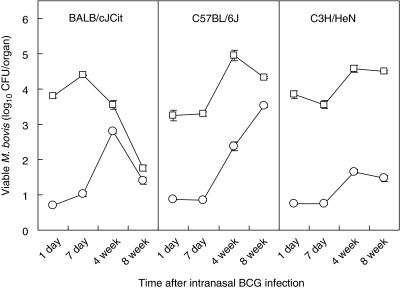
Groups of BALB/cJCit, C57BL/6 J and C3H/HeN strain female mice (n = 10), 6–8 weeks old, were vaccinated intranasally with BCG Pasteur. The persistence of viable BCG in the lungs and spleens was tested after 24 h, 1, 4 and 8 weeks after infection (Fig. 1). There were differences in the response between the three strains of mice used in the experiment, and the pattern of the response by time also differed for each of the mice strains (F-test6,95 = 3·52, P < 0·005). In the lungs, there was a significant relationship between clearance of BCG in the BALB/cJCit with time (slope = −0·0417, n = 30, P < 0·001). In contrast an increase in viable BCG was seen, with time, in the C3H/HeN (slope = 0·016, n = 39, P < 0·05) and C57BL/6 J (slope = 0·023, n = 38, P < 0·05) strains.
Fig. 1.
Time–course of BCG infection in three strains of intranasally inoculated mice (BALB/cJCit, C57BL/6 J, C3H/HeN). Means (n = 10) of CFU counts in the lungs (open squares) and spleens (open circles) at different times after inoculation with 106 CFU BCG-Pasteur strain organisms. Standard errors < ±0·16. Limit of detection 0·7 log CFU/organ.
In the spleen, the linear regressions lines for clearance of BCG, with time, for each strain were significantly different from one another (F-test6,100 = 9·34, P < 0·001). All three strains of mice had a positive correlation, indicating that BCG infection increased in the spleen over the 8-week period with time [BALB/cJCit (slope = 0·015, n = 32, P > 0·05), C3H/HeN (slope = 0·154, n = 40, P < 0·001) and C57BL/6 J (slope = 0·051, n = 40, P < 0·001)]. Notably, however, in BALB/cJCit mice BCG levels began to decline between 4 and 8 weeks in the spleen.
BCG-induced protection against M. tuberculosis challenge
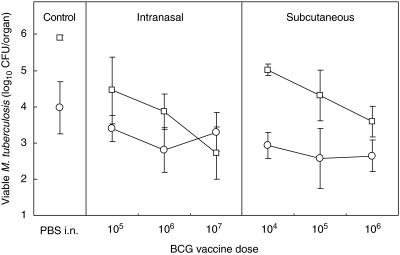
Groups of BALB/cJCit mice (n = 8) were vaccinated subcutaneously with BCG, to give a dose of 104, 105 or 106 CFU. Separate groups of mice (n = 8) were vaccinated intranasally with suspensions of 105, 106 or 107 CFU. Twelve weeks post-vaccination with BCG, mice were challenged with 500 CFU/lung of M. tuberculosis H37Rv by the aerosol route. At 4 weeks post-challenge, mycobacterial CFUs were determined in the lungs and spleens.
A pronounced dose-dependency of protection, evident by the reduction in M. tuberculosis lung CFU, was observed (Fig. 2) in both vaccination groups. Following intranasal delivery, there was a very significant difference between the vaccine groups and the control for the lung counts (t = 10·41, P < 0·001), and no significant difference between the control and vaccine groups in the spleen counts (t = 2·41, P > 0·05). The highest (107) BCG dose imparted stronger protection than 105 or 106 BCG. After fitting a linear regression model to the intranasal vaccine groups (slope = −0·846, 95% CI 0·438–1·254, n = 21, P < 0·001) it was shown that for each unit increase (on the log scale) in vaccination there was a decrease of 0·846 in the log of the lung counts of M. tuberculosis. There was no dose–response in the protective efficacy of vaccination (slope = −0·064, n = 21, P > 0·05) in the spleen.
Fig. 2.
The effect of BCG vaccine dose (CFU/mouse) on protection against virulent tuberculous challenge. BALB/cJCit mice were challenged by exposure to H37Rv aerosol 12 weeks after BCG vaccination. Mean (n = 8) and standard deviation (vertical bars) of CFU counts in the lungs (open squares) and spleens (open circles) at 16 weeks are shown. Limit of detection 0·7 log CFU/organ.
The protective effect of subcutaneous vaccination also showed a linear relationship with a negative slope and hence a decrease of 0·716 in the log of lung counts of M. tuberculosis for each unit increase in vaccination dose (slope = −0·716, 95% CI –0·415, −1·017, n = 21, P < 0·001). Notably, the 105 and 106 doses produced equivalent protection when given either intranasally or subcutaneously. In contrast, in the spleen, there was no significant difference in the protective effect of the different subcutaneous vaccine doses and there was no significant decrease in the spleen counts of M. tuberculosis with each unit increase in subcutaneous vaccination (slope = −0·152, n = 21, P = 0·4).
