Abstract
Low electric field cancer treatment − enhanced chemotherapy (LEFCT-EC) is a new anticancer treatment which utilizes a combination of chemotherapeutic agents and a low electric field. We investigated the antitumour effectiveness of this technique in a model of murine colon carcinoma (CT-26). The low electric field was applied to ∼65 mm3 intracutaneous tumours after intratumoral injection of 5FU, bleomycin or BCNU. We observed significant tumour size reduction and a prolongation of survival time. The complete cure of a significant fraction of animals treated by LEFCT-EC with 5FU (33%), bleomycin (51%) or BCNU (83%) was observed. Mice cured by LEFCT-EC developed resistance to a tumour challenge and their splenocytes had antitumour activity in vivo. Our results suggest that LEFCT-EC is an effective method for treatment of solid tumours.
Keywords: chemotherapy, colon cancer, electrostimulation, immunostimulation, low electric field
INTRODUCTION
Colorectal cancer (CRC) is the second most common malignancy of both sexes in developed countries. Incidence rates have risen (especially in men) since 1985, so that the estimated number of cases in the United States in 2003 was ∼11% [1]. Surgery can cure approximately 50% of patients and the overall 5-year survival of patients with CRC (colorectal cancer) is of the order of 50–55%, with death being due mainly to cancer spread [2]. A considerable number of patients often cannot be operated because of underlying severe complications. For patients with widespread disease and those that cannot undergo surgery, other treatment modalities should be developed.
Chemotherapy alone is not efficient against primary or metastatic CRC. Many chemotherapeutic agents can work only after penetration into the cytoplasm of tumour cells, and their ability to penetrate is often restricted by non-permeable barrier of the cell membrane. To increase the delivery of drugs into tumour cells, two methods based on exposure of primary tumours to electric fields have been suggested.
These methods differ substantially both in their electrical parameters as well as in their underlying mechanisms. The first method, termed electrochemotherapy (ECT), adopted the electroporation setting of high voltage and short duration pulses, where exposure results in permeability increase of the cell membrane due to the formation of membrane pores [3]. The second method stems from our findings that exposure of cells to unipolar trains of low electric fields (20–200 V/cm) induced an efficient non-specific uptake of macromolecules with molecular weights in the range of 1–2000 kDa [4]. The underlying mechanism of this uptake seems to involve an electric field-induced endocytotic-like process [4]. The phenomenon of electroendocytotic uptake by cells after their exposure to 2·5–20 V/cm pulsed low electric fields has been well documented in recent studies [5]. Based on this phenomenon, we developed a novel therapeutic methodology where we expose primary tumours to a low electric field in combination with chemotherapeutic agents in the extracellular compartment of the tumour. The application was designated as ‘low electric field cancer treatment − enhanced chemotherapy’ (LEFCT-EC). The low electric field treatment without chemotherapy was termed ‘low electric field cancer treatment’ (LEFCT). LEFCT-EC was applied recently by us in murine B-16 melanoma with different chemotherapeutic drugs and was found to achieve a cure level up to 30% [6].
In the present study we tested the efficacy of LEFCT-EC against colon carcinoma (CT-26) in vivo in a mouse model. We demonstrate that LEFCT-EC is able to destroy the primary tumour and initiate antitumour immune reactions triggered probably by the antigenic material released from the deteriorating primary lesion.
MATERIALS AND METHODS
Animals
BALB/c male mice (8–12 weeks old) were obtained from the breeding colony of Tel-Aviv University, Israel. Animal care and experimentation was carried out in accordance with Tel-Aviv University guidelines.
Tumour cell line
CT-26 colon carcinoma was induced in a BALB/c mouse by chemical carcinogenesis and was kindly provided by Dr J. Fidler (M.D. Anderson Cancer Institute, Houston, TX, USA). The cell line was found negative for mycoplasma infection as revealed by the VenorGem mycoplasma detection kit 25T (Minerva Biolabs, Berlin, Germany). CT-26 cells were maintained in supplemented Dulbecco's modified Eagle's medium (DMEM), as described previously [7].
