Abstract
The antihuman CD2 MoAb BTI-322 (Lo-CD2a) effectively inhibits T cell responses in vitro to allogeneic cells, which is followed by unresponsiveness to the original stimulator in secondary stimulation. We studied the xenogeneic human antiporcine mixed lymphocyte reaction (MLR), and utilized anti-T cell receptor (TCR) Vβ family antibody-induced cell proliferation to determine the specificity and mechanism. BTI-322 and its humanized version, MEDI-507, effectively inhibited the primary xenogeneic MLR. After suboptimal primary stimulation using lower numbers of xenogeneic stimulator cells, the unresponsiveness in secondary culture was apparent only for xenogeneic stimulator cells of the original SLA haplotype, and not for third-party stimulators or allogeneic cells. The inhibition of primary MLR was not observed for nylon-wool-purified T cells, but was seen after reconstitution of purified T cells with monocytes. Similarly, anti-Vβ family-specific stimulation showed family-specific unresponsiveness in secondary culture. This required the presence of the whole BTI-322 molecule: a F(ab′)2 fragment was not effective. T cells of a distinct Vβ family were depleted after stimulation with an anti-Vβ family-specific antibody and BTI-322. We conclude that the inhibition by BTI-322 of a primary xenogeneic MLR or the response to an anti-TCR Vβ antibody is associated with unresponsiveness upon restimulation, due to activation-associated cell depletion. In this process, the interaction between monocytes and the Fc part of the antibody is involved. This unique characteristic of BTI-322 suggests the potential of the antibody for tolerance induction in vivo, besides the potential use as a T cell depleting agent.
Keywords: CD2 antibodies, cellular activation, human T lymphocytes, tolerance
INTRODUCTION
CD2 is a 45–55 kDa glycoprotein expressed on all mature human T cells, thymocytes, most natural killer cells and a small proportion (9–12%) of bone marrow cells [1]. The molecule has an important role in transmembrane signalling after activation of T lymphocytes via the T cell receptor (TCR), independent of other co-stimulatory molecules such as CD28. In accord with this function, most anti-CD2 antibodies and anti-ligand reagents (e.g. anti-LFA-3 antibody and an LFA-3/human IgG3 Fc fusion protein) inhibit the mixed lymphocyte reaction (MLR) in vitro and graft rejection in vivo[2–4]. This can even result in T cell unresponsiveness, as demonstrated in a murine allogeneic transplantation model [5]. Our studies with a rat antihuman CD2 monoclonal antibody BTI-322, originally named Lo-CD2a [6], have shown not only inhibition of an allogeneic MLR but also subsequent hyporesponsiveness upon allogeneic restimulation while retaining responsiveness to restimulation by xenogeneic antigen, mitogens such as anti-CD3 antibody and phytohaemagglutinin (PHA), and recall antigens such as tetanus toxoid [7–9]. This appears to be a unique characteristic of BTI-322, as all other anti-CD2 antibodies tested to date failed to elicit alloantigen hyporesponsiveness [8]. A humanized version of BTI-322, called MEDI-507, is used currently in clinical trials as part of the conditioning regimen in procedures for immune tolerance induction to solid organ transplants by haematopoietic cell transplantation [10,11] and for refractory haematological malignancies [12].
Effective immunosuppression, either for chronic treatment or as part of a tolerance induction procedure, is an important aspect of xenotransplantation, i.e. models of pig-to-non-human primate xenografts. There is a demand for effective T cell directed immunosuppression, in particular as the role of T cell-independent naturally existing antibodies towards the Galα1,3Gal epitope on pig cells has become obsolete as donors lacking this epitope are available [13,14]. BTI-322 does not recognize CD2 in non-human primate species, but a similar antibody, Lo-CD2b [15], has been included in protocols in pig-to-non-human primate transplantation as an effective T cell-depleting agent, for instance in protocols for tolerance induction upon kidney transplantation [16] and chronic immunosuppression after heterotopic heart transplantation [17]. We therefore initiated studies using BTI-322 in in vitro human antiporcine reactions. We demonstrate here that BTI-322 is able to inhibit the human antiporcine MLR, and induces unresponsiveness in subsequent antigen restimulation. We used the model of anti-TCR Vβ antibody-induced T cell stimulation to evaluate the mechanism of hyporesponsiveness and demonstrate that this occurs by specific activation-associated T cell depletion.
MATERIALS AND METHODS
Animals
Pigs were selected from the Massachusetts General Hospital herd of miniature swine inbred for swine leucocyte antigens (SLA), at 4–8 weeks of age. Animals of SLAaa, SLAcc and SLAdd haplotypes were used.
Antibodies and biologicals
BTI-322 (rat IgG2b-κ), and the humanized version, MEDI-507, were produced and purified by affinity chromatography. A F(ab′)2 preparation was made by pepsin cleavage and subsequent purification by affinity chromatography followed by gel filtration. Other products included a rat IgG2b-κ isotype control Ig (antidinitrophenyl antibody; Zymed, South San Francisco, CA, USA) and purified human IgG1 (Sigma Chemical Co., St Louis, MO, USA), anti-TCR Vβ antibodies (anti-Vβ 8a, mouse IgG2b; anti-Vβ 8b, mouse IgG2a; anti-Vβ 13, IgG1; Endogen, Woburn, MA, USA) and anti-CD2 antibodies (Leu-5b, Becton Dickinson, Mountain View, CA, USA; OKT11, Beckman Coulter, Miami, FL, USA; and MT910, Santa Cruz Biotechnology, Santa Cruz, CA, USA). Anti-CD3 (OKT3) was purified by affinity chromatography from the culture supernatant of the hybridoma cell line (American Tissue Culture Collection, Rockville, MD, USA). All antibodies containing sodium azide were dialysed overnight at 4°C against phosphate-buffered saline (PBS) prior to use.
