Abstract
The phenotype and function of monocyte derived dendritic cells (MdDC) were investigated in 25 patients with common variable immunodeficiency (CVID) to test for abnormalities that might help explain the failure of antibody production. Using MHC class II DR and CD86 as markers of maturation, DCs from the majority of CVID patients were normal. However 5 patients, the majority of whom had affected family members who had previously been shown to have a susceptibility genetic locus in the MHC region, expressed abnormally low levels of DR on repeated testing, in some cases associated with a reduced capacity to support antigen stimulated T cell proliferation; nevertheless costimulatory molecules for production of IL-13, IL-10 and IFN-γ from T cells were intact. In contrast to DCs from healthy donors, DCs from many CVID patients had high spontaneous production of IL-8 and lipopolysaccharide stimulation often caused a reduction in DR expression. Expression of other cytokines (IL-1a, IL-6 and IL-12), either before or after LPS stimulation, was normal. The data suggests there is a fundamental defect in the maturation of MdDCs in a subset of CVID patients that may compromise antigen presentation and subsequent antibody production.
Keywords: Monocyte, dendritic cell, common variable immunodeficiency, MHC II DR function
INTRODUCTION
Common variable immunodeficiency (CVID) is a poorly defined disorder characterized by severe antibody deficiency, recurrent bacterial sino-pulmonary infection and increased frequency of autoimmune and neoplastic diseases [1,2]. The disease is inherited in at least 20% of cases, a major susceptibility genetic locus being within the MHC region on chromosome 6 [3]. Various defects in the differentiation and class switching of B cells have been proposed following investigation of circulating B cells from CVID patients, but B cells from the majority of patients can be induced to secrete immunoglobulins of all isotypes with appropriate in vitro conditions [4–6].
T cell function is disturbed in the majority of CVID patients, as shown by poor delayed hypersensitivity skin reactions to primary and recall antigens, low numbers of circulating antigen primed CD4 T cells after immunization, low IL-2 and high IFN-γ production following in vitro T cell stimulation, and abnormalities in the expression of various lymphocyte surface proteins [7]. We have previously hypothesized that the antibody deficiency in some patients might be due to a marked polarization towards a Th1 type of immune response, a view supported by high production of IL-12 following LPS stimulation in vitro of whole blood [8], increased numbers of circulating T cells expressing IL-12β1 receptors [9], and clinically by a high incidence of granulomatous infiltration of lymphoid and other tissues [7]. Against this background we have investigated the function of monocyte derived dendritic cells (DCs) in CVID patients, which in normal individuals have an important role in the initiation of the immune response and subsequent balance between antibody and cellular protection against infection.
MATERIALS AND METHODS
Patients
Following informed consent and approval of the local Ethics Committee, 25 patients (16 females; mean age 44 years, range 19–70 years) attending the Royal Free clinic with a diagnosis of Common Variable Immunodeficiency (CVID), using IUIS criteria [10], provided heparinized blood samples. Fourteen healthy adult volunteers provided blood for the ‘control’ cultures.
Differentiation of dendritic cells from monocytes
Peripheral blood was collected in lithium heparin tubes, mixed with an equal volume of Xvivo15 (Cambrex, Nottingham, UK) and PBMCs collected over a cushion of a half volume of Lymphoprep (Axis-Shield ProC As, density 1·077 g/ml) after 30 min centrifugation at 280× g. PBMCs were washed twice to reduce platelets. Monocyte derived dendritic cells (MdDC) cells were generated from PBMC adherent to 6 well plates (Nunc 152795) in two successive 2 h incubations (subsequently referred to as MdDC-adh) and from CD14+ cells (MdDC-col) purified with a column (Miltenyi Biotech, 130-042-201) and magnetic beads (120-000-305) as described by the manufacturer. Immature MdDC-adh and MdDC-col were generated after 6 days of culture in Xvivo15 supplemented with 5% foetal bovine serum (FBS, Gibco, Paisley, UK), 100 ng/ml GM-CSF (Insight Biotechnology, Wembley, UK) and 50 ng/ml IL4 (PeproTech EC, 200–04). The medium and reagents used were checked for LPS contamination with the limulus assay (Sigma, Poole, UK) and were negative. Yields of DCs averaged 1 × 106 per 10 × 106 PBMCs for both patients and controls, but depended on the circulating monocyte count which for the patients was within the normal range. CD14 depleted PBMCs were washed and frozen in 10% DMSO/50% FBS/40% Xvivo15 for use in subsequent functional assays. LPS (10 ng/ml – Sigma L6529) was added to cultures at day 6 of culture and supernatant and marker expression were examined after 24 h.