Tumour cell inoculation
Animals were inoculated intracutaneously with 105 CT-26 cells in 100 µl phosphate-buffered saline (PBS) into the low lateral side of the back. Local tumour growth was determined by measuring three mutually orthogonal tumour diameters with a caliper. The volume (V) of the tumours was calculated using the formula: V = D1 × D2 × D3 × π/6, where D1, D2 and D3 represent the three mutually orthogonal tumour diameters.
Low electric field cancer treatment protocol
The exposure of tumours to electric fields was carried out by electrodes, as described previously [6]. Once the tumour reached the size of 5 mm in diameter (∼65 mm3 in volume) (9–12 days after tumour inoculation), it was pierced by four needles (total length 37 mm, diameter of 0·32 mm), one cathode needle in the middle of the tumour and three anodes around it, ∼4 mm from the cathode. The electrodes were connected to an electric pulse generator (GRASS S48 stimulator, GRASS medical instruments, Quincy, MA, USA). The following electric parameters were used: electric field of 20–70 V/cm, repetition frequency of 500 Hz, pulse width of 180 µs and total exposure duration of 12 min. The drug (solution of 100 µl) was injected either into the primary tumour (i.t.) or intravenously (i.v.). Each mouse was treated only once by the low electric field pulses either 2 min after i.t. drug administration or 10 min after i.v. drug administration. The experimental groups that were treated by low electric field in the absence or presence of chemotherapy were designated as LEFCT and LEFCT-EC (name of the drug), respectively. Mice were anaesthetized 10 min before the treatment by i.p. injection of an anaesthetic cocktail composed of imalgen 100 mg/kg and xylazine hydrochloride 6·25 mg/kg in PBS (0·25 ml per mouse).
Chemotherapeutic agents
The following chemotherapeutic drugs were used in this study: bleomycin (Baxter Oncology GmbH, Frankfurt, Germany), BCNU (bis-chloroethyl-nitrosurea, carmustine, Bristol-Myers Squibb Company, Princeton, USA; 33 mg/ml in 96% ethanol) and 5-fluorouracil (5FU, Abic Ltd, Netanya, Israel). The chemotherapeutic drugs were dissolved in PBS to the required concentration before use.
Activity of spleen cells against the tumour cells (Winn assay)
Spleens were removed from normal animals, from the animals bearing 5 mm or 30 mm in diameter CT-26 tumours or from animals cured by different treatments. Splenocytes were mixed in proportions of 100 : 1 (107 : 105) with CT-26 tumour cells and 0·2 ml of the suspension was inoculated into the low lateral side of the backs of normal animals.
Survival measurements
Survival of the experimental animals was measured until 120 days after tumour inoculation (∼ 110 days after treatments). All animals that were alive at this time-point were tumour-free and were considered as cured, as no recurrences occurred there. At post mortem no further metastases were found.
Calculation of energy
The electric energy (E) dissipated during low electric field procedures was calculated using the formula: E = V × I × t, where V: electric voltage, I: electric current, t: time of exposure.
Statistical analysis
The statistical significance (P < 0·05) of the differences between volumes of tumours in the various groups was assessed by applying a two-sided Student's t-test. The plot of survival times (Kaplan–Meir test) and survival comparison between groups (Mantel–Cox test) were carried out using Statsoft statistica software.
RESULTS
Anti-tumoral effect of LEFCT-EC with various cytotoxic drugs
Anti-tumoral effect of LEFCT-EC with 5FU.
We examined the therapeutic efficacy of different chemotherapeutic drugs in combination with the low electric field treatment. We also examined the importance of the mode of drug administration on tumour progression by injecting the chemotherapeutic drug either intratumorally (i.t.) or intravenously (i.v.). Tumour-bearing mice were subjected to the following treatments:
Non-treated tumour-bearing mice (TB mice).
TB mice treated with an electric field of 40 V/cm (LEFCT).
TB mice treated with chemotherapy.
TB mice treated with a combination of chemotherapy and followed by an exposure to electric field of 40 V/cm (LEFCT-EC-drug).