Preparation of peripheral blood mononuclear cells (PBMCs), T cells and monocytes
Heparinized blood of healthy human volunteers or pigs was subjected to Histopaque density gradient centrifugation (Histopaque, Sigma). In flow cytometry (gating for the monocyte population, or staining with anti-CD14), the percentage of monocytes ranged normally between 10 and 20%. Cells were harvested and resuspended in serum-free medium, AIM-V (Gibco BRL, Grand Island, NY, USA). T lymphocytes were purified from PBMCs by nylon wool filtration (Wako Chemicals USA, Richmond, VA, USA): in flow cytometry, purified T cells contained about 85% CD3+ cells and <0·5% CD14+ or CD19+ cells. Monocytes were purified from PBMCs by adherence to plastic during overnight culture, followed by harvest of the adhered population; in flow cytometry this population contained >90% CD14+ cells.
Proliferation assays: primary/secondary MLR, stimulation using anti-CD3 or anti-TCR Vβ antibodies
All assays were carried out in AIM-V medium. Responder cells (human PBMCs or T cells) were stimulated with irradiated (3·5 Grey) stimulator cells including allogeneic cells (human PBMCs), xenogeneic cells (pig PBMCs), anti-CD3 or anti-TCR Vβ antibodies. Responder cells were cultured at 1–2 × 106 cells/ml in 96-well U-bottomed plates (CoStar Corp., Cambridge, MA, USA) and incubated at 37°C in humidified air with 5% CO2. Proliferation was measured in triplicate at different culture times by pulsing the cells with 1 µCi/well [3H]-thymidine ([3H]-TdR), New England Nuclear, Boston, MA, USA) for the last 18 h of the culture period. [3H]-TdR incorporation (counts per minute, cpm) was determined using an automated 96-well plate cell harvester (TomTec, Orange, CT, USA) and a Betaplate counter (Wallac, Gaithersburg, MD, USA). Normally the standard deviation of the triplicate cultures was less than 20% of the mean value: when the standard deviation was more than 20%, the result was rejected and not included in the data presentation.
In primary MLR to assess human antipig (xenogeneic) or antihuman (allogeneic) responses, responder and stimulator cells were cultured at a 1 : 1 ratio (1–2 × 106 cells/ml for each population), unless indicated otherwise, for 7 days. BTI-322 or control rat Ig was added at the beginning of the cultures at 200 ng/ml. Control cultures were performed with anti-CD3 antibody (100 ng/ml) or PHA (1 µg/ml) (Sigma). In stimulation using anti-TCR Vβ antibodies or control Ig, reagents including BTI-322 were used at a concentration of 100 ng/ml, added at the beginning of the culture.
In preparation for secondary MLR, responder cells stimulated for 7 days in primary MLR in 24-well plates (Costar) were harvested and washed extensively, cultured subsequently for 3 days without stimulator cells, and then harvested and readjusted for viable cell numbers. Cultures for the measurement of primary proliferation (day 7) were performed in parallel, in triplicate in 96-well plates. In secondary culture, cells were stimulated with various stimulator populations (1 : 1 ratio, 0·5–1 × 106/ml) including xenogeneic matched cells (the same SLA haplotype as in the primary MLR), xenogeneic mismatched cells (third-party SLA haplotype) or allogeneic cells (human origin). As control, cells were cultured with PHA. There was no BTI-322 or control Ig added in the secondary MLR.
In preparation for restimulation following primary anti-TCR Vβ antibody stimulation, cells were incubated for 7 days in 24-well plates at 1 × 106 cells/ml with 10 µg/ml anti-Vβ antibodies and 100 ng/ml BTI-322 or control Ig. Cultures for the measurement of primary proliferation (day 7) were performed in parallel, in triplicate in 96-well plates. After the primary bulk culture with anti-TCR Vβ antibody with or without BTI-322, cells were washed extensively and in some experiments purified by Histopaque density gradient centrifugation. Cells were then cultured for 3 days without stimulation in 24-well plates at a density of 2 × 106 cells/ml. In flow cytometry, there were approximately 98% CD3+ T cells: there was no remaining anti-TCR Vβ antibody or BTI-322 detectable on the cell surface in direct flow cytometry with a fluorescent secondary goat antirat IgG (Pharmingen), or goat antimouse antibody (data not shown). In secondary stimulation, cells (1 × 105/well, triplicate wells of a 96-well plate) plus an equivalent number of irradiated (3·5 Grey) autologous PBMCs were cultured with anti-TCR Vβ antibodies as described above, either the original stimulating anti-Vβ antibody, or as control another anti-Vβ or anti-CD3 antibody (all at 10 µg/ml). As positive control, PHA stimulation was performed in separate cultures.
Flow cytometry
At various time-points of primary and secondary culture, leucocyte phenotyping was performed for the (responder) cell population. Single or double parameter direct staining was performed using fluorochrome-labelled anti-CD2 (using the Leu-5b antibody, which does not compete with BTI-322 in antigen binding), anti-CD4, anti-CD8, anti-CD3, anti-CD14, anti-CD19, anti-CD25, anti-CD45 or isotype control antibodies (Becton Dickinson). Indirect staining using anti-TCR Vβ was performed using the antibodies described above with the appropriate fluorochrome-labelled goat antimouse IgG isotype-specific F(ab′)2secondary antibody (Southern Biotechnology Associates, Birmingham, AL, USA). Cell viability was assessed by propidium iodide dye exclusion (Sigma). To assess cell depletion, a total of 10 000 cells were collected and only viable cells were analysed using WinList analysis software (Varity Software House, Inc.).
