Abstract
Expression of heat shock protein (HSP)-65 as well as infiltration of T-cells in arterial lesions and raised levels of circulating antibodies against mycobacterial HSP65 (mHSP65) led us to the concept that mHSP65 or its human homologue (hHSP60) might be involved in the etiopathogenesis of Takayasu's arteritis (TA). Therefore, we investigated mHSP65 and hHSP60 reactive peripheral blood T-cell subsets by BrdU incorporation assay and flow cytometry as well as investigating the different isotypes of anti-mHSP65 and hHSP60 antibodies by ELISA. Eighty-four percent (22/26) of the TA patients were observed to show T-cell proliferation to mHSP65 and hHSP60 whereas only 16% (3/18) healthy controls showed such proliferation (P < 0·001). Both HSPs induced proliferation of exclusively CD4+ T-cells and not CD8+ T-cells. We also observed a significantly higher prevalence of only the IgG isotype reactive to mHSP65 and hHSP60 in TA as compared to HC (mHSP65: 92% TA versus 11% HC, P < 0·0001 and hHSP60: 84% versus 22%, P < 0·001). Our data show a significant correlation between mHSP65 and hHSP60 reactive T-cells (CD3+: r = 0·901; CD4+: r = 0·968) as well as anti-mHSP65 and anti-hHSP60 IgG antibodies (r = 0·814) suggesting an infection induced autoimmunity in TA, possibly induced by molecular mimicry between mHSP65 and hHSP60 or other tissue specific antigens.
Keywords: Takayasu's arteritis, mycobacterial HSP65, human HSP60, autoimmunity
INTRODUCTION
Takayasu's arteritis is a chronic granulomatous arteritis affecting large elastic arteries, predominantly the aorta, its main branches, and pulmonary and coronary arteries. Vascular inflammation culminates in intimal thickening, fibrosis and stenosis with or without thrombosis. This eventually results in end organ/tissue ischemia and leads to different clinical manifestations of the disease [1–4]. The disease occurs more commonly in young females than males with peak incidence between 15 and 20 years of age. It is the most common vasculitic disorder in India and the third most common vasculitis after Henoch-Schonlein purpura and Kawasaki disease in the paediatric age group worldwide [5–7].
The aetiopathogenesis of TA is largely unknown but most of the available data suggest that it is an autoimmune disease and both cellular [8,9] as well as humoral [10–13] immune mechanisms are involved in the pathogenesis of the disease. The increased numbers of activated circulating T-cells [8] and their presence in vascular lesions [9] suggest that T-cells have a primary role in initiating the disease. However, the putative antigen(s) that trigger activation and generation of these autoreactive T-cells are still not known. Mycobacterium tuberculosis[14,15] has long been implicated, as a possible aetiological agent in TA but there is no convincing data for this. Expression of heat shock protein (HSP)-65 as well as increased infiltration of T-cells in aortic tissue [16] and raised levels of circulating antimycobacterial HSP65 (mHSP65) antibodies [17,18] in patients indicate that HSP65 whether exogenous or endogenous may be a putative antigen stimulating immune responses in the disease. However, cellular and humoral immune response to mHSP65 and its human homologue, i.e. human heat shock protein-60 (hHSP60) has not yet been evaluated in the disease.
Therefore in the present study, we undertook to investigate the proliferative responses of different T-cell subsets as well as different isotypes of antibodies to mHSP65 and hHSP60 in patients with TA.
SUBJECTS AND METHODS
Subjects
Twenty-six patients with TA (20 female, 6 male; mean age 29·15 ± 9·84 years; range 15–47 years) and 18 age/sex matched healthy controls (HC) after obtaining their informed consent were enrolled in the study, which was approved by the Ethics Committee of Sanjay Gandhi Post-graduate Institute of Medical Sciences, Lucknow, India. All patients included in the study fulfilled at least three of the classification and diagnostic criteria of American College of Rheumatology, 1990 [19] and had an angiographically proven disease.
Except three, all the patients included in the study were on immunosuppressive therapy consisting of prednisolone and azathioprine, which is given for 2 years with tapering of the prednisolone dose as the disease become less active. The detailed clinical data of the patients are given in Table 1.
Table 1.