Detection of cytokines by ELISA
Nunc Maxisorp plates were coated with unconjugated capture mouse antihuman cytokine antibodies to IFN-γ, IL-13, IL-10 and IL4 (Becton Dickinson, Oxford, UK 551221, 554570, 554497 and 554515, respectively) at 1 µg/ml in coating buffer (0·1 m NaHCO3, pH 9) overnight at 4°C. Plates were washed once in PBS/0·05% Tween 20, followed by two washes in PBS. Plates were blocked with 5% BSA (Sigma A7030) in PBS overnight at 4°C. Plates were washed in PBS/0·05% Tween 20 and 60 µl of clarified supernatant samples added. IFN-γ, IL-13, IL-10 and IL-4 cytokine standards (BD 554416, Peprotech EC 200–13, 200–10, 200–04, respectively) were titrated from 4000 to 1 pg/ml in duplicate in the presence of biotin conjugated murine antibodies to human cytokines IFN-γ, IL-13, IL-10 and IL-4 (BD 554550, 555054, 554499, 554483, respectively) at 12·5 ng/ml in 40 µl 1% BSA + 2% AB Serum (Sigma S7148) in PBS/0·05% Tween 20 overnight at 4°C. Plates were washed three times in PBS/0·05% Tween 20, and HRP conjugated Streptavidin (Biosource SNN2004) added at 100 ng/ml in 1% BSA/PBS/0·05% Tween 20 for 40 min at 37°C. Plates were washed twice in wash buffer and twice in PBS. Plates were developed with TMB buffer (Zymed, San Francisco, USA 00–2023), the reaction stopped after 10 minutes in the dark at room temperature by the addition of an equal volume of 1 m HCl. Absorbance was read at 450 nm in a Dynatech MRX plate reader.
Intracellular and surface staining
MdDC-adh cells were stained with antibodies in 96 well plates. Cells were incubated for 4 h with 10 µg/ml brefeldin A (Sigma B6751) and washed twice with 200 µl of cold RPMI/0·01% sodium azide centrifuged at 870 × g for 5 min. The plate was then blotted and the cells resuspended by vortex in 25 µl of fixative solution (reagent A, Serotec BUFO9B) and the plate incubated at room temperature for 15 min. Wells were washed twice, resuspended and stained with 3 antibodies simultaneously in permeabilizing agent (Leukoperm, Serotec BUFO9B) for 1 h at room temperature. MdDC-col cells from some of the patients and controls were also stained for surface markers without permeabilization.
Mouse FITC conjugated anti-human antibodies to MHC II DR, CD83 or CD86 (Pharmingen 555560 and 556855, Serotec MCA 119F, respectively), one of four anti-human cytokine reagents IL-12, IL-6, IL-1a (Pharmingen 559329, 559331 and BD 340514) or IL-8 conjugated to phycoerythrin (Serotec MCA 126PE), and one cy-chrome conjugated antibody against CD40, IL-10, HLA class I ABC (Pharmingen 555590, 554707 and 555554) were premixed in a parallel 96 well plate, diluted 1 : 5 in Leukoperm, and 25 µl applied to pelleted cells in test plates. Controls were stained with mouse isotype control antibodies IgG1 FITC, IgG1 PE and IgG2 Cy-Chrome (BD 349041, 349043 and 349047). Finally, cells were washed twice, resuspended in 100 µl PBS and transferred to LP2 tubes (Life Sciences International, LK00023) and analysed with a Becton Dickinson FACScan.
DC supported T cell proliferation
MdDC-adh cells at day 6 were washed and mixed with 250 µl anti-CD19 (BU12 clone, a gift from D Hardie, Birmingham), 350 µl of anti-CD3 (ATCC-CRL-8001) and 100 µl anti-CD14 (ATCC-HB-247) in 2 ml of Xvivo15 for 20 min at 4°C. Anti-mouse antibody coated magnetic beads (Dynal M-450), 10 µl per 1 × 106 cells, were added for 10 min before cells were depleted with a magnet. MdDC (<5% contaminating CD3+ T cells, CD19+ B cells and CD14+ monocytes) were washed and resuspended in Xvivo15 medium supplemented with 5% FBS, 100 ng/ml GM-CSF and 50 ng/ml IL4 and plated at a concentration of 15 000 cells per well in 96 well round bottom plates. LPS (10 ng–1 µg/ml), E. coli (107 CFU/ml), tetanus toxoid (5–50 µg/ml) and M. tuberculosis PPD (100 ng–10 µg/ml Evans Vaccines dialysed in Xvivo15) was applied in triplicate to MdDC wells and incubated for 48 h. Cells were then washed twice and mixed with T cells (see below).