In each group the survival time and tumour volume were monitored. The results presented in Table 1 indicate that treatment with either 5FU (100 mg/kg) alone or electric field alone (LEFCT) did not cure the animals. However, 33% of the animals treated by the application of 5FU i.t. combined with electric field (LEFCT-EC-5FU i.t.) were completely cured 120 days after tumour inoculation. When the drug was injected i.v. (LEFCT-EC-5FU i.v.), the survival rate was reduced to 16%. We also found a significant increase in the survival time of mice treated either by LEFCT-EC-5FU i.t. or by LEFCT-EC-5FU i.v. compared with 5FU i.t. alone or 5FU i.v. alone (P = 0·03 and P = 0·001, respectively). There was no significant difference in the survival time between LEFCT-EC-5FU i.t. and LEFCT-EC-5FU i.v. treatment groups. The results presented in Table 1 show that treatment with LEFCT-EC delayed the primary tumour growth, even though the primary tumour was not completely eliminated (LEFCT-EC-5FU i.t. versus 5FU i.t., P = 0·03, LEFCT-EC-5FU i.v. versus 5FU i.v., P = 0·0002).
Table 1.
Effect of LEFCT-EC with different chemotherapeutic drugs on the development of CT-26 colon carcinoma
| Average tumour volume (mm3 ± SE)a | ||||||||
|---|---|---|---|---|---|---|---|---|
| Groups | TB mice | LEFCT | 5FU i..t. | 5FU i.v. | LEFCT-EC 5FU i.t. | LEFCT-EC 5FU i.v. | Bleomycin | LEFCT-EC bleomycin |
| Tumour volumeb | 3328 ± 176 | 1782 ± 267 | 780 ± 134 | 461 ± 105 | 118 ± 48 | 119 ± 49 | 1715 ± 208 | 584 ± 146 |
| (68) | (35) | (12) | (6) | (6) | (6) | (19) | (24) | |
| % of survivalc | 0 | 0 | 0 | 0 | 33 | 16 | 0 | 52 |
Average tumour volume in all mice in each group (no. of mice).
Tumour volume was measured 22–24 days after tumour inoculation. Treatment was given when the tumour volume was 65 mm3(9–12 days after tumour inoculation).
Survival 120 days after tumour inoculation.
Anti-tumoral effect of LEFCT-EC with bleomycin.
Treatment of TB mice either with bleomycin (50 U/kg) or with electric field alone cured none of the animals. However, the combined treatment of LEFCT-EC-bleomycin cured 52% of the mice as examined 120 days after tumour inoculation (Table 1). In addition, LEFCT-EC significantly prolonged the survival time of the tumour-bearing mice when compared to all other treatment modes (e.g. LEFCT-EC-bleomycin versus bleomycin, P = 0·0002). Treatment with LEFCT-EC also resulted in a significant shrinkage of the primary tumour volume (LEFCT-EC-bleomycin versus bleomycin, P = 0·00005, Table 1).
Anti-tumoral effect of LEFCT-EC with carmustine (BCNU).
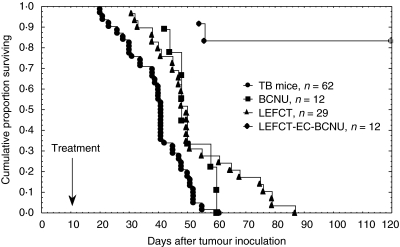
The third cytotoxic agent employed was BCNU. As shown in Fig. 1, BCNU alone or LEFCT alone failed to cure TB mice, although slightly prolonged survival. However, the combined application of BCNU and electric field (LEFCT-EC-BCNU) resulted in a complete cure of 83% of TB mice, as observed 120 days after tumour inoculation. The population of mice, whose primary tumour was not cured following treatment with LEFCT-EC, lived significantly longer than animals from all other groups (e.g. LEFCT-EC-BCNU versus BCNU, P = 0·014), and growth of their tumours was significantly delayed. Average tumour volume measured at 22–24 days after CT-26 inoculation in the group treated by LEFCT-EC-BCNU was 325 ± 38 mm3, whereas in the group treated by BCNU only it was 1077 ± 246 mm3 (LEFCT-EC-BCNU versus BCNU, P = 0·002).
Fig. 1.