Clinical data of the patients
| TA patient | Age/sex | Type of disease | ESR (mm/h) | CRP (mg/dl) | Disease* activity | Treatment (per day) |
|---|---|---|---|---|---|---|
| 1 | 38/F | III | 35 | 10·55 | Active | Untreated |
| 2 | 25/F | III | 51 | 12·24 | Active | Untreated |
| 3 | 31/F | III | 47 | 8·35 | Active | Untreated |
| 4 | 42/F | I | 44 | 5·03 | Active | Prednisolone 10 mg |
| Azathioprine 100 mg | ||||||
| 5 | 28/M | III | 35 | 6·35 | Active | Prednisolone 10 mg |
| Azathioprine 100 mg | ||||||
| 6 | 36/F | III | 25 | 0·25 | Inactive | Prednisolone 5 mg |
| Azathioprine 100 mg | ||||||
| 7 | 24/F | III | 21 | <0·5 | Inactive | Prednisolone 5 mg |
| Azathioprine 100 mg | ||||||
| 8 | 23/M | III | 35 | <0·5 | Inactive | Prednisolone 5 mg |
| Azathioprine 100 mg | ||||||
| 9 | 14/F | III | 20 | <0·5 | Inactive | Prednisolone 5 mg |
| Azathioprine 100 mg | ||||||
| 10 | 28/F | III | 22 | <0·5 | Inactive | Prednisolone 5 mg |
| Azathioprine 100 mg | ||||||
| 11 | 47/M | III | 18 | <0·5 | Inactive | Prednisolone 5 mg |
| Azathioprine 100 mg | ||||||
| 12 | 15/M | III | 35 | <0·5 | Inactive | Prednisolone 5 mg |
| Azathioprine 100 mg | ||||||
| 13 | 42/F | III | 32 | <0·5 | Inactive | Prednisolone 5 mg |
| Azathioprine 100 mg | ||||||
| 14 | 38/F | III | 32 | <0·5 | Inactive | Prednisolone 5 mg |
| Azathioprine 100 mg | ||||||
| 15 | 25/M | III | 50 | 0·41 | Inactive | Prednisolone 5 mg |
| Azathioprine 100 mg | ||||||
| 16 | 16/F | III | 48 | 0·47 | Inactive | Prednisolone 5 mg |
| Azathioprine 100 mg | ||||||
| 17 | 22/F | III | 16 | 0·30 | Inactive | Prednisolone 5 mg |
| Azathioprine 100 mg | ||||||
| 18 | 20/F | III | 15 | 0·31 | Inactive | Prednisolone 5 mg |
| Azathioprine 100 mg | ||||||
| 19 | 23/M | III | 10 | 0·30 | Inactive | Prednisolone 5 mg |
| Azathioprine 100 mg | ||||||
| 20 | 38/F | I | 25 | <0·5 | Inactive | Prednisolone 5 mg |
| Azathioprine 100 mg | ||||||
| 21 | 42/F | III | 29 | 0·31 | Inactive | Prednisolone 5 mg |
| Azathioprine 100 mg | ||||||
| 22 | 28/F | III | 25 | 0·69 | Inactive | Prednisolone 5 mg |
| Azathioprine 100 mg | ||||||
| 23 | 42/F | III | 41 | 0·30 | Inactive | Prednisolone 5 mg |
| Azathioprine 100 mg | ||||||
| 24 | 24/F | III | 16 | <0·5 | Inactive | Prednisolone 5 mg |
| Azathioprine 100 mg | ||||||
| 25 | 33/F | III | 22 | <0·5 | Inactive | Prednisolone 5 mg |
| Azathioprine 100 mg | ||||||
| 26 | 18/F | III | 9 | 0·33 | Inactive | Prednisolone 5 mg |
| Azathioprine 100 mg |
Disease activity was determined according to previously described criteria [39].
Isolation and culture of peripheral blood mononuclear cells (PBMC)
After informed consent, 10 ml of venous blood was obtained from each subject in a sterile glass tube containing 100 IU of heparin (Sigma, St Louis, MO, USA). Within 3 h of the sample collection, PBMC were isolated by standard Ficoll-Hypaque density gradient centrifugation method. The isolated cells were washed twice with plain medium RPMI-1640 and finally resuspended at the concentration of 2 × 106 cells/ml in complete medium consisting of RPMI-1640 supplemented with 3 m m of l-glutamine, 10 m m of HEPES buffer, 1 m m of sodium pyruvate, 10% of heat inactivated fetal bovine serum and bacteriostatic level of penicillin-streptomycin (all from Gibco BRL, USA). Cells were cultured in flat bottom 24 wells tissue culture plate (Nunc, Roskilde, Denmark) at 1 ml/well in the absence or presence of recombinant mHSP65 and hHSP60 (LIONEX Diagnostics & Therapeutics GmbH, Braunschweig, Germany) at the concentration of 10 µg/ml in a humidified environment at 37°C with 5% CO2 in air for 72 h. During last 24 h the cells were pulsed with 30 µg/ml of BrdU to allow its incorporation into DNA of the proliferating cells.
