Abstract
Interferon (IFN)-β reduces the biological activity of multiple sclerosis (MS), a presumably T cell-mediated autoimmune disease of central nervous system (CNS) myelin. Co-stimulatory molecules are necessary for full T cell activation and differential expression of co-stimulatory molecules on antigen-presenting cells is thought to influence the type of effector T cell response (Th1/Th2). In this study we investigated the effects of IFN-β on the expression of co-stimulatory molecules on lymphocytes and monocytes as a potential mechanism of action of IFN-β in MS. Peripheral blood mononuclear cells (PBMCs) were stimulated with IFN-β in vitro and expression of CD80, CD86, CD40 and HLA was examined by flow cytometry and reverse-transcription polymerase chain reaction. Whereas IFN-β had no effect on the expression of these molecules on T and B lymphocytes there was a significant increase on monocytes. Correspondingly, the expression of mRNA increased after 6–18 h. This in vitro response was also observed in untreated MS patients and patients receiving treatment with IFN-β. The increase of co-stimulatory molecules on monocytes was not mediated by interleukin (IL)-10. When IFN-β-stimulated monocytes were used to stimulate autologous T cells an increased secretion of IL-13 was observed. In biopsies taken from IFN-β-induced skin reactions after subcutaneous injection increased expression of CD80 mRNA was detected, indicating that IFN-β also up-regulates this co-stimulatory molecule in vivo. These data provide the background for further studies of IFN-β-induced changes of co-stimulatory molecules in MS patients.
Keywords: co-stimulatory molecules, interferon-β, multiple sclerosis
INTRODUCTION
Multiple sclerosis (MS) is thought to be a T cell-mediated autoimmune disease with peripherally activated T lymphocytes entering the central nervous system (CNS) and causing immune-mediated demyelination and tissue destruction [1]. T cell activation requires recognition of antigen presented by MHC molecules on antigen-presenting cells (APC) as well as additional co-stimulatory signals, particularly CD80 and CD86 [2]. Differential expression of co-stimulatory molecules on APCs is thought to play an important role in directing the T cell response to proinflammatory or regulatory effector functions [2–5]. In animal models of organ-specific autoimmune diseases it has been shown that aberrant expression of co-stimulatory molecules in the tissue is sufficient for the initiation of an autoimmune response [6]. Increased expression of CD80 was observed in early active lesions of MS brain [7]. Mononuclear cells expressing CD40 have been found in perivascular infiltrates in MS and experimental autoimmune encephomyelitis (EAE) but not in normal tissue [8]. Further evidence that co-stimulatory molecules have functional importance in CNS autoimmune disease arises from studies in EAE, where blockade of co-stimulatory molecules CD80, CD86 and CD40 was shown to prevent or ameliorate disease [9–11]. As a consequence, the fusion molecule CTLA-4 Ig, which blocks the CD80 and CD86 pathway, is now evaluated as potential therapy in MS, while its effectiveness in rheumatoid arthritis has already been demonstrated [12].
Interferon (IFN)-β has been shown to reduce the biological activity of relapsing–remitting MS in several clinical class I trials [13–15]. Type I IFNs are produced by almost all cells in the organism in response to viral infection [16,17]. They were first used in MS in view of the propensity of viral infections to trigger relapses. Recent data, however, suggest that their mechanism of action is immunological and complex [16–18]. Type I IFNs protect T cells from apoptosis [19] and are antiproliferative by inhibiting cell cycle progression [20]. Although IFN-β has been used widely in the treatment of MS for several years, the mechanism of action is not well understood. Putatively, IFN-β inhibits the migration of leucocytes across the blood–brain barrier [21–23] and induces alterations of cytokine production [24–27].
Despite the proven beneficial effect of IFN-β in large clinical trials, many patients do not respond to IFN-β therapy. Therefore it is important to find predictive laboratory markers for the clinical response in individual patients. We have shown previously that IFN-β enhances the expression of co-stimulatory molecules on dendritic cells and the stimulatory capacity of DCs [28]. In this study we analysed the effects of IFN-β on the expression of co-stimulatory molecules on lymphocytes and monocytes, as these cells are easily accessible for routine analysis. Because an increased expression of co-stimulatory molecules was induced by stimulation with IFN-β of monocytes we examined further the mechanism and functional relevance on T cells. To confirm that IFN-β has similar effects on the expression of co-stimulatory molecules in vivo we examined tissue from IFN-β-induced injection site reactions (ISR) by reverse-transcription polymerase chain reaction (RT-PCR).
METHODS
Cell preparation and stimulation
Peripheral blood mononuclear cells (PBMCs) were isolated from 10 ml heparinized venous blood from 15 healthy donors (11 female, four male, mean age 37 ± 4), five untreated MS patients (four female, one male, mean age 40 ± 4, duration of disease 5 ± 2 years) and seven patients receiving treatment with IFN-β (five female, two male, mean age 43 ± 5, duration of disease 7 ± 3 years, duration of treatment 15 ± 4 months) by Ficoll gradient centrifugation. T lymphocytes and monocytes were purified further by negative selection using magnetic beads coated with CD22/CD56 and/or CD2 antibodies (Dynal, Hamburg, Germany). Cells were cultured in RPMI-1640 supplemented with 10% fetal calf serum (FCS), 1% HEPES buffer, 1% l-glutamine and 1% penicillin/streptomycin at 1 × 106/ml in humidified air containing 5% CO2 at 37°C. After stimulation of cells with IFN-β1b (0·1–10·000 U/ml, Betaferon, Schering AG, Berlin, Germany; batch number 63211–049) for 24, 48, 72, 96 or 120 h cells were harvested and prepared for flow cytometry analysis.
To investigate functional differences of IFN-β-stimulated monocytes autologous T cells were co-cultured with negatively selected monocytes. Monocytes were stimulated with or without 1000 U/ml IFN-β for 48 h, washed twice and irradiated at 5000 rad; 2 × 104 monocytes were incubated with 2 × 105 autologous T cells in 96-well round-bottomed microtitre plates (Nunc, Wiesbaden, Germany) in medium containing 10% autologous serum for 96 h. Secretion of interleukin (IL)-5, IL-13 and IFN-γ was measured after 2 days in cell culture supernatants. T cell proliferation was measured after 4 days by BrdU incorporation assay (Roche, Mannheim, Germany) using a colourimetric detection system with absorbance at 450 nm, following the manufacturer's instructions. Materials and methods for the enzyme-linked immunosorbent assay (ELISA) technique have been described previously [28].