CD14-depleted PBMC were thawed, washed and mixed for 20 min with 500 µl anti-CD19 and 200 µl anti-CD14 hybridoma supernatants in 2 ml of RPMI 1640. Non-T cells were depleted by magnet after addition of 10 µl/106 cells of anti-mouse antibody coated magnetic beads for 10 min and the cell purity (i.e. >95%) checked by flow cytometry. T cells were washed and resuspended at 15 × 106 cells/ml in 10% FBS/RPMI 1640 and 100 µl aliquots added to wells containing antigen-pulsed MdDC or antigen only. T cell control wells were incubated with 10 µg/ml PHA (phytohaematoglutinin, Sigma L9132), allogeneic MdDC or alone. Plates were incubated in humidified air with 5% CO2 for 7 days with the addition of 1 µCi tritiated thymidine (Pharmacia-Amersham, Chalfont St Giles, Buchinghamshire TRK637) per well for the last 18 h. Incorporated thymidine was captured on glass fibre filters (Perkin Cetus Life Sciences, Cambridge, 1205–401) with a Tomtec cell harvester (Hamden, CT, USA) and measured by a Wallac (Turku, Finland) 145 MicroBeta Trilux scintillation counter.
Dendritic and T cell interaction
The ability of DCs to provide costimulatory signals to T cells was tested as follows: CD/14 column purified monocytes were obtained from 40 ml of blood (see above) and cultured for 6 days in GMCSF and IL4 to induce DC formation. The remaining CD14 negative cells (lymphocytes) were frozen and stored. To test the ability of these DCs to provide a costimulus for cytokine production, particularly IL-13, freshly thawed CD14-depleted PBMC (>85% T cells) were washed thoroughly in and resuspended in XVivo15 medium supplemented with 5% FBS. The CD14 depleted PBMC (5 × 105) and DCs (8·3× 104) were added to wells in 96 well flat bottom plates. The cultures were left untreated or stimulated with anti-CD3 or anti-CD3 and anti-CD28 (clone 28·2) antibodies at 50 ng/ml and 250 ng/ml, respectively, for 48 h after which the plates were centrifuged at 875 × g for 10 min. Supernatants were transferred and stored in plates at −70°C until IFN-γ, IL-13, IL-10 and IL-4 were measured by ELISA.
Statistical analysis
Statistical analyses were performed using SSPS for Windows (version 8·0) and Microsoft Excel 2000. Levels of cytokines and numbers of cells expressing surface molecules were presumed to show a normal distribution and were tested using an independent sample 2-tailed t-test. The Mann–Whitney U-test was used for the proliferation data. P values <0·05 were considered significant.
RESULTS
Maturation of dendritic cells
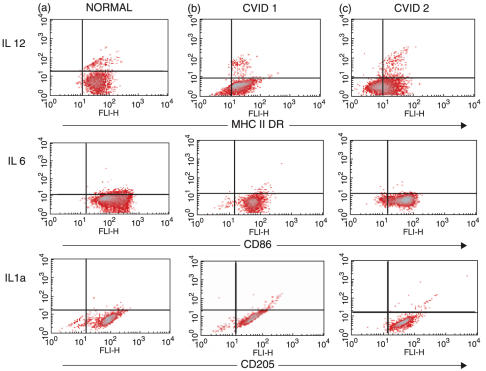
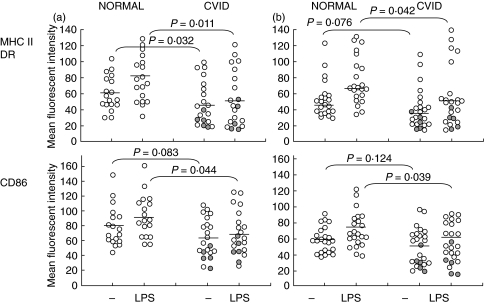
The temporal appearance of large pleomorphic cells with dendrites was qualitatively similar for PBMC cultured with GMCSF and IL-4 from healthy adults and CVID patients. Furthermore, the presence and intensity of DR, CD86 and CD205 (an endocytosis receptor [11]) molecules on the surface of MdDC purified from either adherent or column purified monocytes from most of the CVID patients was similar to MdDC from healthy adults (Fig. 1a,b). However, mature MdDC-adh from some CVID patients (Fig. 1c) showed low expression of DR and CD86 molecules, while CD205 was similar. When comparing the groups of patients and controls, there was a statistically significant reduction in the expression of DR in the CVID group (P = 0·03) (Fig. 2). In contrast to the response of MdDC from normal individuals to LPS, which usually increased the intensity of DR expression, MdDC-adh from 11 CVID patients (52%) showed reduced expression after LPS and the patient mean reduction was different from normal (P = 0·01) (Fig. 2). Over 80% of cells from normal donors were CD86 positive, in contrast to a much wider variation in the patients (40–100%). Co-stimulatory molecule CD 86 expression was not different in the patients (P = 0·08) but 7 CVID patients showed reduced expression after LPS stimulation and the patient mean change in CD86 expression was contrary to the normal LPS maturation response (P = 0·04).