Survival of CT-26-bearing mice following LEFCT-EC-BCNU. Mice bearing 65 mm3 intracutaneous tumours were treated with BCNU (35 mg/kg) intratumorally and/or with low electric fields for 12 min (40 V/cm, 500 Hz, 180 µs). Kaplan–Meir plots (n: animals/group).
Figure 2 shows the mouse-bearing intracutaneous CT-26 colon 2 (2) and 6 (3) weeks after LEFCT-EC-BCNU treatment, compared to the non-treated (1) CT-26-bearing mouse.
Fig. 2.
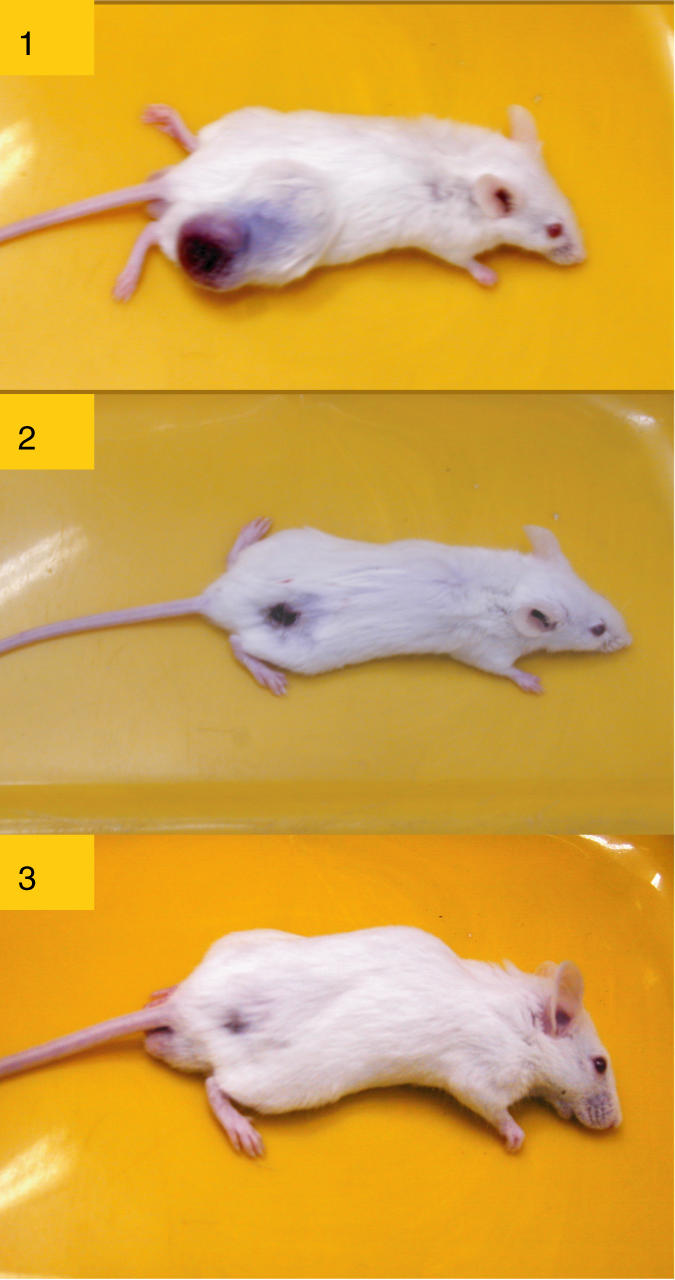
CT-26 carcinoma-bearing mice. (1) CT-26-bearing mice, non-treated. (2) CT-26-bearing mice treated by LEFCT-EC with BCNU (2 weeks after treatment). (3) CT-26-bearing mice cured by LEFCT-EC with BCNU (6 weeks after treatment).
Effect of different intensities of the electric field on the development of CT-26 colon carcinoma
We examined the sole effect of electric field strength on tumour cure. Tumour-bearing animals were subdivided into four groups: non-treated tumour-bearing mice (TB mice) and TB mice treated with an electric field of 20 V/cm (LEFCT 20 V/cm), 40 V/cm (LEFCT 40 V/cm) or 60–70 V/cm (LEFCT 60–70 V/cm).