To investigate whether the effects of IFN-β were mediated by IL-10 we added IL-10 blocking antibody (purified rat antihuman IL-10 monoclonal antibody, clone JES3–19F1, Pharmingen, at 2 µg/ml) or recombinant IL-10 (recombinant human IL-10, Becton Dickinson/Pharmingen, Mountain View, CA, USA, at 25 and 100 ng/ml) during stimulation with IFN-β for 48 h. In order to investigate whether the IFN-β effects were mediated via the IFN-β receptor, IFN-β receptor blocking antibody (clone MMHAR-2, PBL BioMedical Laboratories, Piscataway, NJ, USA) was added at 4 µg/ml during stimulation.
Skin biopsies
Skin biopsies were taken from early visible ISR (median 7 days after injection) from seven MS patients who received IFN-β1b (Betaferon, Schering AG, Berlin, Germany) subcutaneously at a dosage of 8·0 million units on alternate days and from five healthy subjects who had skin removed for plastic surgery, as described previously [29].
Informed consent was obtained from all patients and controls prior to venipuncture or skin biopsy. The study was approved by the local ethics committee.
Flow cytometry analysis
Cells were surface phenotyped by labelling with the following panel of antibodies directed against: CD3 (Cy5, clone UCHT1, Dako, Hamburg, Germany), CD22 (PE, clone 4KB128, Dako), CD28 (PE, clone KOLT; ImmunoTools), CD14 (PE, clone TÜK4; Dako, Hamburg, Germany), CD80 (FITC, clone MAB104, Immunotech, Heidelberg, Germany), CD86 (FITC, clone FUN-1, Becton Dickinson/Pharmingen), CD152 (PE, clone ANC 152·2/8HS, Ancell, Bayport, MN, USA), CD154 (PE, clone 24–31, Bioscience), HLA-DR (PE, clone TÜ36, Becton Dickinson/Pharmingen, Heidelberg, Germany), CD 40 (PE, clone 5C3, Becton Dickinson/Pharmingen) or isotype controls PE-, Cy5 and FITC-labelled (Dako). Cells were examined by flow cytometry using a FACScan (Becton Dickinson). Monocytes and lymphocytes were gated using forward- and side-scatter characteristics and data were analysed using cellquest software. Mean fluorescence intensities (MFI) and percentage of positive cells were determined in order to compare unstimulated and IFN-β-stimulated cells.
RT-PCR
Expression of co-stimulatory molecules was examined at mRNA level by RT-PCR as described previously [30]. In addition the following primer pairs were used: CD40 forward: 5′-AGA AGG CTG GCA CTG TAC GA-3′; CD40 reverse: 5′-CAG TGT TGG AGC CAG GAA GA-3′; IL-10 forward: 5′-AAG CTG AGA ACC AAG ACC CAG ACA TCA AGG CG-3′; IL-10 reverse: 5′-AGC TAT CCC AGA GCC CCA GAT CCG ATT TTG G-3′; HLA-DR forward: 5′-CTG ATG AGC GCT CAG GAA TCA TGG-3′; HLA-DR reverse: 5′-GTT CGT GAG CAC AGT TAC CTC TGG-3′.
PCR cycles were performed at 94°C for 1 min, annealing at 58°C for 1 min and extension at 72°C for 90 s, 35 cycles for co-stimulatory molecules and IL-10 and 24 cycles for β-actin and HLA-DR.
Statistical analysis
Lymphocytes and monocytes with and without IFN-β stimulation were compared using analysis of variance (anova) with sigmaplot 2001 for Windows version 7·0 and sigmastat 1997 for Windows version 2·03 software. P-values < 0·05 were considered significant.
RESULTS
In vitro effects of IFN-β on expression of co-stimulatory molecules
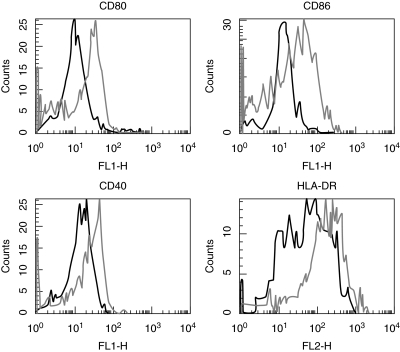
To analyse the effects of IFN-β stimulation on expression of co-stimulatory molecules on monocytes, B cells and T cells, PBMCs from healthy donors were isolated and stimulated as described in Materials and methods. Cells were double-stained with a lineage marker CD3/CD22/CD14 and CD80/CD40/CD86/HLA-DR. Lymphocytes and monocytes were gated using forward- and side-scatter characteristics and analysed by flow cytometry. No changes in the expression of co-stimulatory molecules were observed on T and B lymphocytes; however, IFN-β induced up-regulation of the co-stimulatory molecules CD80, CD86, CD40 and of HLA-DR on monocytes (Fig. 1).
Fig. 1.
Expression of CD40, CD80 and CD86 on monocytes with and without stimulation with IFN-β. PBMC were cultivated for 48 h either without stimulation(black line) or with IFN-β 1000 U/l (grey line). Monocytes were gated using forward- and side-scatter characteristics and analysed by flow cytometry for expression of HLA-DR, CD86, CD80 and CD40 (x-axis). Results are representative of 15 independent experiments (PBMCs from 15 healthy donors).
