Abstract
The role of the Th1/Th2 balance in the pathogenesis of murine Graves' hyperthyroidism is controversial. In BALB/c mice injected with adenovirus expressing TSH receptor (TSHR-adeno model), we found that suppression of TSHR-specific Th1 immune responses by exogenous interleukin-4 (IL-4), α-galactosylceramide or helminth (Schistosoma mansoni) infection was associated with inhibition of hyperthyroidism, indicating the critical role for Th1 cytokines. In contrast, BALB/c IL-4 knockout (KO), but not interferon-γ (IFN-γ) KO mice failed to develop Graves' hyperthyroidism when injected with TSHR-expressing M12 B lymphoma cells (TSHR-M12 model), suggesting the importance of Th2 cytokine IL-4. To reconcile differences in these two models, we used IL-4 KO and IFN-γ KO BALB/c mice in the TSHR-adeno model. Unlike wild-type (wt) BALB/c mice in which 60% developed hyperthyroidism, only 13 and 7% of IL-4 KO and IFN-γ KO mice, respectively, became hyperthyroid. Thyroid stimulating antibodies were positive in most hyperthyroid mice. TSHR antibody titres determined by TSH binding inhibition and ELISA were comparable in all three groups. IgG1 and IgG2a TSHR antibody titres were similar in IFN-γ KO and wt mice, whereas IgG1 TSHR antibody titres and TSHR-specific splenocyte IFN-γ secretion were lower in IL-4 KO than in IFN-γ KO and wt mice, respectively. Our results clearly implicate both IFN-γ and IL-4 in development of hyperthyroidism in the TSHR-adeno model. These data, together with the previous report, also indicate different cytokine requirements in these two Graves' models, with IFN-γ being more important in the TSHR-adeno than the TSHR-M12 model. Moreover, our previous and present observations indicate a difference in the role of exogenous versus endogenous IL-4 in TSHR-adenovirus induced Graves' hyperthyroidism.
Keywords: Graves' hyperthyroidism, thyrotropin receptor, knockout mice, Th1/Th2
INTRODUCTION
The pathogenesis of Graves’ hyperthyroidism is characterized by humoral autoimmune responses, that is, hyperthyroidism is induced by stimulatory autoantibodies against the TSH receptor (TSHR) (thyroid stimulating antibodies, TSAb) [1], suggesting the importance of Th2 responses. However, the role of the Th1/Th2 balance in the pathogenesis of murine experimental Graves’ hyperthyroidism is controversial. In our Graves’ model involving intramuscular injection of adenovirus coding for the TSHR (TSHR-adeno model) [2], as well as in the model using TSHR-expressing B lymphoma M12 cells (TSHR-M12 model) [3], the anti-TSHR immune response is characterized by mixed Th1 and Th2 phenotypes, as demonstrated by TSHR antibodies of subclasses IgG1 (Th2) and IgG2a (Th1) and TSHR antigen-specific splenocyte secretion of Th1 cytokine interferon-γ (IFN-γ) and Th2 cytokines interleukin-4 (IL-4) or IL-10 [4,5]. However, in the TSHR-adeno model, immune deviation away from Th1 by coinjecting IL-4 expressing adenovirus, administration of α-galactosylceramide or helminth (Schistosoma mansoni) infection suppressed hyperthyroidism, indicating a critical role for Th1 responses in disease pathogenesis [4,6]. In contrast, in the TSHR-M12 model, hyperthyroidism was prevented in IL-4 knockout (KO), but not IFN-γ KO mice [5], suggesting the importance of the Th2 cytokine IL-4. To reconcile these contradictory data obtained with different models, in the present study we studied the outcome of immunizing IL-4 KO and IFN-γ KO mice with TSHR-adenovirus.
MATERIALS AND METHODS
Immunization with Ad-TSHR289
Construction, amplification, purification of nonreplicative recombinant adenovirus expressing the human TSHR A subunit (Ad-TSHR289) and determination of the viral particle concentration were performed as previously described [7]. Female wild type (wt) BALB/c mice (eight to 12 weeks old; Charles River Laboratory Inc., Tokyo, Japan) and IFN-γ KO and IL-4 KO BALB/c mice (Jackson Laboratory Inc., Bar Harbor, ME, USA) [8] were injected in the quadriceps with 100 µl PBS containing 1010 particles of Ad-TSHR289 twice at a three-week-interval. Blood, spleens and thyroid tissues were obtained two weeks after the second immunization. Thyroid histology was examined on formalin-fixed tissue sections stained with haematoxylin and eosin. All experiments were conducted in accordance with the principles and procedures outlined in the Guideline for the Care and Use of Laboratory Animals in Nagasaki University. Mice were kept in a conventional housing; our previous data demonstrate no influence of housing conditions on occurrence of hyperthyroidism in our model [9].