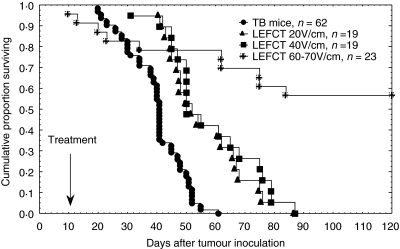
The dependence of survival time on electric field strength is presented in Fig. 3. It is evident that increasing the intensity of the electric field led to a significant increase in the survival rate (LEFCT 20 V/cm versus LEFCT 60–70 V/cm, P = 0·0001; LEFCT 40 V/cm versus LEFCT 60–70 V/cm, P = 0·0002). The treatment with LEFCT 20 V/cm and 40 V/cm extended the survival but did not cure TB mice (LEFCT 20 V/cm or 40 V/cm versus TB, P = 0·00001). However, the treatment with LEFCT 60–70 V/cm not only prolonged survival time but also cured 56% of the animals. Nevertheless, 18% of the animals in the latter group died due to the treatment and were therefore excluded from the survival graph. The electric energy deposited in the different treatments has been calculated and yielded the following results [energy, Joule(J) ± SE]: LEFCT 20 V/cm: 19 J, LEFCT 40 V/cm: 96 ± 8 J, LEFCT 60 V/cm: 209 ± 13 J, LEFCT 70 V/cm: 363 ± 14 J.
Fig. 3.
Survival of CT-26-bearing mice following electric field treatment. Mice bearing 65 mm3 intracutaneous tumours were treated with 20, 40 and 60–70 V/cm for 12 min (frequency 500 Hz, pulse width 180 µs). Kaplan–Meir plots (n: number of animals per group).
Resistance of mice cured from CT-26 carcinoma to a tumour challenge
In order to confirm the development of antitumoral resistance, animals that were cured by LEFCT-EC-BCNU were re-injected intracutaneously with 105 CT-26 tumour cells (cure–challenge). The challenge was given ∼120 days after LEFCT-EC-BCNU treatment. Naive mice inoculated with the same dose of CT-26 cells for the first time served as control (control).
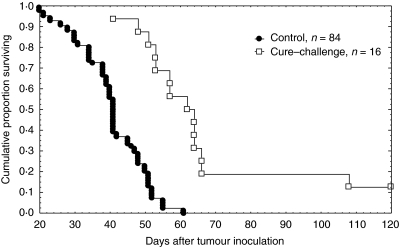
Figure 4 shows a significant prolongation of survival time in the group of cured mice which received tumour challenge compared to the tumour-inoculated mice for the first time (cure–challenge versus control, P = 0·000001). Moreover, 12·5% of the cured and challenged animals did not develop a tumour at all. Tumour growth in the cure–challenged group was significantly slower compared with the control group (control versus cure–challenge, P = 4 × 10−12). Average tumour volume at 18–22 days after tumour inoculation in the group of control mice was 3088 ± 154 mm3, while in the group of cure–challenged mice it was 429 ± 107 mm3.
Fig. 4.
Resistance of mice previously bearing CT-26 and cured by LEFCT-EC to a subsequent inoculation of CT-26 cells. Mice cured by LEFCT-EC-BCNU were challenged with 105 CT-26 cells intracutaneously 120 days after initial inoculation. Kaplan–Meir plots (n: animals/group).
Winn assay
The animals were divided into the following groups:
Group 1: mice inoculated with 105 CT-26 cells only.
Group 2: mice inoculated with 105 CT-26 cells and 107 splenocytes from normal mice.
Group 3: mice inoculated with 105 CT-26 cells and 107 splenocytes from mice bearing 5 mm in diameter CT-26 carcinoma.
Group 4: mice inoculated with 105 CT-26 cells and 107 splenocytes from mice bearing ∼30 mm in diameter CT-26 carcinoma.
Group 5: mice inoculated with 105 CT-26 cells and 107 splenocytes from mice cured by LEFCT.
Group 6:mice inoculated with 105 CT-26 cells and 107 splenocytes from mice cured by LEFCT-EC-BCNU.