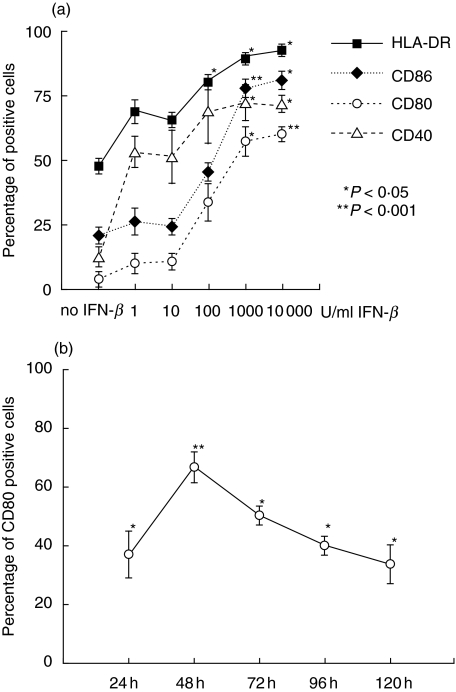
Up-regulation of co-stimulatory molecules was dose-dependent with a maximum effect at a concentration of 1000 U/ml IFN-β (Fig. 2a). Higher concentrations of IFN-β did not result in a further increase. In healthy subjects the mean percentage of positive monocytes increased from 60·8 ± 6·2% without stimulation to 81 ± 6·4% after IFN-β stimulation for HLA-DR (P = 0·004), from 30·1 ± 5·6% to 57 ± 6·0% for CD40 (P = 0·003), from 13·5 ± 3·5% to 55·7 ± 6·5% for CD80 (P < 0·001) and expression of CD86 was up-regulated from 32 ± 4·8% to 63·5 ± 5·2% (P < 0·001) (mean ± s.e.m., n = 15).
Fig. 2.
(a) Dose-dependency of IFN-β induced up-regulation of CD86, CD80, CD40 and HLA-DR on monocytes. PBMCs were stimulated with IFN-β at concentrations from 0 to 10 000 U/ml for 48 h. (b) Kinetics of IFN-β induced up-regulation of co-stimulatory molecules. PBMCs were stimulated with IFN-β 1000 U/ml for 24, 48, 72, 96 and 120 h. Net changes of expression of CD80 are shown. For all experiments monocytes were gated using forward- and side-scatter characteristics and analysed by flow cytometry for expression of HLA-DR (squares), CD86 (diamonds), CD80 (open circles) and CD40 (open triangles). Mean values and standard error from three independent experiments are shown. *P < 0·05, **P < 0·001.
To investigate the kinetics of the up-regulation of co-stimulatory molecules, cells were harvested after 24, 48, 72, 96 and 120 h. Up-regulation of all molecules was detectable after 24 h and peaked at 48 h (shown for CD80 in Fig. 2b); therefore cells were harvested after 48 h in all further experiments. We also investigated the effects of IFN-β on the expression of the CD80/CD86 ligands CD28 and CTLA-4 and the CD40 ligand CD154. However, no changes of these ligands could be observed in vitro (data not shown) and therefore further studies of these molecules were not performed ex vivo.
IFN-β-induced up-regulation of co-stimulatory molecules was also observed in untreated MS patients and was not changed in patients receiving IFN-β treatment (Table 1), in contrast to studies with glatiramer acetate (GA) where treatment with GA reduces the immunological in vitro response to GA [31–33]. The mean percentage of HLA-DR-positive monocytes in unstimulated cells of MS patients was 62·6 ± 9% (range 13–85%) for untreated and 63·5 ± 11·5% (range 35–87%) for IFN-β treated patients; expression of CD86 was 29·4 ± 7% (range 5–41%) for untreated and 26·4 ± 7·4% (range 8–48%) for IFN-β treated patients, CD80 was 13·4 ± 4·9% (range 5–56%) for untreated and 7·5 ± 4·1% (range 4–22%) for IFN-β treated patients and baseline expression of CD40 was 29·8 ± 8·8% (range 8–67%) for untreated and 15·5 ± 4·4% (range 2–32%) for IFN-β treated patients. There were no statistically significant differences between the baseline expression of co-stimulatory molecules on monocytes in healthy controls when compared to MS patients; however, high interindividual variability could be observed. Due to alterations of expression of cell surface molecules, including co-stimulatory molecules by Ficoll separation (unpublished data), comparison of the baseline values should be interpreted with caution.
Table 1.
Comparison of the increase of co-stimulatory molecules on monocytes of untreated MS patients (MS, n = 5) and MS patients under treatment with IFN-β (MS/IFN-β, n = 7). PBMCs were stimulated with IFN-β (1000 U/ml) for 48 h. Net changes are shown as percentage of positive cells ± s.e.m.
| HLA | CD86 | CD80 | CD40 | |
|---|---|---|---|---|
| MS | 13 ± 4 | 18 ± 3 | 23 ± 2 | 15 ± 3 |
| MS/IFN-β | 14 ± 6 | 14 ± 32 | 34 ± 7 | 17 ± 5 |
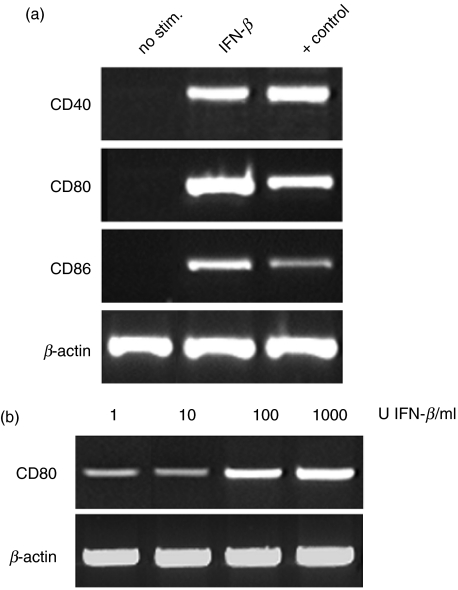
To examine whether the up-regulation of co-stimulatory molecules after stimulation with IFN-β is transcriptionally regulated we further measured the expression of mRNA for co-stimulatory molecules by RT-PCR. As shown in Fig. 3a, mRNA expression of CD80, CD86 and CD40 increased after IFN-β stimulation after 6–18 h. Similar to the effects on surface expression, IFN-β induced the most prominent increase of mRNA of CD80. This effect was also dose-dependent within the tested range for CD80 (Fig. 3b), whereas for CD86 and CD40 mRNA expression was not clearly dose-dependent in three experiments (not shown).
Fig. 3.
mRNA expression of co-stimulatory molecules in PBMCs following stimulation with IFN-β after 18 h. (a) increased mRNA expression of CD40, CD80 and CD86 after stimulation with IFN-β (1000 U/ml). (b) Dose-dependency of IFN-β-induced mRNA expression of CD80. PCR fragment length: CD40 429 bp, CD80 389 bp, CD86 409 bp, β-actin 406 bp.