Pulmonary granuloma formation
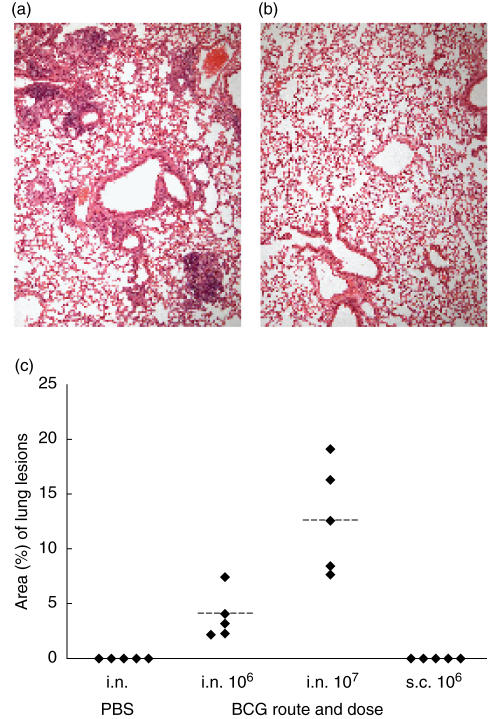
Following intranasal delivery of 106 and 107 BCG, typical granulomatous lesions were observed with a predominantly perivascular and peribronchiolar location (Fig. 3a). Quantitative planimetric evaluation of the area of the lungs affected with these lesions showed that it was significantly greater (P = 0·012, Mann–Whitney test) in mice inoculated with 107 CFU (median 12·55%, interquartile range 8·02–17·66%) than in mice given 106 CFU BCG (median 3·18%, interquartile range 2·26–5·70%) (Fig. 3c). Lung sections from mice which had been vaccinated subcutaneously with 106 BCG, or control mice given PBS intranasally, had no demonstrable granulomatous lesions (Fig. 3b).
Fig. 3.
Morphometric analysis of granulomatous infiltration of the lungs. Representative haematoxylin and eosin stained sections of lungs, harvested 12 weeks after intranasal delivery of 107 CFU organisms (a) or phosphate buffered saline (PBS) (b). Lungs from BALB/cJCit mice given 106 CFU BCG subcutaneous were indistinguishable from the intranasal PBS inoculated controls. (c) Individual values of the relative proportion of lung areas with granulomatous lesions from five mice per group after intranasal (i.n.) or subcutaneous (s.c.) BCG infection and intranasal PBS inoculated controls. Dotted lines = mean values.
DISCUSSION
The fate and organ distribution of BCG following intranasal vaccination varied between the three strains of mice that were studied. Lung CFUs declined substantially from 1 to 8 weeks in BALB/cJCit mice, but remained largely static in the C57BL/6 J and C3H/HeN strains. This pattern was consistent with previously reported Nramp gene control of splenic infection [12] and variations in cytokine production in different strains of mice [13], but not of the susceptibility to chronic infection with virulent M. tuberculosis[14]. Because the declining trend of lung BCG CFUs in BALB/cJCit mice seemed conducive for the evaluation of protection, we used BALB/cJCit mice in the later M. tuberculosis challenge experiments.
As tuberculosis is predominantly a lung disease, the observed BCG dose-dependent protection of the lungs is of particular interest. The beneficial effect of high BCG dose is in agreement with the results of a study in humans in which the frequency and intensity of T cell responses was found to be BCG dose-dependent [15]. As the BCG dose-dependency did not apply to the protection of the spleen, our findings suggest the role of some distinct protective mechanisms in the lungs. Interestingly, the BCG dose-dependent protection was pronounced equally following intranasal and subcutaneous BCG delivery, suggesting that the pulmonary protective mechanisms can be initiated by both local and systemic vaccination. This may involve the regulatory effects of their cytokines [6] from a subset of CD4 T cells, which are recruited to the lungs [16]. Their selective homing may have involved even distant mucosal sites, such as the gut, where a proportion of the higher dose BCG could have reached following intranasal inoculation.
Analysis of the BCG dose–protection relationship suggested that the highest dose of intranasal BCG produced the best protection of the lungs, but it also caused BCG dose-dependent granulomatous pulmonary infiltration. This raises the question as to the degree of lung infiltration that would be acceptable on safety grounds. Sporadic occurrence of lung granulomas of small size may not cause adverse effects, considering that they are probably a common feature of self-healing, i.e. a protective host response, to airborne natural tuberculosis (TB) infections in man. However, more extensive granulomatous infiltration could be classified as a pathological lesion. These concerns over intranasal vaccination are also pertinent to recombinant BCG strains, particularly those expressing cytokines [17,18], since granuloma formation has been attributed to both T cell dependent and independent production of IFN-γ and tumour necrosis factor (TNF)-α[19]. As these cytokines are also known mediators of protection, it may be difficult to dissociate the protective effects from the potentially adverse lung histopathology.