Thyroxine (T4), TSAb and TSH binding inhibiting antibodies (TBIAb) measurements
Total serum T4 was measured with a radioimmunoassay kit (RIA-gnost tT4, Nippon Schering, Osaka, Japan) with 20 µl. The normal range was defined as the mean ± 3 SD. of control untreated mice. TSAb activities in mouse sera were measured with FRTL5 cells and 5 µl sera as previously described [4]. cAMP released into the medium was measured with a cAMP radioimmunoassay kit (Yamasa, Tokyo, Japan). Values greater than 150% of those obtained in control, naïve mice were considered positive. TBIAb values were determined using human recombinant TSHR protein (a TRAb kit; BRAHMS Diagnostica GmbH, Berlin, Germany) and 10 µl sera. TSH binding inhibition >15% compared with TSH binding in control serum was considered positive.
ELISA for anti-TSHR antibodies
ELISA wells were coated overnight with 100 µl TSHR289 protein (1 µg/ml) [5] and incubated with mouse sera (1 : 30 dilution). After incubation with horseradish peroxidase conjugated anti-mouse IgG (diluted 3000×, A3673, Sigma), or subclass-specific anti-mouse IgG1 and IgG2a (diluted 1000× and 1500×; X56 and R19-15, BD PharMingen, San Diego, CA, USA), colour was developed using orthophenylene diamine and H2O2 as substrate and optical density read at 492 nm.
Cytokine assays
Splenocytes were cultured (triplicate aliquots) at 5 × 105 cells per well in a 96-well round bottomed plate in the presence or absence of 5 µg/ml TSHR289 protein or 5 µg/ml concanavalin A (Con A). Four days later, the concentrations of IFN-γ and IL-4 in the culture medium were determined with ELISA kits (Biosource International, Camarillo, CA, USA). Cytokine production was expressed as pg/ml using standard curves of recombinant mouse cytokines.
Statistical analysis
Levels of antibodies and cytokines, and incidence of hyperthyroidism were analysed by a nonparametric Mann–Whitney U-test and/or by χ2 test. A P-value of <0·05 was considered statistically significant.
RESULTS
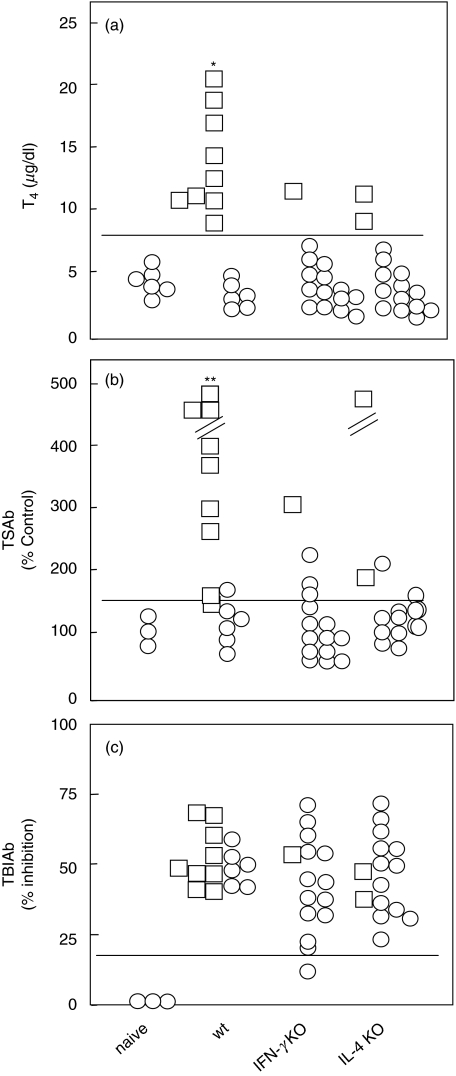
Wt, IFN-γ KO and IL-4 KO BALB/c mice were immunized with 1010 particles/mouse of Ad-TSHR289 twice (three weeks apart) and euthanized two weeks after the second immunization to obtain blood, spleens and thyroid glands. In 9 of 15 (60%) wt mice immunized with Ad-TSHR289, serum T4 levels were elevated compared with the upper limit for naïve mice (mean + 3 SD.) (Fig. 1a). In contrast, T4 levels were only elevated in 1 of 15 (7%) IFN-γ KO mice and 2 of 15 (13%) IL-4 KO mice. Consequently, hyperthyroidism was significantly suppressed in both cytokine KO strains (P < 0·01). Overall, T4 levels in immunized wt mice (8·9 ± 5·0 µg/dl; mean ± S.D) were significantly higher than in naïve mice and in immunized IL-4 KO and IFN-γ KO mice (4·7 ± 1·3, 4·5 ± 2·8 and 4·6 ± 2·4, respectively; P < 0·01). The hyperthyroid mice had diffuse goiters with hyperplasia and hypertrophy of thyroid epithelial cells but no lymphocytic infiltration (data not shown), as previously reported [4,7].
Fig. 1.