To investigate functional differences in IFN-β-stimulated monocytes we co-cultured autologous T cells with monocytes as described in Materials and methods. No changes were observed for T cell proliferation or secretion of IL-5 or IFN-γ. However, IFN-β-stimulated monocytes induced an increased T-cell secretion of IL-13 in three of four independent experiments, mean 14·9 ± 5·3, compared to unstimulated monocytes, mean 4·5 ± 2·8 (Table 2). This difference was not statistically significant (P = 0·08). When antigen-pulsed (tetanus toxoid) monocytes were used under these conditions similar results were obtained (data not shown).
Table 2.
Stimulatory capacity of IFN-β-stimulated monocytes. Monocytes (M) were stimulated with or without IFN-β for 48 h (M/IFN-β) and used as stimulator cells for autologous T cells (T). BrdU incorporation (proliferation) was measured after 4 days (n = 6). IL-5, IL-13, and IFN-γ secretion was measured by ELISA after 2 days (n = 4). Mean values and s.e.m. are shown
| T | M | T + M | T + M/IFN-β | |
|---|---|---|---|---|
| BrdU | 0·25 ± 0·07 | 0·05 ± 0·003 | 0·29 ± 0·03 | 0·3 ± 0·04 |
| IL-13 | 2·8 ± 2·75 | 0 | 4·5 ± 2·8 | 14·9 ± 5·3 |
| IL-5 | 0 | 0 | 0 | 0 |
| IFN-γ | 7·8 ± 7·8 | 0 | 0 | 0 |
Up-regulation of co-stimulatory molecules on monocytes is mediated via the IFN-β receptor and not dependent on lymphocyte–monocyte interaction
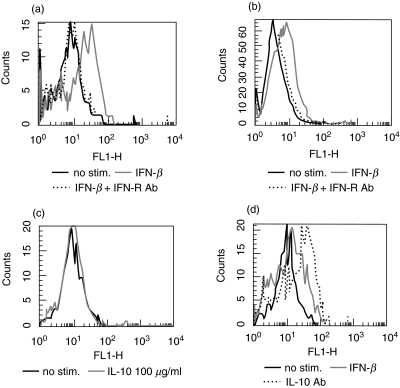
To investigate further the mechanism of IFN-β-induced up-regulation of co-stimulatory molecules we tested whether IFN-β induces this effect directly on monocytes or whether lymphocyte–monocyte interaction is necessary. When monocytes were separated from lymphocytes with magnetic beads and stimulated with IFN-β a similar effect on co-stimulatory molecules was observed, indicating that interaction with lymphocytes is not required (Fig. 4a,b). When PBMCs or isolated monocytes were incubated with IFN-β in the presence of type I IFN-receptor (IFN-R) blocking antibody the IFN-β induced up-regulation of co-stimulatory molecules was inhibited, indicating that the effect is receptor mediated (Fig. 4a,b).
Fig. 4.
Mechanisms of IFN-β-induced up-regulation of co-stimulatory molecules. (a) Blocking of IFN-β receptor reverses IFN-β-mediated up-regulation of CD80. PBMC were cultivated for 48 h, without stimulation (black line) with IFN-β 1000 U/l (grey line) or with IFN-β 1000 U/l and anti-IFN receptor antibody 4 µg/ml (IFN-R antibody: dotted line). (b) IFN-β increases CD80 expression on isolated monocytes. Monocytes were negatively selected using magnetic beads coated with CD2/CD22/CD56 and cultivated for 48 h, without stimulation (black line) with IFN-β 1000 U/l (grey line) or with IFN-β 1000 U/l and anti-IFN receptor antibody 4 µg/ml (IFN-R antibody: dotted line). (c) IL-10 does not increase expression of CD80. PBMCs were cultivated for 48 h without stimulation (black line) or with IL-10 100 µg/ml. (d) Anti-IL-10 does not reverse IFN-β-induced up-regulation of CD80. PBMCs were cultivated for 48 h without stimulation (black line) with IFN-β 1000 U/l (grey line) or with IFN-β 1000 U/l and blocking IL-10 antibody 4 µg/ml (IL-10 antibody: dotted line). Percentage of positive cells was measured by flow cytometry by staining with respective antibodies as described in Materials and methods. Results are representative of three independent experiments.
Up-regulation of co-stimulatory molecules on monocytes is not mediated by IL-10
As IFN-β has been shown to induce IL-10 in vitro and in vivo [26,27,34] we asked whether induction of IL-10 secretion by IFN-β is responsible for the up-regulation of co-stimulatory molecules on monocytes. When IL-10 blocking antibody was added no changes in the expression of CD40, CD86 and HLA-DR were observed, while the expression of CD80 increased even further. When recombinant IL-10 was added to PBMCs expression of CD80, CD86 and CD40 remained unchanged (Fig. 4c,d), while expression of HLA-DR was down-regulated (not shown). Therefore IL-10 does not play a role in IFN-β-mediated up-regulation of co-stimulatory molecules.
Up-regulation of CD80 in IFN-β-induced ISR
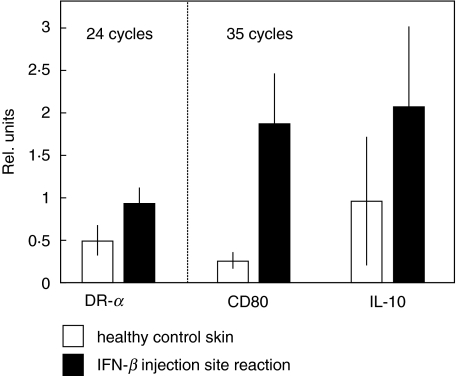
In order to determine whether up-regulation of co-stimulatory molecules can also be observed in vivo, we examined mRNA expression of CD80, HLA-DR and IL-10 by semiquantitative RT-PCR in IFN-β-induced ISR. mRNA for all molecules was detected in normal skin.
Expression of β-actin mRNA was equal in IFN-β-induced ISR and normal skin, while CD80 and IL-10 mRNA expression was increased significantly in ISR compared to normal skin (Fig. 5). HLA-DR mRNA up-regulation was not seen in all patients and median values were not statistically different between ISR and normal skin. These data show that IFN-β also induces a significant up-regulation of CD80 co-stimulatory molecule after injection in vivo.