As shown in Table 2, splenocytes of the mice from all experimental groups could increase the survival rate. However, they had a different capacity. Animals from groups 4 and 6 did not develop tumour entirely. The survival rate of mice from group 5 was 91%, whereas only 41% of mice from group 3 were tumour free. Not one mouse survived in groups 1 and 2. Subsequently, the mice that survived in this experiment were inoculated with a fivefold dose of CT-26 tumour cells (5 × 105 cells per mouse). These animals were immunized to the tumour challenge that appeared in a significant increase of the survival rate and a significant delay in the primary tumour growth compared to the group of first inoculated mice (data not shown).
Table 2.
Activity of spleen cells against the tumour cells (Winn assaya)
| Splenocytesb | None | Normal mice | Mice bearing 30 mm tumours | Mice bearing 5 mm tumours | Mice treated by LEFCTc | Mice treated by LEFCT-EC-BCNUc |
|---|---|---|---|---|---|---|
| % survivald | ||||||
| (12 mice in each group) | 0 | 0 | 41 | 100 | 91 | 100 |
CT-26 cells (105) were mixed in a proportion of 1 : 100 with splenocytes (107) from different groups of mice, and injected to normal mice.
Source of splenocytes
Splenocytes were removed 1 month after treatments.
Survival was determined 120 days after tumour/splenocytes inoculation.
DISCUSSION
It has been reported previously that exposure of cells to a low train of electric fields resulted in an effective uptake of macromolecules via endocytotic-like processes [4,5]. These results led us to suggest that low electric fields can increase the delivery of chemotherapeutic drugs directly into the cytoplasm of cancer cells and thus to increase effectiveness of the drugs against solid tumours. LEFCT-EC was applied in an experimental mouse model of highly metastatic B 16-F10·9 melanoma, and the effectiveness of this new technique was demonstrated clearly [6]. In order to broaden the scope of LEFCT-EC, we examine its effectiveness in another model of metastatic tumour, CT-26 colon carcinoma, and employing several chemotherapeutic drugs. We also examined the role of the electric field strength on the effectiveness of treatment.
5FU alone or in combination with other chemotherapeutic agents is the ‘gold standard’ for the clinical treatment of metastatic colon cancer [8]. We found that intravenous injection of 5FU had no effect on the survival of treated mice, while intratumorally introduced 5FU significantly prolonged survival time, as did treatment with low electric field alone (LEFCT). All treatment modalities decreased tumour growth, but the most pronounced effect was achieved by LEFCT-EC with intratumoral injection of 5FU (33% of complete cure).
Because 5FU is a small molecule that rapidly permeates cell membranes and converts into nucleotides [9], we assessed the potential of LEFCT-EC with a chemotherapeutic drug, which does not permeate effectively into tumour cells.
Bleomycin is a very cytotoxic molecule when introduced inside a cell. However, bleomycin does not diffuse freely through the plasma membrane and normally enters cells through interaction with a membrane protein that mediates its internalization [10]. Electrostimulation of cells allows bleomycin to enter the cytosol directly and to exert fully its cytotoxic potential [11]. Therefore, bleomycin is an excellent candidate for combined treatment with electric fields. We found that treatment with either bleomycin or electric field alone did not cure the animals, while 52% of the mice treated by LEFCT-EC with bleomycin were completely cured. It should be noted that although 5FU alone was more effective against CT-26 than bleomycin, LEFCT-EC-bleomycin had better efficacy than LEFCT-EC-5FU. This finding suggests that electric field increases delivery of drugs into tumour cells.
Other studies of colon cancer electrochemotherapy were performed using either high or low electric fields. Kuriyama et al. [12] reported successful treatment of CT-26 carcinoma-bearing mice by high electric field electrochemotherapy (1000 V/cm, 99 µs, 1 HZ, eight pulses) with 5FU and bleomycin. The survival rate in our experiments was similar to that reported by Kuriyama et al. (∼ 10% survival after LEFCT-EC with 5FU and ∼60% survival after LEFCT-EC with bleomycin). Miyazaki et al. [13] performed a small-scale experiment where they observed an 80% cure of CT-26-bearing mice after combined treatment with bleomycin (0·5 mg per mouse i.t.) and either 50 V/cm or 150 V/cm electric field.