Fig. 1.
Maturation of adherent monocyte-derived dendritic cells (MdDC-adh). Expression of MHC class II, CD86 and CD205 molecules on day 6 DCs are shown on the x axis with simultaneous intracellular cytokine (IL-12p70, IL-6 and IL-1α) expression on the y axis. The percentage of positively staining cells in each plot appears in the upper right quadrants. (a) Normal adults have equivalent marker expression to (b) the majority of individuals with CVID (CVID 1). (c) A proportion of CVID individuals (CVID 2) demonstrate less MHC class II and CD86 molecules but complete expression of the myeloid marker CD205.
Fig. 2.
Distribution and change in MHC and CD86 with LPS stimulation in dendritic cells. (a) Individual mean fluorescent intensity from normal donors and CVID patients; MdDC-adh of normal (n = 18) and CVID (n = 21). (b) MdDC-col of normal (n = 22) and CVID (n = 24). repeat samples from low DR MdDC-adh individuals. Bars indicate median values. P-values from Mann–Whitney U-test.
repeat samples from low DR MdDC-adh individuals. Bars indicate median values. P-values from Mann–Whitney U-test.
Six of the 21 patients tested with low DR expression on the initial testing of MdDC-adh cells were available for re-testing using the MdDC-col technique, each time comparing the results with cells from healthy donors. DCs generated in this way from normal donors generally formed homogenous populations with virtually complete DR and CD86 molecule expression. However, 5 of the 6 patients showed consistently low DR expression on MdDC-col cells on more than one occasion (Table 1), with 4 of these 5 also having low CD86 expression. Similar to the finding with MdDC-adh cells, LPS stimulation did not increase DR expression in these 5 patients. Low surface expression of DR and CD86 was confirmed in patients in the absence of permeabilization. Brief clinical data on the CVID patients with low expression of MHC class II on MdDC (adh or col) cells, together with the frequency of low DR expression on repeated testing is shown in Table 1. There was no obvious difference in the clinical features as compared to the rest of the CVID patients, except for a clustering of patients with affected family members in this group.
Table 1.
Clinical data on patients whose DCs showed poor expression of MHC Class II-DR on initial testing. Family members of 15 of the 25 patients in the cohort were screened for immunoglobulin deficiencies. Five patients had affected family members with CVID and/or selective IgA deficiency (IgAD), 4 of whom appear in this table.
| Serum immunoglobulins | |||||||
|---|---|---|---|---|---|---|---|
| Patient no. | Age (years) | IgG | IgA | IgM | Familial CVID/IgAD | Complications | No. of repeat tests |
| 3 | 50 | <2·0 | <0·1 | <0·1 | Yes | Granulomatous | 1 (ad)* |
| lung disease | 2 (col)† | ||||||
| 8 | 57 | 4·5 | <0·1 | 0·6 | ND | Bronchitis | 1 (ad) |
| 3 (col) | |||||||
| 13 | 48 | 4·1 | 0·4 | 0·6 | Yes | Severe bronchiectasis | 1 (ad) |
| 2 (col) (N) | |||||||
| 17 | 51 | 2·2 | <0·2 | 0·4 | ND | Severe enteropathy | 1 (ad) |
| 2 (col) | |||||||
| 20 | 38 | 4·4 | <0·1 | 0·3 | Yes | Very high | 1 (ad) |
| anti-IgA antibodies | 3 (col) | ||||||
| 25 | 44 | <1·5 | <0·1 | <0·1 | Yes | Gastritis with | 2 (ad) |
| vitamin B12 deficiency | 2 (col) | ||||||
ad, MdDC-adh;
CD14 MdDC-Col ND, Family members not screened N denotes the only patient (no. 13) whose DCs showed normal DR expression when subsequently tested using column separated monocyte precursors.
CD83 expression was variable and usually very low on both column and adherent purified MdDC from both normal and CVID patients and usually increased after LPS stimulation in both groups.