Fig. 5.
mRNA expression of HLA-DR, IL-10 and CD80 in interferon-1b injection site reactions (black bars) compared to healthy control skin (white bars); mean data of all seven patients and three healthy controls and standard errors are shown. Results are shown in relation to the corresponding β-actin signal. The mRNA for HLA-DR, CD80 and IL-10 is induced at the injection sites.
DISCUSSION
In this study we investigated the effects of IFN-β on the expression of co-stimulatory molecules on PBMCs in healthy controls and patients with MS. We show that IFN-β increases the expression of co-stimulatory molecules on monocytes but not on lymphocytes via direct interaction with the high-affinity IFN-β receptor. This effect is not dependent on lymphocyte–monocyte interaction or on IL-10. Up-regulation of mRNA expression indicates that up-regulation is transcriptionally regulated. When IFN-β-stimulated monocytes were used to stimulate autologous T cells increased secretion of IL-13 was observed. IFN-β also increases the expression of CD80 in vivo, as shown by elevated levels of CD80 mRNA in tissue from IFN-β skin injection sites.
The role of co-stimulatory molecules in MS is not well understood. However, several studies indicate that increased expression, particularly of CD80 and CD40, might be of relevance in the pathogenesis. In MS CD80 expression is up-regulated on T lymphocytes in brain and B lymphocytes in cerebrospinal fluid (CSF) in early disease or during acute illness, while CD86 expression was also detected in inflammatory ischaemic infarcts and in stable MS [7,35,36]. Similarly, mononuclear cells expressing CD40 have been found in perivascular infiltrates in MS and EAE but not in normal tissue [8], and CD40 mRNA expression was increased in PBMCs in MS patients [37]. In EAE, in most studies blockade of CD80 and CD86 resulted in amelioration or suppression of disease, but disease exacerbation has also been reported [38]. In Theiler's encephalomyelitis, blockade of CD80 but not CD86 resulted in suppression of disease indicating that in this animal model CD80 also has an important role in the disease pathogenesis [39]. On first view our data showing IFN-β-induced expression, particularly of CD80, seems to conflict with these findings. However, IFN-β induces expression of co-stimulatory molecules only on monocytes but not on T cells or B cells, whereas in MS brain CD80 is expressed mainly on T cells [7]. In a recent study of the effects of IFN-β treatment on expression of co-stimulatory molecules ex vivo a decrease of CD80 expression on monocytes was reported in relapsing–remitting but not in secondary–progressive MS (n = 6). This effect was seen only after 12 months of treatment, whereas the values at 3, 6 and 9 months were not different [40]. The significance of these findings, particularly with regard to larger patient groups and clinical response to therapy, is as yet unclear.
The cellular expression patterns of co-stimulatory molecules are likely to play a role not only in the regulation of the immune response, but also of their ligands CD28 and CTLA-4. Whereas CD28 ligation induces activation, IL-2 secretion and clonal expansion, ligation of CTLA-4 has down-regulatory effects [41]. IFN-β has the potential to induce regulatory cytokines via its effect on APCs. When IFN-β stimulated monocytes were used as antigen-presenting cells to stimulate autologous T cells, cytokine secretion of IL-13 was increased in three of four experiments. This finding indicates that the IFN-β-induced effects on monocytes are functionally relevant in the activation of T cells by altering the pattern of cytokine secretion. Similar effects were seen in previous experiments when dendritic cells (DCs) were used as APCs. We showed that IFN-β increases the expression of co-stimulatory molecules during maturation of dendritic cells resulting in an enhanced cytokine secretion of IL-13 and IL-5 in DC-stimulated autologous T cells [28]. The stimulatory capacity of monocytes is much weaker when compared to DCs and is usually not sufficient to induce a primary T cell response. It is therefore interesting that in our experiments IFN-β seemed to increase the stimulatory capacity of monocytes, resulting in increased IL-13 secretion of autologous T cells. It will also be of interest to follow-up on this finding in MS patients using antigen-specific T cell lines or clones as responder cells to increase the sensitivity. Our results so far would support the hypothesis that one mechanism of action of IFN-β is by altering the stimulatory function of APCs and the resulting T cell response in the periphery.
In human myeloid DCs type I IFN did not induce IL-12, but augmented IL-10 production and priming of regulatory T cells [42]. When IFN-β is injected into the skin, resident Langerhans cells, a member of the DC family, are exposed to high local concentrations of IFN-β. Therefore the effects of IFN-β on DCs might be of particular relevance for its immunomodulatory function in vivo.
The in vivo effects of IFN-β are also influenced by spatial and temporal expression patterns of the co-stimulatory molecules. CD86 is expressed constitutively on monocytes and B-cells and is up-regulated on T cells early after activation, whereas expression of CD80 is more restricted [43,44]. In EAE, using single versus multiple injections of CTLA-4 Ig it was shown that there are essential timing requirements for the co-ordinated interaction of CD80 and CD86 and CD28 family receptors, and that disruption of this critical timing can have opposing results on the outcome of an immune response [5]. Moreover, there are qualitative differences in the signals induced by CD80 and CD86, as CD86 but not CD80 was shown to co-stimulate preferentially the initial production of IL-4 [3]. Taken together, there is evidence that up-regulation of co-stimulatory molecules by IFN-β might have immunoregulatory anti-inflammatory effects in autoimmune disease depending on multiple factors including type of APC, expression of T cell ligands, local factors at injection site as well as timing requirements.
We investigated further the mechanism of IFN-β-induced up-regulation of co-stimulatory molecules in vitro. We show that the effect is mediated directly via the IFN-β receptor as it can be abrogated by receptor blocking antibodies. There is no difference in the response to IFN-β when PBMCs or negatively isolated monocytes are used, indicating that lymphocyte–monocyte contact is not necessary.