RESULTS
Primary xenogeneic MLR in the presence of BTI-322 followed by unresponsiveness in a subsequent secondary MLR
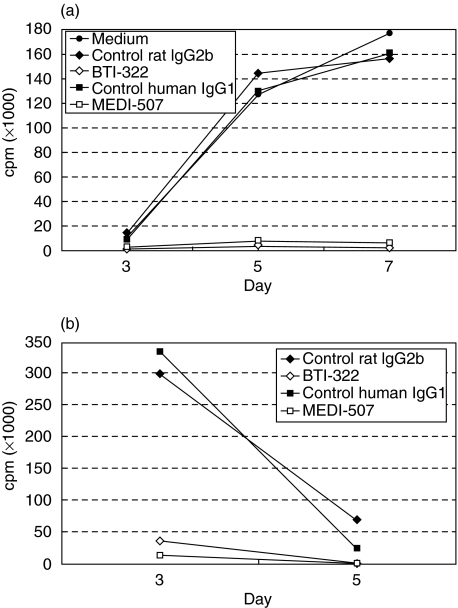
Data from a representative experiment (n > 5) are presented in Fig. 1. In the primary xenogeneic MLR using human PBMCs as responders, the response measured by [3H]-TdR incorporation peaked between days 5 and 7: the proliferative response normally exceeded 100–150 × 103 cpm (Fig. 1a). This was observed irrespective of the haplotype of porcine PBMC stimulator cells (SLAaa, SLAcc and SLAdd). In our experience this response is stronger than that seen in allogeneic MLR, which under similar conditions is 50–80% of the xenogeneic response. BTI-322 completely inhibited the response. The results presented in Fig. 1 are from an experiment in which the humanized version of BTI-322 was also tested along with an appropriate control (human IgG1): both BTI-322 and MEDI-507 inhibited the primary MLR. BTI-322 also inhibited the production of cytokines in the culture supernatant by >85%, including interleukin-2, tumour necrosis factor, interleukin-10 and interferon-γ (data not shown). In the absence of stimulator cells, BTI-322 itself did not elicit a proliferative response or cytokine release (data not shown).
Fig. 1.
Effect of BTI-322 on primary and secondary xenogeneic MLR. (a) Human PBMCs (1 × 106/ml) were cultured at a 1 : 1 ratio with irradiated SLAdd porcine PBMCs, in the presence of 200 ng/ml BTI-322 or the humanized version MEDI-507, or control isotype-matched Ig, followed by measurement of [3H]-TdR incorporation (cpm) at days 3, 5, and 7. (b) Cells harvested after primary xenogeneic stimulation in the presence of these antibodies or controls were cultured for 3 days without stimulant, and then subjected to a secondary xenogeneic MLR, followed by measurement of [3H]-TdR incorporation (cpm) at days 3 and 5.
In a secondary xenogeneic MLR, cells cultured with control Ig during the first culture showed a peak response normally at day 3, which is typical for a secondary response (Fig. 1b). There was no response in the secondary MLR in the case where the primary culture was performed in the presence of either BTI-322 or MEDI-507.
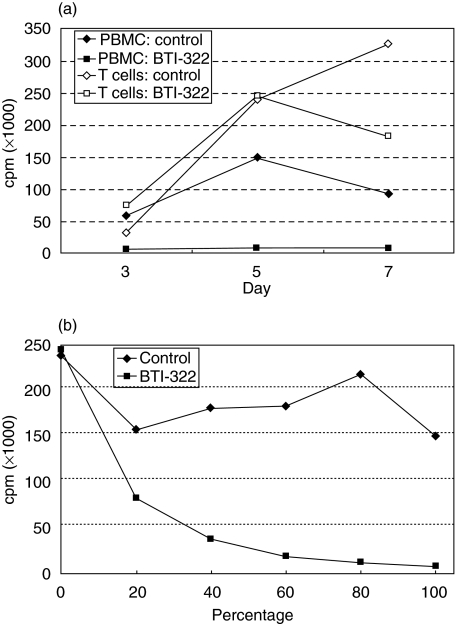
Using purified T cells as responder cells, BTI-322 had no clear inhibitory effect in the primary MLR (Fig. 2a shows a representative experiment, n > 5). This observation is compatible with the absent response of purified T cells to anti-CD3 stimulation, which needs the presence of monocytes. This also applies for BTI-322: upon addition of monocytes (adherent cells) to the culture, the inhibitory activity of BTI-322 was recovered (Fig. 2b): about 50% inhibition was observed when the number of monocytes was 20% of the original percentage in the PBMC population, and an almost complete inhibition was observed when monocytes were supplemented to 100% of the original percentage.
Fig. 2.
Use of T cells as responder cells and effect of monocytes. (a) PBMCs or nylon-wool-purified T cells (both 1 × 106/ml) were cultured at a 1 : 1 ratio with irradiated SLAdd porcine PBMCs, in the presence of 200 ng/ml BTI-322 or control Ig, followed by measurement of [3H]-TdR incorporation (cpm) at days 3, 5, and 7. (b) Purified responder T cells were supplemented with monocytes (adherent cells) at relative proportions [100% reflects the original proportion in the original PBMC preparation (10–20%)].