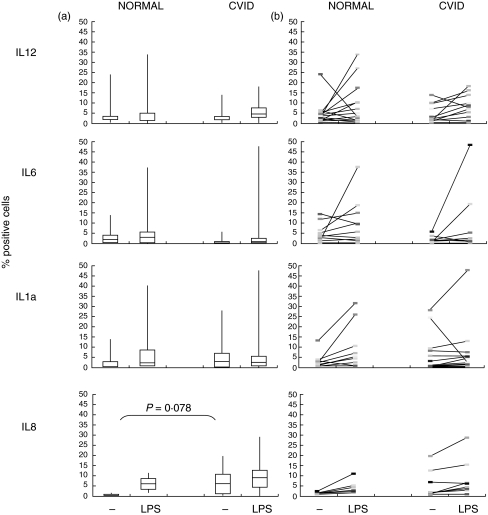
The production of intracellular IL-12p70, IL-6 and IL-1a was consistently limited to a minor percentage of MdDC-adh or MdDC-col cells in both patients and controls. There was only a small increase in cells containing IL-12p70 or IL-6 after LPS stimulation in both healthy controls and CVID patients, with a smaller increase for IL-12 and a lower baseline for IL-6 in CVID patients although these differences did not reach significance (Fig. 3).
Fig. 3.
Change in cytokine and chemokine expression with LPS stimulation in normal and CVID MdDC-adh cells. (a) bar shows median, 25–75% in boxes and complete ranges (|) in percent positive expression on DCs from normal donors and CVID patients. (b) individual changes in IL-12, IL-6, IL-1a (18 controls and 21 patients) and IL-8 expression (8 controls and patients) with LPS stimulation. P values from Mann–Whitney U-test.
IL-8 was produced by only a small proportion of cells from healthy controls without stimulation (mean 0·98%) with an average 6·4% fold increase in positive cells after LPS stimulation. However, there were more cells expressing IL-8 without stimulation in CVID patients (P = 0·03), with only a very small increase after LPS stimulation (Fig. 3). Four of the 5 patients whose DCs expressed low levels of DR had high numbers of DCs spontaneously expressing IL-8.
Normal costimulation of T cell cytokines by DCs from CVID patients
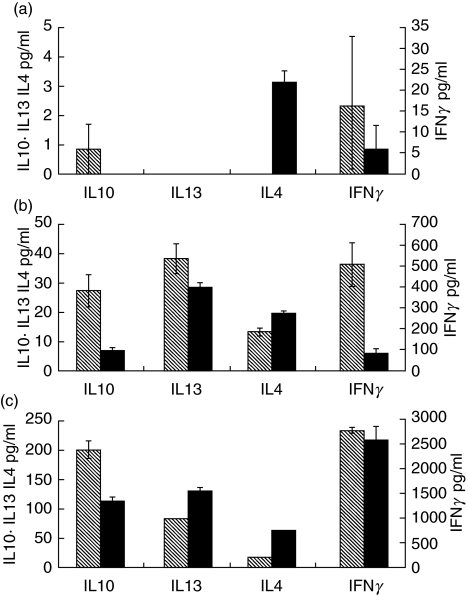
Four patients and controls were tested in this system, two of the patients being selected from the 5 patients with consistently low expression of DR on their DCs. The 48 h cytokine production of MdDC-col stimulated autologous T cells was normal in all 4 patients, suggesting normal function of the B7 costimulatory ligand on DCs (Fig. 4).
Fig. 4.
Cytokine profile of Control ( ) and Patient (▪) T cells stimulated with (a) anti-CD3 alone, (b) anti-CD3 and anti-CD28 or (c) anti-CD3 and dendritic cells. Error bars show SEM of triplicated experiments. Note differences in scales.
) and Patient (▪) T cells stimulated with (a) anti-CD3 alone, (b) anti-CD3 and anti-CD28 or (c) anti-CD3 and dendritic cells. Error bars show SEM of triplicated experiments. Note differences in scales.
T cell proliferation to antigen presented by MdDC
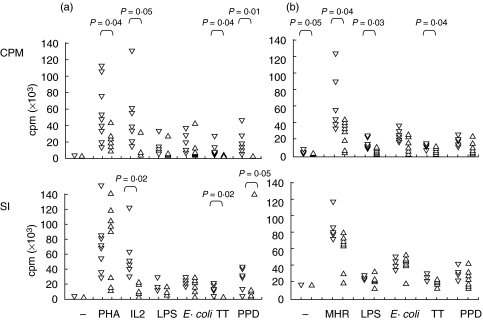
Lymphoproliferative responses of 9 CVID individuals, including 2 with low MHC Class II expression from DCs, was lower than that of the 9 healthy subjects, in regards to the thymidine uptake in both unstimulated and tetanus toxoid, PPD, IL-2 or PHA stimulated T cells (Fig. 5). However, thymidine uptake was similar with LPS and E.coli stimulation in both healthy controls and patients.