Flow cytometric analysis of T-cell proliferation
Following the incorporation of BrdU, cells were harvested directly in FACS tubes and washed once in PBS, fixed with FACS Lysing solution and permeablized with 0·1% saponin for 15 min at room temperature. Thereafter 50 Kunitz U/ml DNAse-I, activity 400–600 KU/ml protein (Sigma) dissolved in Earl's Balance Salt Solution (Gibco BRL, USA) was added. DNA was digested for 10 min at 37°C. Cells were washed, resuspended in 100 µl PBS containing 2% bovine serum albumin and incubated with FITC-conjugated antibody to BrdU (Becton Dickinson, CA, USA) at room temperature for 45 min. Following this step cells were washed once in PBS-BSA; resuspended in 300 µl PBS-BSA; divided in three equal parts in different FACS tubes and immunostained with PE-conjugated CD3, CD4 and CD8 monoclonal antibodies (Becton Dickinson) at room temperature for 30 min. Finally cells were washed twice with PBS-BSA and resuspended in 500 µl of PBS containing 1% paraformaldehyde and analysed by flow cytometry (FACS calibre, Becton Dickenson) using Cell-Quest software. A subject was considered to have a positive T-cell proliferative response to mHSP65 or hHSP60 if the percentage of BrdU positive T-cells of the subject was more than mean + 2 standard deviation (SD) of the BrdU positive T-cells of healthy controls to a given HSP.
Detection of different isotypes of anti-HSP antibodies
Antibodies against mHSP65 and hHSP60 were investigated in the sera of patients and controls by ELISA as described [18]. Briefly, 100 µl of 10 µg/ml of mHSP65/hHSP60 in carbonate-bicarbonate buffer (pH 9·6) was added to each well of 96-well microtitre plates (Nunc). Following overnight incubation at 4°C, the plates were washed thrice with PBS and blocked with 200 µl/well of PBS-BSA (2% BSA) for 2 h at 37°C. Plates were then washed with PBS containing 0·05% Tween-20 (PBS-T). For detecting antibodies to HSP, 1 : 200 dilution of test serum was used for IgG, IgM, and IgA isotypes. One hundred microliters of the appropriately diluted samples were added to the wells in duplicate and incubated for 1 h at 37°C. Following washing with PBS-T, 100 µl/well of alkaline phosphatase-conjugated rabbit anti-human IgG/IgM/IgA (Sigma) diluted 1 : 2000 in PBS-BSA was added and incubated for 1 h at 37°C. After washing with PBS-T, the colour reaction was developed by adding 100 µl/well of p-nitrophenyl phosphate (1 mg/ml, Sigma) and absorbance was read in an automated ELISA reader (Tecan Spectra, Austria) at 405 nm. The cut off value for stating that a sample is positive for HSP antibodies was taken as mean + 2SD of the optical densities (OD) of the normal healthy controls.
Statistical analysis
Statistical comparisons between TA patients and healthy controls for cellular proliferative responses and anti-HSP-antibodies levels were performed by Mann–Whitney U-test. Z-statistics was used for comparing the prevalence between the groups. Correlation analyses between HSP responses were done by Spearman's rank correlation test. The data was expressed as mean ± SD and a P-value of <0·05 was considered to be statistically significant.
RESULTS
Proliferative responses of T-cells to mHSP65 and hHSP60
Twenty-two out of 26 (84%) of the TA patients were observed to have T-cell proliferation to mHSP65 and hHSP60 whereas, only 3 (16%) of 18 healthy controls showed T-cell proliferation (P < 0·001).
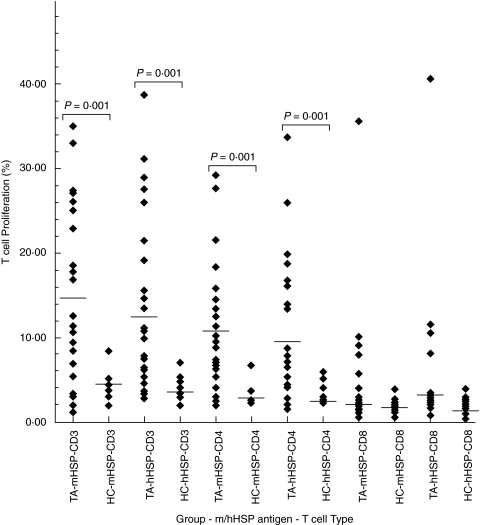
The magnitude of proliferative response of CD3+ T-cells to mHSP65 and hHSP60 was significantly higher in patients as compared to healthy controls (mHSP65: 14·72 ± 9·98% versus 4·19 ± 2·20%, P < 0·001 and hHSP60: 13·25 ± 10·05% versus 3·71 ± 1·51%, P < 0·001). Among the CD3+ T-cells, the percentage of mHSP65 and hHSP60 reactive CD4+ T-cell was significantly higher in patients as compared to the healthy controls (mHSP65: 11·10 ± 8·26% versus 3·49 ± 1·55%, P < 0·001 and hHSP60: 10·67 ± 8·50% versus 3·30 ± 1·20%, P < 0·001). However, CD8+ T-cell showed no significant proliferation to any of the HSPs in both groups (mHSP65: 3·15 ± 2·85% versus 2·85 ± 0·89%, P > 0·05 and hHSP60: 3·95 ± 1·75% versus 2·05 ± 0·93%, P > 0·05).