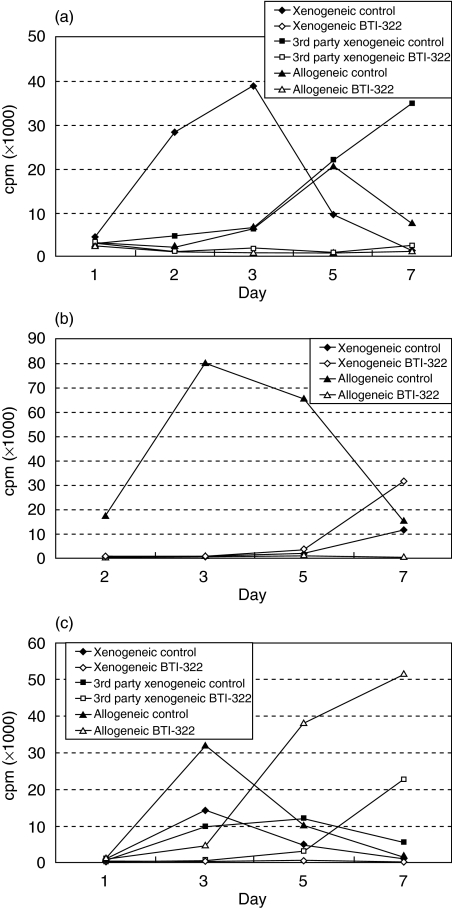
To address the specificity of unresponsiveness in secondary MLR, cultures were stimulated with xenogeneic cells of the same SLA haplotype, third-party SLA xenogeneic cells or allogeneic (human) cells. Figure 3a shows a representative experiment (n > 5). In the case of a primary culture in the presence of control Ig, the response in the secondary stimulation by SLA cells of the same haplotype showed the kinetics of a secondary reaction peaking at day 3. According to expectation, the response to third-party SLA cells or human allogeneic cells followed the pattern of a primary response, which peaked later and could be higher than that of the secondary response. When a primary culture contained BTI-322, however, there was no response upon secondary stimulation with any of these three stimulator cell populations. This result was obtained irrespective of SLA haplotype, i.e. using SLAcc or SLAdd cells in primary stimulation and SLAdd, SLAcc or SLAaa cells, respectively, as third-party stimulators in secondary stimulation. These data contrast with data in the allogeneic system in which BTI-322 induced unresponsiveness only to secondary allogeneic stimulation, but not to xenogeneic stimulation (Fig. 3b). We hypothesized that this phenomenon was due to a higher level of activation in the primary xenogeneic MLR compared with allogeneic MLR, related presumably to a higher frequency of responding T cells in xenogeneic stimulation, e.g. including alloresponsive cells.
Fig. 3.
Effect of BTI-322 on secondary MLR: SLAcc, third-party SLAdd and allogeneic restimulation after primary stimulation with SLAdd cells. (a) Cells (1 × 106/ml) from primary 7-day xenogeneic MLR (1 : 1 responder : stimulator ratio) in the presence of 200 ng/ml BTI-322 or control Ig were cultured in secondary MLR with cells of the original SLA haplotype, third-party xenogeneic cells and allogeneic cells. Data shown are [3H]-TdR incorporation at various time-points during secondary culture, for cells cultured during the primary MLR. (b) The same experiment, using allogeneic stimulation in primary MLR. (c) The same experiment using suboptimal xenogeneic stimulation in primary MLR (1 : 0·125 responder : stimulator ratio).
To test this hypothesis, primary xenogeneic stimulation was performed under suboptimal conditions, by lowering the number of stimulator cells. This resulted in a reduced response which could be associated with delayed kinetics of the response but which did not affect the profound inhibitory action of BTI-322 (Table 1, presenting a representative experiment, n > 5). Cells harvested from these primary cultures in the presence of BTI-322 were still unresponsive to xenogeneic secondary stimulation to the same SLA haplotype, but showed a response to third-party xenogeneic stimulation and allogeneic stimulation. This response in secondary stimulation to third-party or allogeneic stimulation could even be higher for cells harvested from primary cultures in the presence of BTI-322 than for cells harvested from primary cultures in the presence of control Ig, in particular the response on days 5 or 7 after secondary stimulation. This is illustrated in Fig. 3c for a representative experiment of a series of five experiments. The most probable explanation for this phenomenon is a change in frequency of responsive cells remaining after primary culture: assuming that depletion of the responsive cells in primary culture occurs in the presence of BTI-322, as documented in the TCR Vβ system (see below), there is enrichment in the remaining cell population for cells with other specificities including alloreactive cells and third-party SLA-reactive cells. This phenomenon of selective enrichment might also apply for the results presented in Fig. 3b: a possible BTI-322-induced depletion of alloreactive cells in primary stimulation might have resulted in a higher frequency of xenoreactive cells, hence a higher level of proliferation upon xenogeneic stimulation in secondary culture. In all cases, including those showing unresponsiveness, there was a response to control stimulation with anti-CD3 antibody or PHA (data not shown).
Table 1.
The effect of stimulator : responder ratio in primary xenogeneic MLR on the efficacy of BTI-322 in inducing hyporesponsiveness during a secondary MLR to cells of the orginal xenogeneic SLA haplotype, to third-party xenogeneic stimulator cells or to allogeneic stimulator cellsa
| Primary xenogeneic MLR (SLAcc stimulation) | ||||
|---|---|---|---|---|
| Proliferation (cpm) | Secondary MLR (SLAcc, SLAdd, or allogeneic stimulation) | |||
| Responder : stimulator | Control Ig | BTI-322 | Stimulator | BTI-322 in primary culture (% of cpm of control Ig) |
| 1 : 1 | 101 500 | 1400 | SLAcc | 3 |
| SLAdd | 2 | |||
| Allogeneic | 5 | |||
| 1 : 0·5 | 56 600 | 1000 | SLAcc | 3 |
| SLAdd | 470 | |||
| Allogeneic | 490 | |||
| 1 : 0·25 | 22 000 | 700 | SLAcc | 3 |
| SLAdd | 40 | |||
| Allogeneic | 180 | |||
| 1 : 0·125 | 4 800 | 700 | SLAcc | 4 |
| SLAdd | 410 | |||
| Allogeneic | 370 | |||
| 1:0 | 400 | 500 | SLAcc or SLAdd | NA |
| Allogeneic | NA | |||
The concentration of responder cells in the primary MLR was 1 × 106/ml, and xenogeneic SLAcc stimulator cells were used at varying responderstimulator ratios. Proliferation data are [3H]TdR incorporation (cpm) at day 5 of the primary MLR in the presence of BTI-322 or control Ig. After harvest and a 3-day culture without stimulation, a secondary MLR was performed with xenogeneic stimulator cells (original SLAcc haplotype or third-party SLAdd), or with allogeneic cells at a 1 : 1 responder : stimulator ratio. The response of cells from primary culture in the presence of BTI-322 is presented as a percentage of that from primary culture in the presence of control Ig: due to the variability in primary and secondary responses this percentage shows variability for the various responder : stimulator ratios.