Another question was whether the effect is mediated by IL-10, as several studies have reported an up-regulation of IL-10 by IFN-β both in vitro and in vivo [26,27,34,45] and IL-10 has been shown to influence the activation of T cells through the CD28–CD80/86 pathway [46]. When IL-10 was blocked during IFN-β stimulation, IFN-β-induced up-regulation of CD80 was enhanced even further, while expression of the other co-stimulatory molecules was not significantly changed, indicating that the up-regulation of co-stimulatory molecules on monocytes by IFN-β is independent of its effects on IL-10 secretion.
In patients treated with GA, another immunomodulatory treatment for MS, down-regulation of the in vitro response to GA has been observed [31–33] and changes in the in vitro response have been correlated with the clinical response to therapy [47]. We therefore examined whether the in vitro response to IFN-β would be altered during treatment with IFN-β in MS patients. When comparing the IFN-β-induced up-regulation of co-stimulatory molecules in untreated and IFN-β-treated MS patients there was no difference, indicating that reactivity of monocytes to IFN-β in vitro does not change during IFN-β treatment.
In order to investigate whether similar IFN-β-induced changes seen in our cell culture experiments could be observed in vivo, we examined mRNA expression of HLA-DR, CD80 and IL-10 in IFN-β-induced skin lesions (ISR). ISR occurs frequently (5%) in patients treated with subcutaneous IFN-β1b (Betaferon®) or IFN-β1a (Rebif®) [48]. The severity varies greatly between individuals, ranging from mild inflammatory responses to rare cutaneous necroses (eight of 150 000 injections). The mechanisms of injection site inflammation are unknown. They are currently thought to represent an inflammatory response to IFN-β [49]. The histopathological examination of the skin lesions used in this study showed a perivascular infiltration of activated (HLA-DRα+ CD3+) T cells (>90%) and CD68+ macrophages and increased expression of adhesion molecules ELAM-1 and VCAM-1 on endothelial cells. Mast cells and Langerhans cells were unchanged compared to normal skin (Hilse et al., manuscript in preparation). In this study we found a strong up-regulation of CD80 and IL-10 mRNA expression, indicating that IFN-β also induces expression of CD80 in vivo. Up-regulation of CD80 expression in skin, however, is not a specific effect of IFN-β but has also been observed in other inflammatory skin diseases, e.g. psoriasis, allergic contact dermatitis or lichen planus [49,50]. In these diseases expression of CD80 was detected mainly on APCs and not on T cells. Due to our limited sample size it was not possible to conduct further immunostaining on our biopsies and determine which cells up-regulate CD80 expression in ISRs. Because similar ISRs were not observed in patients receiving placebo in the large multicentre studies [14,15], it can be assumed that the effect is mediated by IFN-β and not other compounds in the injection solution. Our finding of IFN-β-induced expression of CD80, however, does not explain why only a small percentage of patients develops the ISR.
In conclusion, it can be hypothesized that the timed and differential up-regulation of co-stimulatory molecules by IFN-β in vivo on monocytes and DCs influence the secretion of IL-13, IL-5 and IL-10 and other regulatory T cell effector functions that might mediate the therapeutic effect of IFN-β in MS. To test this hypothesis, further studies are now under way in our clinic to determine changes of co-stimulatory molecules directly ex vivo from peripheral blood monocytes before and during IFN-β therapy and to correlate these changes with the clinical course of disease. Because monocytes can be obtained easily from patients the examination of co-stimulatory molecules might be a valuable immunological marker during IFN-β therapy.
Acknowledgments
We thank Franziska Bode for excellent technical assistance and F. Heidenreich for supporting the project and for helpful discussions.
References
- 1.Compston A, Coles A. Multiple sclerosis. Lancet. 2002;359:1221–31. doi: 10.1016/S0140-6736(02)08220-X. [DOI] [PubMed] [Google Scholar]
- 2.Chang TT, Kuchroo VK, Sharpe AH. Role of the B7-CD28/CTLA-4 pathway in autoimmune disease. Curr Dir Autoimmun. 2002;5:113–30. doi: 10.1159/000060550. [DOI] [PubMed] [Google Scholar]
- 3.Freeman GJ, Boussiotis VA, Anumanthan A, et al. B7-1 and B7-2 do not deliver identical co-stimulatory signals, since B7-2 but not B7-1 preferentially co-stimulates the initial production of IL-4. Immunity. 1995;2:523–32. doi: 10.1016/1074-7613(95)90032-2. [DOI] [PubMed] [Google Scholar]
- 4.Perrin PJ, Scott D, June CH, Racke MK. B7-mediated co-stimulation can either provoke or prevent clinical manifestations of experimental allergic encephalomyelitis. Immunol Res. 1995;14:189–99. doi: 10.1007/BF02918216. [DOI] [PubMed] [Google Scholar]
- 5.Racke MK, Scott DE, Quigley L, et al. Distinct roles for B7-1 (CD-80) and B7-2 (CD-86) in the initiation of experimental allergic encephalomyelitis. J Clin Invest. 1995;96:2195–203. doi: 10.1172/JCI118274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harlan DM, Hengartner H, Huang ML, et al. Mice expressing both B7-1 and viral glycoprotein on pancreatic beta cells along with glycoprotein-specific transgenic T cells develop diabetes due to a breakdown of T lymphocyte unresponsiveness. Proc Natl Acad Sci USA. 1994;91:3137–41. doi: 10.1073/pnas.91.8.3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Windhagen A, Newcombe J, Dangond F, et al. Expression of co-stimulatory molecules B7-1 (CD80), B7-2 (CD86), and interleukin 12 cytokine in multiple sclerosis lesions. J Exp Med. 1995;182:1985–96. doi: 10.1084/jem.182.6.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laman JD, Claassen E, Noelle RJ. Functions of CD40 and its ligand, gp 39 (CD40L) Crit Rev Immunol. 1996;16:59–108. doi: 10.1615/critrevimmunol.v16.i1.40. [DOI] [PubMed] [Google Scholar]