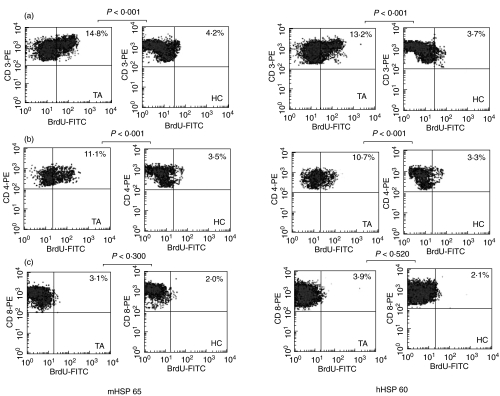
Representative dot plots and box plot of CD3+ T-cells and their CD4+ and CD8 + subsets to mHSP65 and hHSP60 are presented as Figs 1 and 2, respectively.
Fig. 1.
Representative flow cytometric dot plots showing proliferative response of CD3+ T-cells and their CD4+ and CD8+ subsets to mycobacterial heat shock protein-65 (mHSP65) and human heat shock protein-60 (hHSP60) in patients with Takayasu's arteritis (TA) and healthy controls (HC). The proliferative response observed in TA as compared to HC to mHSP65 as well as hHSP60 was significantly higher in (a) CD3+ T-cells and (b) CD4+ T-cells but not in (c) CD8+ T-cells.
Fig. 2.
Dot-plots showing magnitude of proliferative response of CD3+ T-cells and their CD4+ and CD8+ subsets to mycobacterial heat shock protein-65 (mHSP) and human heat shock protein-60 (hHSP) in patients with Takayasu's arteritis (TA) and healthy controls (HC). The proliferative response observed in TA as compared to HC to mHSP65 as well as hHSP60 was significantly higher only in CD3+ T-cells and CD4+ T-cells. Each dot represents the data of an individual subject and horizontal line crossing dots represents mean percentage of proliferative response in each group.
Antibodies to mHSP65 and hHSP60
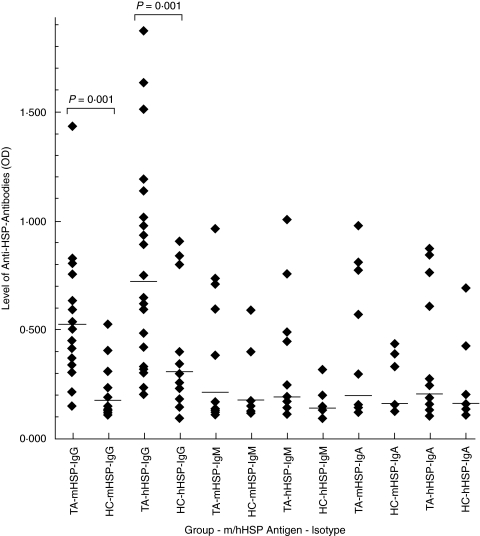
The prevalence of IgG anti-mHSP65 and IgG anti-hHSP60 antibodies was significantly higher in TA as compared to healthy controls (IgG-mHSP65: 92% (24/26) versus 11% (2/18), P < 0·0001 and IgG-hHSP60: 84% (22/26) versus 22% (4/18), P < 0·001). There was no significant difference in the prevalence of IgM-mHSP65 and IgM-hHSP60 isotype (IgM-mHSP65: 19% (5/26) versus 11% (2/18), P > 0·05 and IgM-hHSP60: 15% (4/26) versus 11% (2/18), P > 0·05) as well as IgA-mHSP65 and IgA-hHSP60 isotype (IgA-mHSP65: 15% (4/26) versus 17% (3/18), P > 0·05 and IgA-hHSP60: 15% (4/26) versus 11% (2/18), P > 0·05) between the groups.
Titers of IgG-mHSP65 and IgG-hHSP60 antibodies were also significantly higher in patients as compared to controls (IgG-mHSP65: 0·534 ± 0·343 versus 0·217 ± 0·111, P < 0·001 and IgG-hHSP60: 0·769 ± 0·467 versus 0·330 ± 0·243, P < 0·001). The titres of IgM-mHSP65 and IgM-hHSP60 [IgM-mHSP65: 0·225 ± 0·220 versus 0·178 ± 0·115, P = 0·400 and IgM-hHSP60: 0·219 ± 0·204 versus 0·166 ± 0·072, P = 0·890] as well as those of IgA-mHSP65 and IgA-hHSP60 (IgA-mHSP65: 0·237 ± 0·230 versus 0·182 ± 0·093, P = 0·789 and IgA-hHSP60: 0·233 ± 0·223 versus 0·184 ± 0·139, P < 0·797) antibodies in both the groups were comparable (Fig. 3).