Primary T cell activation via TCR Vβ epitopes and subsequent unresponsiveness mimics the xenogeneic system
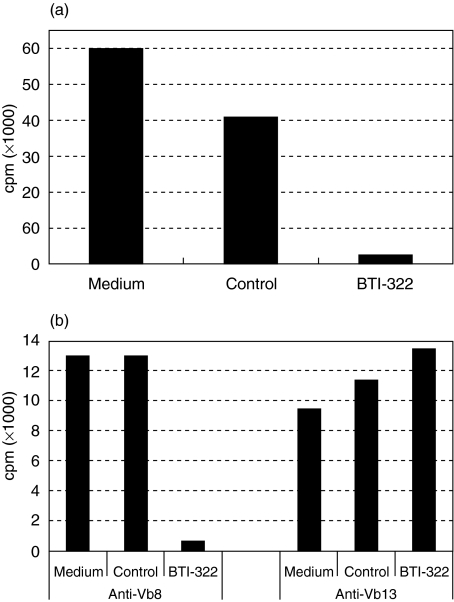
The broad and ill-defined antigenic spectrum in a xenogeneic MLR does not allow an unequivocal specificity assessment. We therefore used anti-TCR Vβ antibodies to study the effect of BTI-322 on activation of T cells of a distinct Vβ family. This approach was based on our experience that the addition of BTI-322 to cultures stimulated with anti-CD3 antibody or PHA, i.e. polyclonal T cell activation, result in massive T cell depletion (>90%, unpublished data) and unresponsiveness in restimulation. Antibodies or combinations of antibodies were selected which stained the greatest percentage of CD3+ T cells (between 5 and 10%) and which induced cell proliferation in primary assays. Data from a representative experiment (n > 5) are presented in Fig. 4. BTI-322 inhibited the primary proliferation of PBMCs to soluble anti-Vβ8 (Fig. 4a), anti-Vβ13 or other antibodies (data not shown), whereas control Ig had no effect. This effect of BTI-322 in a primary culture was also apparent for purified T cells (data not illustrated). In analogy to the MLR experiments described above, cells were stimulated with a distinct anti-Vβ antibody during the primary culture, and subjected to a secondary culture with the same antibody or antibodies to a different Vβ family. In parallel control experiments PHA stimulation was performed. Cells from a primary culture with anti-Vβ8 stimulation in the presence of BTI-322 were unresponsive to anti-Vβ8 stimulation in the secondary culture, but the response to anti-Vβ13 stimulation was not affected (Fig. 4b). Similar results were observed for other anti-Vβ antibodies or using purified T cells primary stimulation (data not shown). Other anti-CD2 antibodies (Leu-5b, OKT11, MT910) inhibited the primary anti-Vβ response but failed to elicit hyporesponsiveness in secondary stimulation. The unresponsiveness upon secondary stimulation with the same anti-Vβ antibody required the presence of the complete BTI-322 molecule in the primary culture: a F(ab′)2 preparation of BTI-322 was not effective, which confirmed our results in allogeneic MLR (Table 2). In flow cytometry there was no coating with BTI-322 of cells before adding these to the secondary culture; also, residual anti-Vβ antibody coating was not detectable (data not shown).
Fig. 4.
Effect of BTI-322 on primary and secondary stimulation with anti-TCR Vβ family-specific antibodies. (a) PBMCs were incubated in primary culture with 10 µg/ml anti-TCR Vβ8 antibody, with or without BTI-322 or control Ig (100 ng/ml): [3H]-TdR incorporation was measured at day 7. (b) Cells after primary stimulation as in (a) were washed and cultured for 3 days without stimulator, and then subjected to secondary stimulation with the original anti-Vβ8 antibody or an irrelevant anti-Vβ13 antibody (10 µg/ml): [3H]-TdR incorporation was measured at day 3.
Table 2.
Effect of F(antibody′)2 fragments of BTI-322 in primary stimulation on unresponsiveness in secondary anti-TCR Vβ stimulationa
| Secondary culture cpm ± s.e.m. (within brackets percentage of control antibody in primary culture) | ||
|---|---|---|
| Antibody supplementation in primary culture with anti-TCR Vβ8 antibody | Anti-Vβ8 | Anti-Vβ13 |
| Control antibody | 20 900 ± 3000 (100) | 20 600 ± 500 (100) |
| BTI-322 | 2700 ± 300 (13) | 17 700 ± 200 (86) |
| BTI-322 F(ab′)2 | 39 000 ± 600 (190) | 61 400 ± 2500 (300) |
The primary culture was performed for PBMCs (1 × 106/ml) with anti-Vβ8 antibody and BTI-322 or control Ig, all at 100 ng/ml, for 7 days. After a subsequent 3-day culture without stimulant, a secondary culture was performed with anti-Vβ8 or Vβ13 antibody: [3H]-TdR incorporation was measured after 3 days.