- 9.Chang TT, Jabs C, Sobel RA, Kuchroo VK, Sharpe AH. Studies in B7-deficient mice reveal a critical role for B7 co-stimulation in both induction and effector phases of experimental autoimmune encephalomyelitis. J Exp Med. 1999;190:733–40. doi: 10.1084/jem.190.5.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cross AH, Girard TJ, Giacoletto KS, et al. Long-term inhibition of murine experimental autoimmune encephalomyelitis using CTLA-4-Fc supports a key role for CD28 co-stimulation. J Clin Invest. 1995;95:2783–9. doi: 10.1172/JCI117982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Howard LM, Miga AJ, Vanderlugt CL, et al. Mechanisms of immunotherapeutic intervention by anti-CD40L (CD154) antibody in an animal model of multiple sclerosis. J Clin Invest. 1999;103:281–90. doi: 10.1172/JCI5388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kremer JM, Westhovens R, Leon M. Treatment of rheumatoid arthritis by selective inhibition of T cell activation with fusion protein CTLA4Ig. N Engl J Med. 2003;349:1907–15. doi: 10.1056/NEJMoa035075. Diet al. [DOI] [PubMed] [Google Scholar]
- 13.Jacobs LD, Cookfair DL, Rudick RA, et al. Intramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis. The Multiple Sclerosis Collaborative Research Group (MSCRG) Ann Neurol. 1996;39:285–94. doi: 10.1002/ana.410390304. [DOI] [PubMed] [Google Scholar]
- 14.Paty DW, Li DK. Interferon beta-1b is effective in relapsing-remitting multiple sclerosis. II. MRI analysis results of a multicenter, randomized, double-blind, placebo-controlled trial. UBC MS/MRI Study Group and the IFNB Multiple Sclerosis Study Group. Neurology. 1993;43:662–7. doi: 10.1212/wnl.43.4.662. [DOI] [PubMed] [Google Scholar]
- 15.Prevention of Relapses and Disability by Interferon beta-1a Subcutaneously in Multiple Sclerosis (PRISMS) Study Group. Randomised double-blind placebo-controlled study of interferon beta-1a in relapsing/remitting multiple sclerosis. Lancet. 1998;352:1498–504. [PubMed] [Google Scholar]
- 16.Rudick RA, Ransohof RM. Biologic effects of interferons: relevance to multiple sclerosis. Mult Scler. 1995;1(Suppl. 1):S12–6. [PubMed] [Google Scholar]
- 17.Tilg H, Kaser A. Interferons and their role in inflammation. Curr Pharm Des. 1999;5:771–85. [PubMed] [Google Scholar]
- 18.Biron CA. Role of early cytokines, including alpha and beta interferons (IFN-alpha/beta), in innate and adaptive immune responses to viral infections. Semin Immunol. 1998;10:383–90. doi: 10.1006/smim.1998.0138. [DOI] [PubMed] [Google Scholar]
- 19.Marrack P, Kappler J, Mitchell T. Type I interferons keep activated T cells alive. J Exp Med. 1999;189:521–30. doi: 10.1084/jem.189.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cataldi A, Santavenere E, Vitale M, et al. Interferon affects cell growth progression by modulating DNA polymerase activity. Cell Prolif. 1992;25:225–31. doi: 10.1111/j.1365-2184.1992.tb01397.x. [DOI] [PubMed] [Google Scholar]
- 21.Bever CT, Jr, Rosenberg GA. Matrix metalloproteinases in multiple sclerosis: targets of therapy or markers of injury? Neurology. 1999;53:1380–1. doi: 10.1212/wnl.53.7.1380. [DOI] [PubMed] [Google Scholar]
- 22.Brown KA. Factors modifying the migration of lymphocytes across the blood–brain barrier. Int Immunopharmacol. 2001;1:2043–62. doi: 10.1016/s1567-5769(01)00129-1. [DOI] [PubMed] [Google Scholar]
- 23.Dhib-Jalbut S, Jiang H, Williams GJ. The effect of interferon beta-1b on lymphocyte–endothelial cell adhesion. J Neuroimmunol. 1996;71:215–22. doi: 10.1016/s0165-5728(96)00156-7. [DOI] [PubMed] [Google Scholar]
- 24.Boxel-Dezaire AH, Trig T, Hoff SC, et al. Contrasting responses to interferon beta-1b treatment in relapsing–remitting multiple sclerosis: does baseline interleukin-12p35 messenger RNA predict the efficacy of treatment? Ann Neurol. 2000;48:313–22. doi: 10.1002/1531-8249(200009)48:3<313::aid-ana5>3.3.co;2-0. [DOI] [PubMed] [Google Scholar]
- 25.Byrnes AA, McArthur JC, Karp CL. Interferon-beta therapy for multiple sclerosis induces reciprocal changes in interleukin-12 and interleukin-10 production. Ann Neurol. 2002;51:165–74. doi: 10.1002/ana.10084. [DOI] [PubMed] [Google Scholar]
- 26.Kozovska ME, Hong J, Zang YC, et al. Interferon beta induces T helper 2 immune deviation in MS. Neurology. 1999;53:1692–7. doi: 10.1212/wnl.53.8.1692. [DOI] [PubMed] [Google Scholar]
- 27.Rudick RA, Ransohoff RM, Lee JC, et al. In vivo effects of interferon beta-1a on immunosuppressive cytokines in multiple sclerosis. Neurology. 1998;50:1294–300. doi: 10.1212/wnl.50.5.1294. [DOI] [PubMed] [Google Scholar]
- 28.Wiesemann E, Sonmez D, Heidenreich F, Windhagen A. Interferon-beta increases the stimulatory capacity of monocyte-derived dendritic cells to induce IL-13, IL-5 and IL-10 in autologous T cells. J Neuroimmunol. 2002;123:160–9. doi: 10.1016/s0165-5728(01)00482-9. [DOI] [PubMed] [Google Scholar]