We tested the effect of LEFCT-EC with another chemotherapeutic agent, carmustine (BCNU), which is a small, lipid-soluble molecule, approved mainly for the treatment of brain tumours and lymphomas [14]. It was observed that LEFCT-EC with BCNU completely cured 83% of tumour-bearing animals. This strong effect can be explained by the strong effect of BCNU alone that was more effective then bleomycin or 5FU alone.
In addition, we examined the effect of electric field strength alone on the development of primary CT-26 carcinoma. Electric field intensities of either 20 V/cm or 40 V/cm delayed tumour growth and extended survival time, but did not cure the treated animals due to the fact that recurrence occurred in all treated mice. In contrast, treatment with electric fields of higher intensity (60–70 V/cm) completely cured 56% of the animals. However, the application of such high electric fields was accompanied by increased mortality soon after the treatment (mortality rate was 18%). This lethal effect probably occurred because of an electric shock that caused serious damage to the vital systems. Thus, it may be suggested that under our experimental conditions there is a critical level of energy, approximately between 100 J and 200 J, which causes massive enough damage to CT-26 thereby preventing recurrence of the primary tumour in part of the treated animals. This level of energy allows us to suggest that 20 V/cm can also be applied on larger tumours (10–30 mm in diameter) without side effects.
In our previous study we demonstrated that B16 melanoma-bearing mice, cured by LEFCT-EC, developed resistance to a tumour challenge, and splenocytes from these animals were able to delay tumour growth in a Winn assay. Furthermore, a part of the animals was cured completely by LEFCT-EC of both the primary tumour and lung metastases [6]. The development of antitumour immune response after application of low electric field chemotherapy was also reported by Miyazaki et al. [13]. Thus, we postulated that destruction of the primary tumour by LEFCT-EC might release antigenic material [15,16] from the tumour cells, which can trigger the immune response [17]. Indeed, we found a significant prolongation in the survival of mice cured by LEFCT-EC and challenged with CT-26 cells compared to naive control animals. Moreover, 12·5% of the cured animals developed a complete resistance to the tumour challenge. Also, tumour growth in the cured group was significantly slower than in the control group.
In the Winn assay we found that splenocytes from the mice bearing 5 mm CT-26 tumour had antitumour activity. This activity was increased with increase of tumour burden. However, electric field alone or combined with chemotherapy significantly enhanced antitumour capacity of splenocytes. The data demonstrates clearly that LEFCT-EC can induce an antitumoral immune response.
Our results indicate that LEFCT-EC can directly destroy CT-26 tumours and facilitate the destruction of residual disease, probably by eliciting an antitumoral innate and/or adaptive immune response. Thus, LEFCT-EC is a new method of treating tumours complementary to surgery or, in the case of non-operable tumours, replacing surgery. It is with these findings that we are now initiating a pilot study in humans focusing on subcutaneous metastasis such as in breast cancer and skin malignancies such as malignant melanoma, Kaposi's sarcoma, etc. We will evaluate a new range of drugs such as doxorubicin, vinblastine and others in combination with LEFCT. Patients with multiple nodules will serve as their own self-controls, as only one or two nodules will undergo LEFCT while the others will simultaneously receive only systemic chemotherapy.
Acknowledgments
This study was supported in part by Hamer Medical Research Fund and the Horowitz-Ramot Fund. This work was performed in partial fulfillment of the requirements towards a PhD degree of Alexander Plotnikov, Sackler Faculty of Medicine, Tel-Aviv University.