Fig. 5.
Lymphocyte proliferation in 9 normal adults (▿) and CVID patients (▵). (a) PBMC were incubated with antigens directly or (b) purified T cells with MdDC-col pulsed for 48 h with antigens. T cell incorporation of tritiated thymidine (cpm) for 18 h was measured in triplicate after culture of cells for 7 days; maximum uptake on concentration curve shown for each antigen. Stimulation index (SI) was derived by dividing cpm values by the basal proliferation rate of unstimlated PBMC or unpulsed dendritic cells. Where results are similar in different individuals, only one triangle is shown. Comparisons made using Mann–Whitney U-test.
A different pattern of response to antigens was seen in the presence of DCs. The response to unpulsed dendritic cells alone was approximately one third lower in CVID T cells as compared to healthy controls, and there were significant reductions in thymidine incorporation in CVID T cells when mixed with allogeneic dendritic cells (P = 0·04), or when autologous DCs were pulsed with LPS (P = 0·03) and tetanus toxin (P = 0·04), but not with E. coli or PPD. However, the statistical differences in regard to stimulation indices between patients and controls were lost for all antigens when the adjustment was made for a lower basal proliferation rate in CVID patients. The T cells from CVID patients with repatedly low MHC Class II expression on DCs showed particularly low proliferation to antigen presented by DCs.
DISCUSSION
Monocytes from the majority of CVID patients differentiated into dendritic cells, as defined by typical morphology and high surface expression of MHC Class II and CD86. However, we were able to show that DCs from 5 of the patients with low DR expression on initial testing were consistently abnormal on more than one occasion when tested again using CD14 column purified monocytes as DC precursors. Since there were 5 other patients whose DCs showed low expression of DR on the first screening, it is possible that up to 50% of CVID patients have persistently reduced expression of DC maturation markers. However, DCs from healthy donors showed wide variation in marker expression and the results on re-testing one patient were not consistent. Therefore the in vitro system we used is probably too insensitive to identify subtle defects in DC maturation, only identifying those with severe defects.
CD86 expression was low in most of those with low DR expression, suggesting that there is failure of DC maturation in some CVID patients, rather than a specific defect in the expression or fixation of surface DR on DCs. Our preliminary studies have shown normal expression of DR on B cells from some of the same CVID patients in this study (data not shown) and DR expression on T cells and monocytes from CVID patients appears to be normal in previously published work [7,8]. In those patients tested, the DCs with reduced DR expression were functional in their ability to provide a costimulus for anti-CD3 to stimulate the production of IL-13, IL-4 and IFN-γ from autologous T cells; this suggests that these DCs expressed functional B7 molecules [12]. DCs from most patients were also able to present a variety of antigens to autologous T cells and support normal proliferation; as expected the T cells alone generally showed poor proliferation to these antigens, as has been previously described [13,14]. The stimulation index for autologous T cells stimulated with E coli, PPD and tetanus toxoid in the presence of DCs, or the MLR with allogeneic T cells, was very low when DCs were used from two of the patients with the lowest DR expression, suggesting that these cells are compromised in their ability to present antigen on Class II molecules.
What could be the mechanism for the low DR expression on DCs from some CVID patients? Contaminating T cells producing inhibitors for DC maturation could explain the phenomenon with MdDC-adh cells. IFN-γ is a candidate since this has been shown to inhibit DC differentiation if present in the earlier stages of the culture [15], and when lymphocytes from CVID patients are stimulated in vitro, more cells express IFN-γ than from normal individuals [16]. However, the fact that the abnormality persists when purified CD14+ monocytes are used as DC precursors does not support this explanation.
Another possibility is that the circulating monocytes in some CVID patients have been influenced by exposure in vivo to bacterial lipopolyssacharide (LPS) due to a failure to produce neutralizing antibody to LPS, perhaps exacerbated by increased numbers of LPS producing bacteria in the gut. This scenario is supported by the finding that LPS can prevent DC differentiation in vivo in a mouse model [17] and impaired maturation, APC function and cytokine production of human monocytes when added in vitro [18]. Although it is likely that all the CVID patients in our cohort fail to make antibodies to LPS, the amount of LPS produced by the bacterial flora in the gut may vary between patients.