Fig. 3.
Dot-plots showing levels of IgG, IgM and IgA isotypes of antimycobacterial heat shock protein-65 (mHSP) antibodies and anti-human heat shock protein-60 (hHSP) antibodies in patients with Takayasu's arteritis (TA) and healthy control (HC). The levels of only IgG antibodies to both the HSPs were significantly higher in TA as compared to HC. Each dot represents data of an individual subject and horizontal line crossing dots represents mean OD of each group.
Correlation between responses to mHSP65 and hHSP60
A significant correlation between mHSP65 and hHSP60 reactive T-cells (CD3+: r = 0·901; P < 0·001 and CD4+: r = 0·968; P < 0·001) as well as anti-mHSP65 and anti-hHSP60 IgG antibodies (r = 0·814; P < 0·001) were observed in patients with TA.
DISCUSSION
A number of immune abnormalities including increased number of circulating activated T-cells [8,9], different autoantibodies [10–13] and inflammatory cytokines [20,21] have been implicated in the pathogenesis of TA. However, the initial stimulus that triggers these immune abnormalities in the disease is not yet clear.
In the present study we have investigated T-cell proliferative response and antibodies to mHSP65 as well as its 60 kD human homologue in patients with TA. We observed mHSP65 and hHSP-60 reactive T-cells as well as IgG anti-mHSP65 and IgG anti-hHSP60 antibodies in most of the TA patients, suggesting the role of mycobacterial HSP65 in the aetiology of the disease. To the best of our knowledge, this is the first study reporting detection of cellular and humoral responses to hHSP60 in TA and also first in the detection of the immune responses to mHSP65 and hHSP60 simultaneously, enabling evaluation of correlation between cellular and humoral immune responses to both the HSPs.
The cellular response to a panel of mycobacterial antigens has previously been studied in a single TA patient by Moraes et al. [22]. These authors detected increased proliferation of peripheral blood lymphocytes exclusively to mHSP65 and not to any other mycobacterial antigens. However, they did not study the phenotypes of proliferating lymphocytes. We have analysed proliferative response of CD3+ T-cells as well as their CD4+ and CD8+ subsets and observed that CD3+ and CD4+ T-cells exhibit a proliferative response to mHSP65 as well as hHSP60. However, there was no reactivity to either of the HSPs in the CD8+ T-cells. Similar findings were also reported in other vasculitides including Kawasaki disease and Behcet's disease. In Kawasaki disease, Sireci et al. [23] have shown clonal proliferation of CD4+ and CD8+ T-cell to an epitope of mHSP65, spanning amino acid 65–85. Direskeneli et al. [24] in Behcet's disease reported an enhanced T-cell reactivity to the eight synthetic peptides derived from mHSP65 and hHSP60.
In another study on Behcet's disease a significant fraction of γδ T-cell were shown to proliferate against mHSP65 [25]. Although, we have not evaluated proliferative responses of these cells in our study but a proliferative response of a population of CD3+ T-cells other than CD4+ and CD8+ T-cells points towards proliferation of γδ T-cells in TA. Expression of 65 kD heat shock protein and increased infiltration of γδ T-cell in arterial lesion of TA also support this observation [16].
We also investigated T-cells reactivity to hHSP60, which shows sequence homology with mHSP65 and found that proliferative responses of CD3+ T-cells as well as their CD4+ and CD8+ subsets were similar to the mHSP65 reactivity. There is no previous study on T-cells reactivity to hHSP60 in TA. However, in Behcet's disease increased T-cell response to 336–351 peptide fragment of hHSP60 has been reported showing proliferation exclusively of CD4+ T-cells and not of CD8+ T-cells [26].
In addition, we also investigated different isotypes of antibodies reactive to mHSP65 and hHSP60 in the patients. We found IgG antibodies to mHSP65 in 92% of patients. There are two previous studies on anti-mHSP65 antibodies in TA. In corroboration with our findings, Hernandez-Pando et al. [17] have also reported significantly raised IgG antibodies to mHSP65 in 78% patients with TA. On the contrary, Aggarwal et al. [18] have detected increased level of only IgA antibodies to mHSP65 in 91·6% patients. One of the reasons for this difference between the data of Aggarwal et al. [17] and that of Hernandez-Pando et al. [18] and our may be due to existence of different isotypes of anti-HSP65 antibodies in different stages of the disease. Similar to our findings, increased IgG anti-mHSP65 isotype have also been reported in Kawasaki disease [27]. The existence of anti-hHSP60 antibodies has not been studied in TA before. However, Yokota et al. [27] have detected antibodies reactive to a specific epitope of human HSP65 (equivalent to hHSP60) in Kawasaki disease but they did not specify the isotypes. We have investigated IgG, IgM and IgA isotypes of anti-hHSP60 antibodies and detected significant increase in anti-hHSP60 IgG-isotype in TA.