REFERENCES
- 1.Jemal A, Murray T, Samuels A, Ghafoor A, Ward E, Thun MJ. Cancer statistics, 2003. CA Cancer J Clin. 2003;53:5–26. doi: 10.3322/canjclin.53.1.5. [DOI] [PubMed] [Google Scholar]
- 2.Langman M, Boyle P. Chemoprevention of colorectal cancer. Gut. 1998;43:578–85. doi: 10.1136/gut.43.4.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ho SY, Mittal GS. Electroporation of cell membranes: a review. Crit Rev Biotechnol. 1996;16:349–62. doi: 10.3109/07388559609147426. [DOI] [PubMed] [Google Scholar]
- 4.Rosemberg Y, Korenstein R. Incorporation of macromolecules into cells and vesicles by low electric fields: induction of endocytotic-like processes. Bioelectrochem Bioenerg. 1997;42:275–81. [Google Scholar]
- 5.Antov U, Barbul A, Korenstein R. Electroendocytosis: stimulation of adsorptive and fluid-phase uptake by pulsed low electric fields. Exp Cell Res. 2004;297:348–62. doi: 10.1016/j.yexcr.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 6.Entin I, Plotnikov A, Korenstein R, Keisari Y. Tumor growth retardation, cure, and induction of anti tumor immunity in B16 melanoma bearing mice by low electric field enhanced chemotherapy. Clin Cancer Res. 2003;9:3190–7. [PubMed] [Google Scholar]
- 7.Xiang R, Lode HN, Gillies D, Reisfeld RA. T cell memory against colon carcinoma is long-lived in the absence of antigen. J Immunol. 1999;163:3676–83. [PubMed] [Google Scholar]
- 8.Haskell CM. Principles of cancer chemotherapy. In: Haskell CM, editor. Cancer treatment. 5. Philadelphia: WB Saunders Co.; 2001. pp. 731–4. [Google Scholar]
- 9.Wohlhueter RW, McIvor RS, Plagemann PGW. Facilitated transport of uracil and 5-fluouracil, and permeation of orotic acid into cultured mammalian cells. J Cell Physiol. 1980;104:309–19. doi: 10.1002/jcp.1041040305. [DOI] [PubMed] [Google Scholar]
- 10.Pron G, Belehradek J, Mir LM. Identification of a plasma membrane protein that specifically bind bleomycin. Biochem Biophys Res Comm. 1993;194:333–7. doi: 10.1006/bbrc.1993.1824. [DOI] [PubMed] [Google Scholar]
- 11.Pron G, Mahrour N, Orlowski S, et al. Internalization of bleomycin molecules responsible for bleomycin toxicity: a receptor-mediated endocytosis mechanism. Biochem Pharmacol. 1999;57:45–56. doi: 10.1016/s0006-2952(98)00282-2. [DOI] [PubMed] [Google Scholar]
- 12.Kuriyama S, Matsumoto M, Mitoro A, et al. Electrochemotherapy for colorectal cancer with commonly used chemotherapeutic agents in a mouse model. Dig Dis Sci. 2000;45:1568–77. doi: 10.1023/a:1005565027969. [DOI] [PubMed] [Google Scholar]
- 13.Miyazaki S, Gunji Y, Matsubara H, et al. Possible involvement of antitumor immunity in the eradication of colon 26 induced by low-voltage electrochemotherapy with bleomycin. Surg Today. 2003;33:39–44. doi: 10.1007/s005950300006. [DOI] [PubMed] [Google Scholar]
- 14.Haskell CM. Principles of cancer chemotherapy. In: Haskell CM, editor. Cancer treatment. 5. Philadelphia: WB Saunders Co.; 2001. p. 119. [Google Scholar]
- 15.Toyoshima M, Nakajima M, Yamori T, Tsuruo T. Purification and characterization of the platelet-aggregating sialoglycoprotein gp44 expressed by highly metastatic variant cells of mouse colon adenocarcinoma 26. Cancer Res. 1995;55:767–73. [PubMed] [Google Scholar]
- 16.Shigeoka H, Karsten U, Okuno K, Yasutomi M. Inhibition of liver metastases from neuraminidase-treated colon 26 cells by anti-Thomsen–Friedenreich-specific monoclonal antibody. Tumor Biol. 1999;20:139–46. doi: 10.1159/000030056. [DOI] [PubMed] [Google Scholar]
- 17.Boon T, Coulie P, Van den Eynde B. Tumor antigens recognized by T cells. Immunol Today. 1997;18:267. doi: 10.1016/s0167-5699(97)80020-5. [DOI] [PubMed] [Google Scholar]