The increased numbers of DCs expressing IL-8 in CVID might again reflect in vivo exposure of circulating monocytes to LPS. This pro-inflammatory chemokine is mainly produced by macrophages, neutrophils and plasmacytoid dendritic cells [19] but has been detected in Langerhans cells after CD40 ligation [20] and in monocyte derived DCs [21]. The numbers of IL8 producing cells was amplified 6·5 fold on average in normal cells by LPS exposure and may be a clearer indicator of dendritic cell maturation than other cytokine responses. Oxidative stress is reported to elevate the production of IL8 from MdDC [22] and there is evidence of increased oxidative stress in monocytes from CVID patients [23].
DCs from 9 of the CVID patients responded abnormally to LPS stimulation, in that DR expression decreased; the same effect was seen with DCs from only one normal control. This could be explained by a defect in stabilizing Class II on the DC surface during maturation [24], possibly associated with particular MHC Class II alleles. This possibility should be investigated further, particularly since there was a clustering of familial CVID cases in the subgroup with low DR expression, these same families providing evidence for a CVID genetic susceptibility locus within the MHC Class II region [3].
In conclusion, the maturation of DCs from monocytes appears to be impaired in a subset of CVID patients, and there may be an additional problem in the fixation of Class II molecules on the surface of DCs in some patients. These abnormalities could contribute to the failure to generate antigen specific CD4+ T cell responses reported in CVID, without which antibody production is likely to be severely impaired.
Acknowledgments
We thank Sisters Sarita Workman, Cilla Freud and other nurses for providing blood from the patients, and Dr M Ritter (RPMS, London) for providing the Mab to CD205. The work was part funded by a European Union grant (IMPAD).
REFERENCES
- 1.Cunningham-Rundles C. Clinical and immunologic analyses of 103 patients with common variable immunodeficiency. J Clin Immunol. 1989;9:22–33. doi: 10.1007/BF00917124. [DOI] [PubMed] [Google Scholar]
- 2.Hermaszewski RA, Webster AD. Primary hypogammaglobulinaemia: a survey of clinical manifestations and complications. Q J Medical. 1993;86:31–42. [PubMed] [Google Scholar]
- 3.Kralovicova J, Hammarstrom L, Plebani A, Webster AD, Vorechovsky I. Fine-scale mapping at IGAD1 and genome-wide genetic linkage analysis implicate HLA-DQ/DR as a major susceptibility locus in selective IgA deficiency and common variable immunodeficiency. J Immunol. 2003;170:2765–75. doi: 10.4049/jimmunol.170.5.2765. [DOI] [PubMed] [Google Scholar]
- 4.Bryant A, Calver NC, Toubi E, Webster AD, Farrant J. Classification of patients with common variable immunodeficiency by B cell secretion of IgM and IgG in response to anti-IgM and interleukin-2. Clin Immunol Immunopathol. 1990;56:239–48. doi: 10.1016/0090-1229(90)90145-g. [DOI] [PubMed] [Google Scholar]
- 5.Ochs HD, Hollenbaugh D, Aruffo A. The role of CD40L (gp39) /CD40 in T/B cell interaction and primary immunodeficiency. Semin Immunol. 1994;6:337–41. doi: 10.1006/smim.1994.1042. [DOI] [PubMed] [Google Scholar]
- 6.Warnatz K, Denz A, Drager R, et al. Severe deficiency of switched memory B cells (CD27(+)IgM(−)IgD(−) in subgroups of patients with common variable immunodeficiency: a new approach to classify a heterogeneous disease. Blood. 2002;99:1544–51. doi: 10.1182/blood.v99.5.1544. [DOI] [PubMed] [Google Scholar]
- 7.Webster ADB. Common variable immunodeficiency. In: Roifman C, editor. Humoral Deficiencies. Vol. 21. Philadelphia, USA: Immunology and Allergy Clinics of North America; 2001. pp. 1–22. [Google Scholar]
- 8.Cambronero R, Sewell WA, North ME, Webster AD, Farrant J. Up-regulation of IL-12 in monocytes: a fundamental defect in common variable immunodeficiency. J Immunol. 2000;164:488–94. doi: 10.4049/jimmunol.164.1.488. [DOI] [PubMed] [Google Scholar]