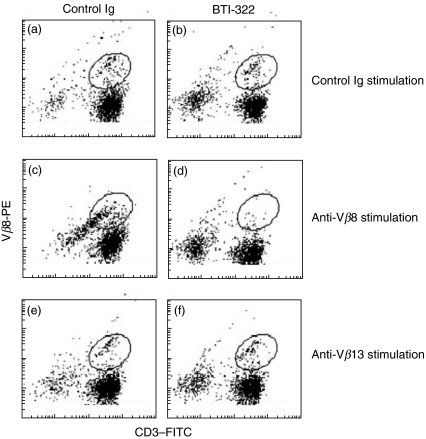
The results in the anti-TCR Vβ system were similar to those observed in the xenogeneic MLR, and this prompted us to use the anti-Vβ system to determine the fate of specific T cells activated by anti-Vβ antibody in the presence of BTI-322. Markers were analysed that could potentially be modulated by activation (TCR Vβ and CD3) or BTI-322 treatment (CD2) or that were assumed to remain relatively constant during culture (CD4 and CD8). In the presence of BTI-322 during the primary culture with a distinct anti-Vβ antibody, a specific depletion of cells expressing this Vβ family was observed and is shown for the Vβ8 family in Fig. 5. In CD3-Vβ8 double staining, CD3+Vβ8+ cells were reduced substantially after stimulation of PBMCs by anti-Vβ8 in the presence of BTI-322 (Fig. 5d), whereas such cells were present after culture without stimulant irrespective of the presence of BTI-322 (Fig. 5a,b) and after anti-Vβ8 stimulation in the presence of control Ig (Fig. 5c). CD3+Vβ8+ cells were detectable when the anti-Vβ8 antibody was replaced by an anti-Vβ13 antibody, irrespective of the presence of BTI-322 (Fig. 5e,f). The same results were observed for the reciprocal experiment, i.e. CD3+Vβ13+ cells were no longer detectable after culture with an anti-Vβ13 antibody in the presence of BTI-322, while there was no effect on CD3+Vβ8+ cells upon anti-Vβ13 stimulation. In these experiments the double-negative CD3–Vβ8– population representing the non-T cell population was reduced in cultures supplemented with the control antibody, which is ascribed to the expansion of T cells in these cultures. Using double labelling with CD4 plus CD8 antibodies in combination with an anti-Vβ8 antibody, a similar pattern of TCR Vβ-specific depletion was observed, i.e. CD4+CD8+Vβ8+ cells were depleted in cultures of PBMCs upon stimulation with an anti-Vβ8 antibody in the presence of BTI-322 (data not illustrated).
Fig. 5.
Presence of CD3+Vβ8+ cells after stimulation of PBMCs with anti-TCR Vβ8 or anti-TCR Vβ13 antibody in the presence of BTI-322 or control Ig. PBMCs (1 × 106/ml) were stimulated for 7 days by control antibody (a,b), anti-Vβ8 antibody (c,d) or anti-Vβ13 antibody (e,f) at 100 ng/ml, in the presence of 100 ng/ml control antibody (a,c,e) or BTI-322 (b,d,f). Subsequently, flow cytometry was performed with a fluorescein isothiocyanate (FITC)-conjugated anti-CD3 antibody in combination with an anti-TCR Vβ8 antibody [indirect staining using a phycoerythrin (PE)-conjugated secondary antibody]. The position of the CD3+Vβ8+ double-positive population in the individual plots is indicated by circles, determined by flow cytometry on freshly isolated PBMCs from the same donor with appropriate isotype-matched control antibody and single-antibody staining. The CD3+Vβ8+ double-positive population comprises approximately 4·5% of the viable cells in (a,b,e and f); approximately 14% in (c); and approximately 1% in (d).
In the xenogeneic system unresponsiveness could be demonstrated at low concentrations of BTI-322 (10 ng/ml), and at this concentration there was no substantial cell depletion observed (data not shown). In a concentration–response experiment in anti-Vβ8 antibody stimulation, supplementation with BTI-322 at various concentrations (1, 10 and 100 ng/ml) resulted in a substantial reduction of CD3+Vβ8+ cells at the lowest concentration of 1 ng/ml. This was apparent in the analysis of all cells after culture, and was even more clear when only viable cells were analysed by propidium iodide dye exclusion.
DISCUSSION
The anti-CD2 antibody BTI-322 is well known for its potent immunosuppressive activity in vitro and effective T cell depletion in vivo. It has distinct characteristics not shared with other anti-CD2 antibodies, namely the generation of unresponsiveness upon restimulation with the same antigen after primary stimulation in the presence of BTI-322. Even the antibody 35·1 (mouse IgG2a-κ, ATCC), that competes with BTI-322 in CD2 binding, does not show this effect (unpublished data from our group). We have reported this phenomenon previously for the allogeneic human in vitro MLR and anti-CD3-induced cell proliferation [6,9], and now show this in the present study for the human antiporcine MLR (Figs 1–3) and for anti-TCR Vβ antibody stimulation (Fig. 4). In the allogeneic MLR, unresponsiveness was observed upon restimulation with the same allogeneic donor or a third-party allogeneic donor, but not to restimulation with xenogeneic cells, mitogens or unrelated antigens. In contrast, unresponsiveness after primary stimulation by xenogeneic cells was observed for both allogeneic and xenogeneic stimulation in the secondary culture (Fig. 3a). This difference is explained most probably by the higher proliferative response upon primary xenogeneic stimulation than allogeneic stimulation; the xenoreactive population could potentially include alloreactive cells. Evidence for this explanation was obtained in primary cultures under suboptimal conditions, by lowering the number of xenogeneic stimulator cells. Under these conditions the proliferation after primary stimulation was substantially lower (Table 1), and upon restimulation unresponsiveness was observed only for porcine stimulator cells of the same SLA haplotype as used in primary culture, and not for third-party porcine stimulator cells or allogeneic stimulator cells (Fig. 3c). These data fit with the general knowledge that SLA antigens are a major stimulator in xenogeneic human antiporcine MLR, and that there is a certain level of cross-reactivity between HLA and SLA in stimulation of human T cells: on the other hand, the human antiporcine response can differentiate between distinct SLA haplotypes [18,19], and in vivo sensitization to HLA does not necessarily lead to a heightened anti-SLA reactivity [20]. Therefore, the response under suboptimal conditions in the secondary MLR to third-party porcine cells or allogeneic stimulator cells by cells rendered unresponsive to porcine cells using low numbers of stimulator cells in primary culture follows the kinetics of a primary MLR (Fig. 3b).