To alleviate the possible adverse effects from intranasal vaccination, it could be of interest to use BCG variants lacking the granuloma-inducing trehalose-6,6′-dimycolate [20] or auxotrophic strains of M. tuberculosis[21] with diminished granuloma-inducing capacity. Alternatively, antigenic subunits instead of BCG could be used. DNA inoculation leads to relatively poor pulmonary T cell responses [22], while proteins combined with strong adjuvants such as cholera toxin [23] may also cause adverse pulmonary infiltration and IgE response [5]; interestingly, the latter can be reduced by systemic inoculation of BCG [24], thus favouring vaccination by combined intranasal and systemic routes.
Despite the long history and widespread use of BCG vaccination in humans (although with limited protective efficacy), the optimal vaccine dose and route of delivery have not yet been confirmed. The described BCG dose-dependent protection of lungs, but not of the spleen, suggests the role of different organ-specific mechanisms of protection. When comparing BCG doses, intranasal delivery was as protective as subcutaneous inoculation. Although higher doses of intranasally delivered BCG imparted better protection against the infection of the lungs, this was associated with enhanced granulomatous infiltration. This finding suggests that intranasal vaccination would need to be constrained to those BCG doses avoiding undue pathology of the lungs, and also indicates an advantage of vaccine delivery to sites not draining directly to the lungs. In conclusion, in our experiments intranasal vaccination, although attractive as a delivery route, could not provide better protection than subcutaneous inoculation without the associated lung pathology. A possible way forward may be to explore whether genetically modified strains of mycobacteria provide protection without inducing granuloma formation in the lung.
Acknowledgments
Funding was obtained from a grant QLK2-1999-00367 of the Fifth Framework of the European Commission, and from the Department of Health, UK. The views expressed in the publication are those of the authors and not necessarily those of the funding bodies. We are grateful for the technical assistance of staff from the Biological Investigations Group, HPA, Porton Down and for help with the evaluation of histological sections from Professor Eddy Odell at the Guy's campus of Kings College London. We also thank Dr Jim Todd, London School of Hygiene and Tropical Medicine, for performing the statistical analyses.
REFERENCES
- 1.Falero-Diaz G, Challacombe S, Banerjee D, Douce G, Boyd A, Ivanyi J. Intranasal vaccination of mice against infection with Mycobacterium tuberculosis. Vaccine. 2000;18:3223–9. doi: 10.1016/s0264-410x(00)00134-1. [DOI] [PubMed] [Google Scholar]
- 2.Lyadova IV, Vordermeier HM, Eruslanov EB, Khaidukov SV, Apt AS, Hewinson RG. Intranasal BCG vaccination protects BALB/c mice against virulent Mycobacterium bovis and accelerates production of IFN-gamma in their lungs. Clin Exp Immunol. 2001;126:274–9. doi: 10.1046/j.1365-2249.2001.01667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen L, Wang J, Zganiacz A, Xing Z. Single intranasal mucosal Mycobacterium bovis BCG vaccination confers improved protection compared to subcutaneous vaccination against pulmonary tuberculosis. Infect Immun. 2004;72:238–46. doi: 10.1128/IAI.72.1.238-246.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nuermberger EL, Yoshimatsu T, Tyagi S, Bishai WR, Grosset JH. Paucibacillary tuberculosis in mice after prior aerosol immunization with Mycobacterium bovis BCG. Infect Immun. 2004;72:1065–71. doi: 10.1128/IAI.72.2.1065-1071.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hodge LM, Marinaro M, Jones HP, McGhee JR, Kiyono H, Simecka JW. Immunoglobulin A (IgA) responses and IgE-associated inflammation along the respiratory tract after mucosal but not systemic immunization. Infect Immun. 2001;69:2328–38. doi: 10.1128/IAI.69.4.2328-2338.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Power CA, Wei G, Bretscher PA. Mycobacterial dose defines the Th1/Th2 nature of the immune response independently of whether immunization is administered by the intravenous, subcutaneous, or intradermal route. Infect Immun. 1998;66:5743–50. doi: 10.1128/iai.66.12.5743-5750.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gruppo V, Orme IM. Dose of BCG does not influence the efficient generation of protective immunity in mice challenged with Mycobacterium tuberculosis. Tuberculosis (Edinb) 2002;82:267–73. doi: 10.1054/tube.2002.0340. [DOI] [PubMed] [Google Scholar]