- 9.McQuaid A, Tormey VJ, Trafford B, Webster AD, Bofill M. Evidence for increased expression of regulatory cytokine receptors interleukin-12R and interleukin-18R in common variable immunodeficiency. Clin Exp Immunol. 2003;134:321–7. doi: 10.1046/j.1365-2249.2003.02271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conley ME, Notarangelo LD, Etzioni A. Diagnostic criteria for primary immunodeficiencies. Clin Immunol. 1999;93:190–7. doi: 10.1006/clim.1999.4799. [DOI] [PubMed] [Google Scholar]
- 11.Freysdottir J, Ritter MA. Antibodies to human thymic epithelium form part of the murine autoreactive repertoire. Dev Immunol. 2001;8:75–93. doi: 10.1155/2001/59678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jordan WJ, Ritter MA. Optimal analysis of composite cytokine responses during alloreactivity. J Immunol. 2002;260:1–14. doi: 10.1016/s0022-1759(01)00490-2. [DOI] [PubMed] [Google Scholar]
- 13.Stagg AJ, Funauchi M, Knight SC, Webster AD, Farrant J. Failure in antigen responses by T cells from patients with common variable immunodeficiency (CVID) Clin Exp Immunol. 1994;96:48–53. doi: 10.1111/j.1365-2249.1994.tb06228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Funauchi M, Farrant J, Moreno C, Webster AD. Defects in antigen-driven lymphocyte responses in common variable immunodeficiency (CVID) are due to a reduction in the number of antigen-specific CD4+ T cells. Clin Exp Immunol. 1995;101:82–8. doi: 10.1111/j.1365-2249.1995.tb02281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Delneste Y, Charbonnier P, Herbault N, Magistrelli G, Caron G, Bonnefoy JY, Jeannin P. Interferon-gamma switches to monocyte differentiation from dendritic cells to macrophages. Blood. 2003;101:143–50. doi: 10.1182/blood-2002-04-1164. [DOI] [PubMed] [Google Scholar]
- 16.North ME, Webster AD, Farrant J. Primary defect in CD8+ lymphocytes in the antibody deficiency disease (common variable immunodeficiency). abnormalities in intracellular production of interferon-gamma (IFN-gamma) in CD28+ (‘cytotoxic’) and. Clin Exp Immunol. 1998;111:70–5. doi: 10.1046/j.1365-2249.1998.00479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rotta G, Edwards EW, Sangaletti S, et al. Lipopolysaccharide or whole bacteria block the conversion of inflammatory monocytes into dendritic cells in vivo. J Exp Med. 2003;198:1253–63. doi: 10.1084/jem.20030335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xie J, Qian J, Wang S, Freeman ME, Epstein J, Yi Q. Novel and detrimental effects of lipopolysaccaride on in vitro generation of immature dendritic cells: involvement of mitogen- activated protein kinase p38. J Immuol. 2003;171:4792–800. doi: 10.4049/jimmunol.171.9.4792. [DOI] [PubMed] [Google Scholar]
- 19.Rissoan MC, Soumelis V, Kadowaki N, et al. Reciprocal control of T helper cell and dendritic cell differentiation. Science. 1999;283:1183–6. doi: 10.1126/science.283.5405.1183. [DOI] [PubMed] [Google Scholar]
- 20.Caux C, Massacrier C, Vanbervliet B, Dubois B, Van Kooten C, Durand I, Banchereau J. Activation of human dendritic cells through CD40 cross-linking. J Exp Med. 1994;180:1263–72. doi: 10.1084/jem.180.4.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buelens C, Verhasselt V, De Groote D, Thielemans K, Goldman M, Willems F. Human dendritic cell responses to lipopolysaccharide and CD40 ligation are differentially regulated by interleukin-10. Eur. J Immunol. 1997;27:1848–52. doi: 10.1002/eji.1830270805. [DOI] [PubMed] [Google Scholar]
- 22.Verhasselt V, Goldman M, Willems F. Oxidative stress up-regulates IL-8 and TNF-alpha synthesis by human dendritic cells. Eur J Immunol. 1998;28:3886–90. doi: 10.1002/(SICI)1521-4141(199811)28:11<3886::AID-IMMU3886>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 23.Aukrust P, Berge RK, Muller F, Ueland PM, Svardal AM, Froland SS. Elevated plasma levels of reduced homocysteine in common variable immunodeficiency – a marker of enhanced oxidative stress. Eur J Clin Invest. 1997;27:723–30. doi: 10.1046/j.1365-2362.1997.18807328.x. [DOI] [PubMed] [Google Scholar]
- 24.Villadangos JA. Presentation of antigens by MHC class II molecules. getting the most out of them. Mol Immunol. 2001;38:329–46. doi: 10.1016/s0161-5890(01)00069-4. [DOI] [PubMed] [Google Scholar]