The finding of general unresponsiveness at high numbers of stimulator cells and SLA haplotype-restricted unresponsiveness using low numbers of stimulator cells complicates studies on the specificity. We therefore shifted to a model of TCR Vβ family-specific stimulation. Similar to the xenogeneic MLR, Vβ family-specific unresponsiveness after primary stimulation with anti-Vβ antibody in the presence of BTI-322 was evident (Fig. 4). This was documented for both the Vβ8 and the Vβ13 family. The advantage of this model is the possibility to follow T cells expressing a distinct Vβ family during primary stimulation. Depletion of T cells (defined either by CD3 expression or the expression of CD4 and CD8) of a distinct Vβ family was observed upon anti-Vβ antibody stimulation in the presence of BTI-322 (Fig. 5) at concentrations as low as 1 ng/ml. Thus, the unresponsiveness upon secondary stimulation with a distinct anti-Vβ antibody is associated with the specific depletion of T cells of this particular Vβ family during primary culture with anti-Vβ antibody and BTI-322.
We conducted exploratory studies on the mechanism by which responding cells are depleted during primary culture in the presence of BTI-322. Stimulation of the responsive T cell population during primary culture appears to be required: for instance, stimulation of cells in primary culture with autologous cells does not result in measurable proliferation, and supplementation of such cultures with BTI-322 does not result in unresponsiveness to allogeneic or xenogeneic stimulation in secondary culture (unpublished data). Also, BTI-322 itself does not activate T cells. The presence of a stimulating agent (xenogeneic cells or anti-Vβ antibody) together with BTI-322 does not result in a proliferative response, as this response was inhibited almost completely in the presence of BTI-322. Purified T cells are not sensitive to BTI-322-induced unresponsiveness, and monocytes (adherent cells in the PBMC population) are required to generate unresponsiveness (Fig. 2). The intact BTI-322 molecule is also required as an F(ab′)2 antibody fragment was not efficacious (Table 2). This indicates that the simple binding of BTI-322 during T cell activation needs the interaction between the Fc fragment of the antibody and accessory cells to mediate T cell depletion. Various mechanisms have been proposed, such as antibody-dependent cell-mediated cytotoxicity of cells rendered vulnerable to lysis when being in a state of activation [21]. Recent in vivo studies in severe combined immunodeficient/non-obese diabetic mice grafted with human fetal skin and then injected with human allogeneic blood lymphocytes have shown that the skin graft rejection by allogeneic lymphocytes is inhibited adequately by treatment with BTI-322: this inhibition of rejection did not occur when F(ab′)2 antibody fragments were used, or when blood lymphocytes were depleted by negative selection using anti-CD56 and anti-CD16 antibodies [22]. These data are in accordance with our findings in the in vitro model, and show that the interaction between the Fc part of BTI-322 and CD16+CD56+ natural killer cells is required in the elimination of alloreactive cells.
It remains to be established whether specific unresponsiveness as observed in MLR secondary responses also occurs in vivo following treatment with BTI-322 or its human analogue. The general T cell-depleting capacity of the antibody has been demonstrated in dosing studies in chimpanzees (unpublished results) and is being utilized in clinical trials (the humanized version MEDI-507 [10–12]). A similar antibody but with broader primate reactivity (called Lo-CD2b; [16,17]) has also been used in transplantation in large animals such as pig-to-baboon xenotransplantation. As this is normally conducted as part of a complex immunosuppressive regimen, it is difficult to assess the specific role of the antibody in the induction of tolerance. However, as the antibody is administered in the immediate post-transplant period when the first activation of the immune system occurs, there is the hypothetical possibility that the antibody specifically eliminates activated T cells apart from a general T cell depletion and as such could contribute to immune tolerance induction.