The cellular and humoral immune responses observed in the present study are not only specific to TA but have been documented in atherosclerosis [28,29] and other inflammatory autoimmune disorders as well [30,31]. We have also investigated immune responses of TA patients to purified protein derivative (PPD) of Mycobacterium tuberculosis as a control mycobacterial antigen and observed that similar to HSP, most of the patients also have T-cell and antibody reactivity to PPD (Unpublished Data). Similar to our observation, studies in other vasculitides using PPD as a control antigen have shown T-cell reactivity to mHSP65 as well as to PPD [23].
An important finding of our study is a significant correlation between mHSP65 and hHSP60 reactive T-cells as well as antibodies in the patients. These findings may suggest an infection-induced autoimmunity in TA. Exposure of individuals to microbial infections may be the primary pathogenic event in TA. The immune response generated in the host against microbial HSP65, which is an immunodominant antigen, may cross react with autologous HSP60 or other tissue-specific proteins containing similar epitopes leading to development of autoimmunity to arterial components. Increased expression of HSP65 in vascular lesions [16], sequence homology of hHSP60 with variety of autoantigens [32] and demonstration of mycobacterial antigen [14] as well as Epstein-Barr virus [33] in arterial tissues support our hypothesis. However, inflammation is another important factor that can lead to tissue stress and consequently to up-regulation of HSP60 expression [34]. Thus the immune response to mycobacterial HSP65 may also be due to raised responses to self HSP-60 [35].
There are reports in other autoimmune/inflammatory diseases that HSP-reactive T-cells cause cytotoxicity of target cells expressing HSP60 or other HSPs [36,37]. Similarly the HSP60/65 specific IgG antibodies have been shown to mediate cytotoxicity of vascular endothelial cells in other vasculopathies [38]. We although have not demonstrated the pathogenic relevance of HSP reactive T-cells and IgG antibodies observed in the present study but these studies and expression of HSP65 in the vascular lesions in TA [16] together suggest an important role of these cellular and humoral components in the disease. However, the exact pathogenic role of HSP-reactive T-cells and antibodies is not known and need to be investigated.
In conclusion, we have demonstrated the presence of T-cells as well as IgG antibodies reactive to mHSP65 and its human homologue suggesting that cross reactivity of immune response between mHSP65 and hHSP60 or related arterial antigens may be an important cause of development of autoimmunity in TA. Future studies focused on cellular and humoral responses to specific epitopes of HSP65/60 and identification of homologous arterial antigens would be important to delineate the exact etiopathogenesis of the disease.
Acknowledgments
The authors are thankful to the Indian Council of Medical Research, New Delhi for providing funds for project on ‘Role of αβ and γδ T-cells in Takayasu's arteritis’ and Council of Scientific and Industrial Research, India for awarding Research Fellowship to the first author.
REFERENCES
- 1.Kerr GS. Takayasu's arteritis. Rheum Dis Clin North Am. 1995;21:1041–58. [PubMed] [Google Scholar]
- 2.Moriwaki R, Noda M, Yajima M, Sharma BK, Numano F. Clinical manifestations of Takayasu arteritis in India and Japan: new classification of angiographic findings. Angiology. 1997;48:369–79. doi: 10.1177/000331979704800501. [DOI] [PubMed] [Google Scholar]
- 3.Mohan N, Kerr G. Takayasu's Arteritis. Curr Treat Options Cardiovasc Medical. 1999;1:35–42. doi: 10.1007/s11936-999-0005-9. [DOI] [PubMed] [Google Scholar]
- 4.Johnston SL, Lock RJ, Gompels MM. Takayasu arteritis: a review. J Clin Pathol. 2002;55:481–6. doi: 10.1136/jcp.55.7.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jain S, Kumari S, Ganguly NK, Sharma BK. Current status of Takayasu arteritis in India. Int J Cardiol. 1996;54(Suppl.):S111–6. doi: 10.1016/s0167-5273(96)88780-8. [DOI] [PubMed] [Google Scholar]