- 8.Abolhassani M, Lagranderie M, Chavarot P, Balazuc AM, Marchal G. Mycobacterium bovis BCG induces similar immune responses and protection by rectal and parenteral immunization routes. Infect Immun. 2000;68:5657–62. doi: 10.1128/iai.68.10.5657-5662.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lagranderie M, Chavarot P, Balazuc AM, Marchal G. Immunogenicity and protective capacity of Mycobacterium bovis BCG after oral or intragastric administration in mice. Vaccine. 2000;18:1186–95. doi: 10.1016/s0264-410x(99)00386-2. [DOI] [PubMed] [Google Scholar]
- 10.James BW, Williams A, Marsh PD. The physiology and pathogenicity of Mycobacterium tuberculosis grown under controlled conditions in a defined medium. J Appl Microbiol. 2000;88:669–77. doi: 10.1046/j.1365-2672.2000.01020.x. [DOI] [PubMed] [Google Scholar]
- 11.Druett HA. A mobile form of the Henderson apparatus. J Hygiene (Lond) 1969;67:437–48. doi: 10.1017/s0022172400041851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Skamene E. The Bcg gene story. Immunobiology. 1994;191:451–60. doi: 10.1016/S0171-2985(11)80451-1. [DOI] [PubMed] [Google Scholar]
- 13.Wakeham J, Wang J, Xing Z. Genetically determined disparate innate and adaptive cell-mediated immune responses to pulmonary Mycobacterium bovis BCG infection in C57BL/6 and BALB/c mice. Infect Immun. 2000;68:6946–53. doi: 10.1128/iai.68.12.6946-6953.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Medina E, Rogerson BJ, North RJ. The Nramp1 antimicrobial resistance gene segregates independently of resistance to virulent Mycobacterium tuberculosis. Immunology. 1996;88:479–81. doi: 10.1046/j.1365-2567.1996.d01-700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lowry PW, Ludwig TS, Adams JA, et al. Cellular immune responses to four doses of percutaneous bacille Calmette–Guérin in healthy adults. J Infect Dis. 1998;178:138–46. doi: 10.1086/515614. [DOI] [PubMed] [Google Scholar]
- 16.Palendira U, Bean AG, Feng CG, Britton WJ. Lymphocyte recruitment and protective efficacy against pulmonary mycobacterial infection are independent of the route of prior Mycobacterium bovis BCG immunization. Infect Immun. 2002;70:1410–6. doi: 10.1128/IAI.70.3.1410-1416.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murray PJ, Aldovini A, Young RA. Manipulation and potentiation of antimycobacterial immunity using recombinant bacille Calmette–Guérin strains that secrete cytokines. Proc Natl Acad Sci USA. 1996;93:934–9. doi: 10.1073/pnas.93.2.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marshall BG, Wangoo A, O'Gaora P, Cook HT, Shaw RJ, Young DB. Enhanced antimycobacterial response to recombinant Mycobacterium bovis BCG expressing latency-associated peptide. Infect Immun. 2001;69:6676–82. doi: 10.1128/IAI.69.11.6676-6682.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith D, Hansch H, Bancroft G, Ehlers S. T-cell-independent granuloma formation in response to Mycobacterium avium: role of tumour necrosis factor-alpha and interferon-gamma. Immunology. 1997;92:413–21. doi: 10.1046/j.1365-2567.1997.00384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Actor JK, Olsen M, Hunter RL, Jr, Geng YJ. Dysregulated response to mycobacterial cord factor trehalose-6,6′-dimycolate in CD1D-/- mice. J Interferon Cytokine Res. 2001;21:1089–96. doi: 10.1089/107999001317205222. [DOI] [PubMed] [Google Scholar]
- 21.Smith DA, Parish T, Stoker NG, Bancroft GJ. Characterization of auxotrophic mutants of Mycobacterium tuberculosis and their potential as vaccine candidates. Infect Immun. 2001;69:1142–50. doi: 10.1128/IAI.69.2.1142-1150.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.D'Souza S, Rosseels V, Denis O, et al. Improved tuberculosis DNA vaccines by formulation in cationic lipids. Infect Immun. 2002;70:3681–8. doi: 10.1128/IAI.70.7.3681-3688.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodriguez A, Troye-Blomberg M, Lindroth K, Ivanyi J, Singh M, Fernandez C. B- and T-cell responses to the mycobacterium surface antigen PstS-1 in the respiratory tract and adjacent tissues. Role of adjuvants and routes of immunization. Vaccine. 2003;21:458–67. doi: 10.1016/s0264-410x(02)00478-4. [DOI] [PubMed] [Google Scholar]
- 24.Kumar M, Behera AK, Matsuse H, Lockey RF, Mohapatra SS. A recombinant BCG vaccine generates a Th1-like response and inhibits IgE synthesis in BALB/c mice. Immunology. 1999;97:515–21. doi: 10.1046/j.1365-2567.1999.00782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]