REFERENCES
- 1.Bierer BE, Sleckman BP, Ratnofsky SE, Burakoff SJ. The biologic roles of CD2, CD4, and CD8 in T cell activation. Annu Rev Immunol. 1989;7:579–99. doi: 10.1146/annurev.iy.07.040189.003051. [DOI] [PubMed] [Google Scholar]
- 2.Olive D, Cerdan C, Ragueneau M, Mawas C. Functional activity of the CD2 panel mAbs: relationships between epitope clustering and selected mitogenic pairs. In: Reinherz E, Hayes B, Nadler L, Bernstein I, editors. Leucocyte typing II. New York: Springer-Verlag; 1986. pp. 150–60. [Google Scholar]
- 3.Miller GT, Hochman PS, Meier W, et al. Specific interaction of lymphocyte function-associated antigen 3 with CD2 can inhibit T cell responses. J Exp Med. 1993;178:211–22. doi: 10.1084/jem.178.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sultan P, Schechner JS, McNiff JM, et al. Blockade of CD2–LFA−3 interactions protects human skin allografts in immunodeficient mouse/human chimeras. Nat Biotechnol. 1997;15:759–62. doi: 10.1038/nbt0897-759. [DOI] [PubMed] [Google Scholar]
- 5.Punch JD, Tono T, Qin L, Bishop DK, Bromberg JF. Tolerance induction by anti-CD2 plus anti-CD3 monoclonal antibodies: evidence for an IL-4 requirement. J Immunol. 1998;161:1156–62. [PubMed] [Google Scholar]
- 6.Latinne D, De La Parra D, Nizet Y, et al. An anti-CD2 mAb induces immunosuppression and hyporesponsiveness of CD2+ human T cells in vitro. Int Immunol. 1996;8:1113–9. doi: 10.1093/intimm/8.7.1113. [DOI] [PubMed] [Google Scholar]
- 7.Xia H, Ravoet AM, Latinne D, et al. Rat hybridomas and rat monoclonal antibodies. Boca Raton: CRC Press; 1990. Rat monoclonal antibodies specific for human T lymphocytes; pp. 309–11. [Google Scholar]
- 8.Schad V, Greenstein JL, Giovino-Barry V, et al. An anti-CD2 monoclonal antibody that elicits alloantigen-specific hyporesponsiveness. Transplant Proc. 1996;28:2051–3. [PubMed] [Google Scholar]
- 9.Nizet Y, Chentoufi AA, De la Parra B, et al. The experimental (in vitro) and clinical (in vivo) immunosuppressive effects of a rat IgG2b anti-human CD2 mAb, LO-CD2a/BTI-322. Transplantation. 2000;69:1420–8. doi: 10.1097/00007890-200004150-00036. [DOI] [PubMed] [Google Scholar]
- 10.Sachs DH, Cosimi AB. Renal allograft tolerance through mixed chimerism. 2004 doi: 10.1097/MOT.0b013e3283484b2c. [ http://www.immunetolerance.org/research/solidorgan/trials/sachs1.html] [DOI] [PMC free article] [PubMed]
- 11.Koenecke C, Shaffer J, Alexander SI, et al. NK cell recovery, chimerism, function, and recognition in recipients of haploidentical hematopoietic cell transplantation following nonmyeloablative conditioning using a humanized anti-CD2 mAb, MEDI-507. Exp Hematol. 2003;31:911–23. doi: 10.1016/s0301-472x(03)00224-8. [DOI] [PubMed] [Google Scholar]
- 12.Spitzer TR, McAfee SL, Dey BR, et al. Nonmyeloablative haploidentical stem-cell transplantation using anti-CD2 monoclonal antibody (MEDI-507)-based conditioning for refractory hematologic malignancies. Transplantation. 2003;75:1748–51. doi: 10.1097/01.TP.0000064211.23536.AD. [DOI] [PubMed] [Google Scholar]
- 13.Phelps CJ, Koike C, Vaught TD, et al. Production of alpha 1,3-galactosyltransferase-deficient pigs. Science. 2003;299:411–4. doi: 10.1126/science.1078942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kolber-Simonds D, Lai L, Watt SR, et al. Production of α-1,3-galactosyltransferase null pigs via nuclear transfer with fibroblasts bearing loss of heterozygosity mutations. Proc Natl Acad Sci USA. 2004;101:7335–40. doi: 10.1073/pnas.0307819101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dehoux JP, De la Parra B, Latinne D, Bazin H, Gianello P. Effect in vitro and in vivo of a rat anti-CD2 monoclonal antibody (LO-CD2b) on pig-to-baboon xenogeneic cellular (T and natural killer cells) immune response. Xenotransplantation. 2001;8:193–201. doi: 10.1034/j.1399-3089.2001.0o092.x. [DOI] [PubMed] [Google Scholar]
- 16.Barth RN, Yamamoto S, LaMattina JC, et al. Xenogeneic thymokidney and thymic tissue transplantation in a pig-to-baboon model. I. Evidence for pig-specific T cell unresponsiveness. Transplantation. 2003;75:1615–24. doi: 10.1097/01.TP.0000064335.50622.20. [DOI] [PubMed] [Google Scholar]
- 17.Kuwaki K, Knosalla C, Dor FJMF, et al. Suppression of natural and elicited antibodies in pig-to-baboon heart transplantation using a human anti-human CD154 mAb-based regimen. Am J Transplant. 2004;4:363–72. doi: 10.1111/j.1600-6143.2004.00353.x. [DOI] [PubMed] [Google Scholar]
- 18.Satake M, Kawagishi N, Möller E. Direct activation of human responder T cells by porcine stimulator cells leads to T cell proliferation and cytotoxic T cell development. Xenotransplantation. 1996;3:198–206. [Google Scholar]
- 19.Yamada K, Sachs DH, DerSimonian H. Human anti-porcine xenogeneic T cell response. Evidence for allelic specificity of mixed leukocyte reaction and for both direct and indirect pathways of recognition. J Immunol. 1995;155:5249–56. [PubMed] [Google Scholar]
- 20.Oostingh GJ, Davies HFS, Bradley JA, Taylor CJ. Comparison of allogeneic and xenogeneic in vitro T cell proliferative responses in sensitized patients awaiting kidney transplantation. Xenotransplantation. 2003;10:545–51. doi: 10.1034/j.1399-3089.2003.00089.x. [DOI] [PubMed] [Google Scholar]
- 21.Nizet Y, Chentoufi AA, Havaux X, et al. Apoptosis of human naive NK cells mediated by a rat IgG2b anti CD2 mAb through a fractricidal ADCC reaction. Immunol Lett. 1999;68:229–35. doi: 10.1016/s0165-2478(99)00069-3. [DOI] [PubMed] [Google Scholar]
- 22.Snanoudj R, Pouleau M, Bidère N, et al. A role for CD2 antibodies (BTI-322 and its humanized form) in the in vivo elimination of human T lymphocytes infiltrating and allogeneic human skin graft in SCID mice: an Fcã receptor-related mechanism involving co-injected human NK cells. Transplantation. 2004;78:50–8. doi: 10.1097/01.tp.0000128235.04297.43. [DOI] [PubMed] [Google Scholar]