- 6.Murangan MN, Bavdekar SB, More V, Desmukh H, Tripathy M, Vaswani R. Study of Takayasu's arteritis in children: clinical profile and management. J Post Grad Med. 2000;46:3–8. [PubMed] [Google Scholar]
- 7.Brogan PA, Dillon MJ. Vasculitis from the pediatric perspective. Curr Rheumatol Rep. 2000;2:411–6. doi: 10.1007/s11926-000-0041-7. [DOI] [PubMed] [Google Scholar]
- 8.Nityanand S, Giscombe R, Srivastava S, Hjelmstrom P, Sanjeevi CB, Sinha N, Grunewald J, Lefvert AK. A bias in the alphabeta T cell receptor variable region gene usage in Takayasu's arteritis. Clin Exp Immunol. 1997;107:261–8. doi: 10.1111/j.1365-2249.1997.295-ce1186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seko Y, Takahashi N, Tada Y, Yagita H, Okumura K, Nagai R. Restricted usage of T-cell receptor Vgamma-Vdelta genes and expression of costimulatory molecules in Takayasu's arteritis. Int J Cardiol. 2000;75(Suppl. 1):S77–7. doi: 10.1016/s0167-5273(00)00194-7. [DOI] [PubMed] [Google Scholar]
- 10.Sima D, Thiele B, Turowski A, Wilke K, Hiepe F, Volk D, Sonnichsen N. Anti-endothelial antibodies in Takayasu arteritis. Arthritis Rheum. 1994;37:441–3. doi: 10.1002/art.1780370323. [DOI] [PubMed] [Google Scholar]
- 11.Nityanand S, Mishra K, Shrivastava S, Holm G, Lefvert AK. Autoantibodies against cardiolipin and endothelial cells in Takayasu's arteritis. prevalence and isotype distribution. Br J Rheumatol. 1997;36:923–4. doi: 10.1093/rheumatology/36.8.923. [DOI] [PubMed] [Google Scholar]
- 12.Baltazares M, Mendoza F, Dabague J, Reyes PA. Antiaorta antibodies and Takayasu arteritis. Int J Cardiol. 1998;66(Suppl. 1):S183–7. doi: 10.1016/s0167-5273(98)00166-1. [DOI] [PubMed] [Google Scholar]
- 13.Tripathy NK, Sinha N, Nityanand S. Antimonocyte antibodies in Takayasu's arteritis: prevalence of and relation to disease activity. J Rheumatol. 2003;30:2023–6. [PubMed] [Google Scholar]
- 14.Morrison R, Milner LS, Jacobs D, Thompson PD, Kala U, Franklin J, Ninin D. The role of mycobacteria in Takayasu's arteritis (TA) Kidney Int. 1989;35:913. [Google Scholar]
- 15.Kothari SS. Aetiopathogenesis of Takayasu's arteritis and BCG vaccination: the missing link? Med Hypotheses. 1995;45:227–30. doi: 10.1016/0306-9877(95)90109-4. [DOI] [PubMed] [Google Scholar]
- 16.Seko Y, Minota S, Kawasaki A, et al. Perforin-secreting killer cell infiltration and expression of a 65-kD heat-shock protein in aortic tissue of patients with Takayasu's arteritis. J Clin Invest. 1994;93:750–8. doi: 10.1172/JCI117029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hernandez-Pando R, Reyes P, Espitia C, Wang Y, Rook G, Mancilla R. Raised agalactosyl IgG and antimycobacterial humoral immunity in Takayasu's arteritis. J Rheumatol. 1994;21:1870–6. [PubMed] [Google Scholar]
- 18.Aggarwal A, Chag M, Sinha N, Naik S. Takayasu's arteritis. role of Mycobacterium tuberculosis and its 65 kDa heat shock protein. Int J Cardiol. 1996;55:49–55. doi: 10.1016/0167-5273(96)02660-5. [DOI] [PubMed] [Google Scholar]
- 19.Arend WP, Michel BA, Bloch DA, et al. The American College of Rheumatology 1990 criteria for classification of Takayasu's arteritis. Arthritis Rheum. 1990;33:1129–34. doi: 10.1002/art.1780330811. [DOI] [PubMed] [Google Scholar]
- 20.Sharma BK, Jain S, Ganguly NK. Intracellar signal transduction in T-cells in Takayasu's arteritis. Ann NY Acad Sci. 1996;793:453–5. doi: 10.1111/j.1749-6632.1996.tb33540.x. [DOI] [PubMed] [Google Scholar]
- 21.Noris M, Pharm DC, Diana E, Gamba S, Bonazzola S, Remuzzi G. Interleukin-6 and RANTES in Takayasu arteritis. A guide for therapeutic decisions? Circulation. 1999;100:55–60. doi: 10.1161/01.cir.100.1.55. [DOI] [PubMed] [Google Scholar]
- 22.Moraes MF, Ordway D, Oliveira L, Costa IL, Badura R, Pinheiro MN, da Graca JM, Ventura FA. Cellular immune responses to Mycobacterium tuberculosis in a patient with Takayasu's arteritis. Rev Port Cardiol. 1999;18:359–67. [PubMed] [Google Scholar]
- 23.Sireci G, Dieli F, Salerno A. T cells recognize an immunodominant epitope of heat shock protein 65 in Kawasaki disease. Mol Med. 2000;6:581–90. [PMC free article] [PubMed] [Google Scholar]