- 29.Werfel T, Kirchhoff K, Wittmann M, et al. Activated human T lymphocytes express a functional C3a receptor. J Immunol. 2000;165:6599–605. doi: 10.4049/jimmunol.165.11.6599. [DOI] [PubMed] [Google Scholar]
- 30.Windhagen A, Maniak S, Gebert A, Ferger I, Wurster U, Heidenreich F. Human polymorphonuclear neutrophils express a B7-1-like molecule. J Leukoc Biol. 1999;66:945–52. doi: 10.1002/jlb.66.6.945. [DOI] [PubMed] [Google Scholar]
- 31.Brenner T, Arnon R, Sela M, et al. Humoral and cellular immune responses to Copolymer 1 in multiple sclerosis patients treated with Copaxone. J Neuroimmunol. 2001;115:152–60. doi: 10.1016/s0165-5728(01)00250-8. [DOI] [PubMed] [Google Scholar]
- 32.Farina C, Then BF, Albrecht H, et al. Treatment of multiple sclerosis with Copaxone (COP). Elispot assay detects COP-induced interleukin-4 and interferon-gamma response in blood cells. Brain. 2001;124:705–19. doi: 10.1093/brain/124.4.705. [DOI] [PubMed] [Google Scholar]
- 33.Wiesemann E, Klatt J, Wenzel C, Heidenreich F, Windhagen A. Correlation of serum IL-13 and IL-5 levels with clinical response to Glatiramer acetate in patients with multiple sclerosis. Clin Exp Immunol. 2003;133:454–60. doi: 10.1046/j.1365-2249.2003.02238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rudick RA, Ransohoff RM, Peppler R, VanderBrug MS, Lehmann P, Alam J. Interferon beta induces interleukin-10 expression: relevance to multiple sclerosis. Ann Neurol. 1996;40:618–27. doi: 10.1002/ana.410400412. [DOI] [PubMed] [Google Scholar]
- 35.Monteyne P, Guillaume B, Sindic CJ. B7-1 (CD80), B7-2 (CD86), interleukin-12 and transforming growth factor-beta mRNA expression in CSF and peripheral blood mononuclear cells from multiple sclerosis patients. J Neuroimmunol. 1998;91:198–203. doi: 10.1016/s0165-5728(98)00180-5. [DOI] [PubMed] [Google Scholar]
- 36.Svenningsson A, Dotevall L, Stemme S, Andersen O. Increased expression of B7-1 co-stimulatory molecule on cerebrospinal fluid cells of patients with multiple sclerosis and infectious central nervous system disease. J Neuroimmunol. 1997;75:59–68. doi: 10.1016/s0165-5728(96)00234-2. [DOI] [PubMed] [Google Scholar]
- 37.Huang WX, Huang P, Hillert J. Systemic upregulation of CD40 and CD40 ligand mRNA expression in multiple sclerosis. Mult Scler. 2000;6:61–5. doi: 10.1177/135245850000600201. [DOI] [PubMed] [Google Scholar]
- 38.Neville KL, Dal Canto MC, Bluestone JA, Miller SD. CD28 co-stimulatory blockade exacerbates disease severity and accelerates epitope spreading in a virus-induced autoimmune disease. J Virol. 2000;74:8349–57. doi: 10.1128/jvi.74.18.8349-8357.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Inoue A, Koh CS, Yamazaki M, Yagita H. Effect of anti-B7-1 and anti-B7-2 mAb on Theiler's murine encephalomyelitis virus-induced demyelinating disease. J Immunol. 1999;163:6180–6. [PubMed] [Google Scholar]
- 40.Shapiro S, Galboiz Y, Lahat N, Kinarty A, Miller A. The ‘immunological-synapse’ at its APC side in relapsing and secondary–progressive multiple sclerosis: modulation by interferon-β. J Neuroimmunol. 2003;144:116–24. doi: 10.1016/j.jneuroim.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 41.Greenwald RJ, Boussiotis VA, Lorsbach RB, Abbas AK, Sharpe AH. CTLA-4 regulates induction of anergy in vivo. Immunity. 2001;14:145–55. doi: 10.1016/s1074-7613(01)00097-8. [DOI] [PubMed] [Google Scholar]
- 42.Ito T, Amakawa R, Inaba M, Ikehara S, Inaba K, Fukuhara S. Differential regulation of human blood dendritic cell subsets by IFNs. J Immunol. 2001;166:2961–9. doi: 10.4049/jimmunol.166.5.2961. [DOI] [PubMed] [Google Scholar]
- 43.Boussiotis VA, Freeman GJ, Gribben JG, Nadler LM. The role of B7-1/B7-2: CD28/CLTA-4 pathways in the prevention of anergy, induction of productive immunity and down-regulation of the immune response. Immunol Rev. 1996;153:5–26. doi: 10.1111/j.1600-065x.1996.tb00918.x. [DOI] [PubMed] [Google Scholar]
- 44.Fleischer J, Soeth E, Reiling N, Grage-Griebenow E, Flad HD, Ernst M. Differential expression and function of CD80 (B7-1) and CD86 (B7-2) on human peripheral blood monocytes. Immunology. 1996;89:592–8. doi: 10.1046/j.1365-2567.1996.d01-785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu Z, Pelfrey CM, Cotleur A, Lee JC, Rudick RA. Immunomodulatory effects of interferon beta-1a in multiple sclerosis. J Neuroimmunol. 2001;112:153–62. doi: 10.1016/s0165-5728(00)00403-3. [DOI] [PubMed] [Google Scholar]
- 46.Mitra RS, Judge TA, Nestle FO, Turka LA, Nickoloff BJ. Psoriatic skin-derived dendritic cell function is inhibited by exogenous IL-10. Differential modulation of B7-1 (CD80) and B7-2 (CD86) expression. J Immunol. 1995;154:2668–77. [PubMed] [Google Scholar]
- 47.Farina C, Wagenpfeil S, Hohlfeld R. Immunological assay for assessing the efficacy of glatiramer acetate (Copaxone) in multiple sclerosis: a pilot study. J Neurol. 2002;249:1587–92. doi: 10.1007/s00415-002-0904-0. [DOI] [PubMed] [Google Scholar]
- 48.Walther EU, Hohlfeld R. Multiple sclerosis: side effects of interferon beta therapy and their management. Neurology. 1999;53:1622–7. doi: 10.1212/wnl.53.8.1622. [DOI] [PubMed] [Google Scholar]
- 49.Nickoloff BJ, Nestle FO, Zheng XG, Turka LA. T lymphocytes in skin lesions of psoriasis and mycosis fungoides express B7-1: a ligand for CD28. Blood. 1994;83:2580–6. [PubMed] [Google Scholar]
- 50.Simon JC, Dietrich A, Mielke V, et al. Expression of the B7/BB1 activation antigen and its ligand CD28 in T cell-mediated skin diseases. J Invest Dermatol. 1994;103:539–42. doi: 10.1111/1523-1747.ep12395743. [DOI] [PubMed] [Google Scholar]