- 24.Direskeneli H, Eksioglu-Demiralp E, Yavuz S, Ergun T, Shinnick T, Lehner T, Akoglu T. T cell responses to 60/65 kDa heat shock protein derived peptides in Turkish patients with Behcet's disease. J Rheumatol. 2000;27:708–13. [PubMed] [Google Scholar]
- 25.Hasan A, Fortune F, Wilson A, et al. Role of gamma delta T cells in pathogenesis and diagnosis of Behcet's disease. Lancet. 1996;347:789–94. doi: 10.1016/s0140-6736(96)90868-5. [DOI] [PubMed] [Google Scholar]
- 26.Kaneko S, Suzuki N, Yamashita N, Nagafuchi H, Nakajima T, Wakisaka S, Yamamoto S, Sakane T. Characterization of T cells specific for an epitope of human 60-kD heat shock protein (hsp) in patients with Behcet's disease (BD) in Japan. Clin Exp Immunol. 1997;108:204–12. doi: 10.1046/j.1365-2249.1997.3611265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yokota S, Tsubaki K, Kuriyama T, et al. Presence in Kawasaki disease of antibodies to mycobacterial heat shock protein HSP65 and autoantibodies to epitopes of human HSP65 cognate antigen. Clin Immunol Immunopathol. 1993;67:163–70. doi: 10.1006/clin.1993.1060. [DOI] [PubMed] [Google Scholar]
- 28.Xu Q. Role of heat shock proteins in atherosclerosis. Arterioscler Thromb Vasc Biol. 2002;22:1547–59. doi: 10.1161/01.atv.0000029720.59649.50. [DOI] [PubMed] [Google Scholar]
- 29.Xu Q, Kleindienst R, Waitz W, Dietrich H, Wick G. Increased expression of heat shock protein 65 coincides with a population of infiltrating T lymphocytes in atherosclerotic lesions of rabbits specifically responding to heat shock protein 65. J Clin Invest. 1993;91:2693–702. doi: 10.1172/JCI116508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Res PC, Schaar CG, Breedveld FC, van Eden W, van Embden JD, Cohen IR, de Vries RR. Synovial fluid T cell reactivity against 65 kD heat shock protein of mycobacteria in early chronic arthritis. Lancet. 1988;2:478–80. doi: 10.1016/s0140-6736(88)90123-7. [DOI] [PubMed] [Google Scholar]
- 31.Tsoulfa G, Rook GA, Bahr GM, et al. Elevated IgG antibody levels to the mycobacterial 65-kDa heat shock protein are characteristic of patients with rheumatoid arthritis. Scand J Immunol. 1989;30:519–27. doi: 10.1111/j.1365-3083.1989.tb02459.x. [DOI] [PubMed] [Google Scholar]
- 32.Jones DB, Coulson AF, Duff GW. Sequence homologies between hsp60 and autoantigens. Immunol Today. 1993;14:115–8. doi: 10.1016/0167-5699(93)90210-C. [DOI] [PubMed] [Google Scholar]
- 33.Kazuhiro M, Ohsawa M, Hu S, Kanno H, Aozasa K, Nose M. Large-vessel arteritis associated with chronic active Epstein-Barr virus infection. Artheritis Rheum. 1998;41:369–73. doi: 10.1002/1529-0131(199802)41:2<369::AID-ART22>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 34.Kaufmann SH. Heat shock proteins and the immune response. Immunol Today. 1990;11:129–36. doi: 10.1016/0167-5699(90)90050-j. [DOI] [PubMed] [Google Scholar]
- 35.Anderton SM, van der Zee R, Goodacre JA. Inflammation activates self hsp60-specific T cells. Eur J Immunol. 1993;23:33–8. doi: 10.1002/eji.1830230107. [DOI] [PubMed] [Google Scholar]
- 36.Weiss RA, Madaio MP, Tomaszewski JE, Kelly CJ. T cells reactive to an inducible heat shock protein induce disease in toxin-induced interstitial nephritis. J Exp Med. 1994;180:2239–50. doi: 10.1084/jem.180.6.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ugrinovic S, Mertz A, Wu P, Braun J, Sieper J. A single nonamer from the Yersinia 60-kDa heat shock protein is the target of HLA-B27-restricted CTL response in Yersinia-induced reactive arthritis. J Immunol. 1997;159:5715–23. [PubMed] [Google Scholar]
- 38.Schett G, Xu Q, Amberger A, Van der Zee R, Recheis H, Willeit J, Wick G. Autoantibodies against heat shock protein 60 mediate endothelial cytotoxicity. J Clin Invest. 1995;96:2569–77. doi: 10.1172/JCI118320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tripathy NK, Sinha N, Nityanand S. Anti-annexin-V antibodies in Takayasn's arteritis: prevalence and relationship with disease activity. Clin Exp Immunol. 2003;134:360–64. doi: 10.1046/j.1365-2249.2003.02282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]