(a) T4, (b) TSAb and (c) TBIAb in wt, IFN-γ KO and IL-4 KO BALB/c mice immunized with AdTSHR289. Mice were immunized twice with AdTSHR289 with a three weekly interval and studied two weeks after the second injection. Data are the means of duplicate determinations. ○ euthyroid and □ hyperthyroid mice; horizontal lines, the upper limits of each assay in naïve mice; *P < 0·01 versus other three groups by chi-square and Mann–Whitney U-tests; **P < 0·01 versus other three groups by chi-square test.
In TSHR antibody assays, TSAb was positive in most hyperthyroid mice (Fig. 1b). The TSAb positivity was significantly higher than other three groups (P < 0·01). However, unlike the striking differences in T4 and TSAb levels in wt mice versus cytokine knockout mice, TBIAb levels were comparable in all three groups (Fig. 1c).
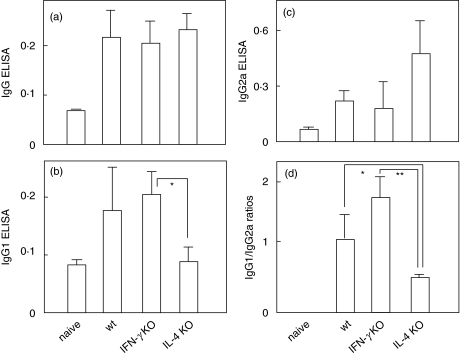
TSHR antibody titres determined by ELISA were also similar in all three groups of Ad-TSHR289 immunized mice (Fig. 2a). IgG1 and IgG2a TSHR antibody levels were comparable in wt and IFN-γ KO mice (Fig. 2b,c). In IL-4 KO mice, IgG1 TSHR antibody levels tended to be lower and IgG2a levels higher than in the other two groups. However, because of variability in antibody titres between individual mice, only IgG1 TSHR antibody levels were significantly lower in IL-4 KO than in IFN-γ KO mice (Fig. 2b). Finally, the ratios of IgG1 to IgG2a TSHR antibodies in IL-4 KO mice were significantly lower than in wt and IFN-γ mice (Fig. 2d).
Fig. 2.
Anti-TSHR antibody titres analysed by ELISA for IgG class and IgG1 and IgG2a subclasses. Data are the means of duplicate determinations. P < *0·05 and **0·01 by Mann–Whitney U-tests.
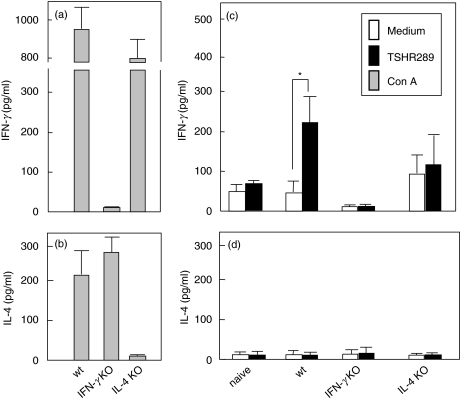
In assays for splenocyte cytokine secretion, as expected, Con A failed to induce IFN-γ secretion in IFN-γ KO mice (Fig. 3a) or IL-4 in IL-4 KO mice (Fig. 3b), while both cytokines were produced by splenocytes from wt mice. In response to in vitro stimulation with TSHR289 protein, splenocytes from immunized wt mice produced IFN-γ, as previously described [4]. However, unlike wt mice, the IFN-γ response of splenocytes from IL-4 KO mice challenged with TSHR289 protein was impaired (Fig. 3c). Indeed, this cytokine was secreted spontaneously by splenocytes incubated in medium alone and was not significantly increased in response to TSHR289 protein. IL-4 was not secreted in response to TSHR289 protein by splenocytes from any group (Fig. 3d).
Fig. 3.
IFN-γ and IL-4 production by splenocytes from wt, IFN-γ KO and IL-4 KO BALB/c mice immunized with AdTSHR289 and naïve wt mice. Splenocytes were prepared from immunized mice two weeks after the second immunization and were stimulated with Con A (5 µg/ml) or TSHR289 protein (5 µg/ml) for five days. IFN-γ and IL-4 levels in the culture supernatants were measured by ELISA. Data are the means ± S.E. (3 mice in each group). Con-A and TSHR289 protein stimulated cytokine productions are shown in A and B, and C and D, respectively. *P < 0·01 by Mann–Whitney U-tests.
DISCUSSION
The present study was designed to explore the difference in Th1/Th2 bias reported for two models of Graves’ hyperthyroidism induced in BALB/c mice. Recently, Dogan et al. [5] prevented induction of hyperthyroidism using TSHR-expressing M12 B cells in IL-4 KO but not in IFN-γ KO mice. In contrast, we now show that both IL-4 KO and IFN-γ KO mice are protected from developing hyperthyroidism induced using TSHR-adenovirus. Taken together, these findings indicate different cytokine requirements for the two murine models. In particular, IFN-γ appears to be more critical for induction of hyperthyroidism in the TSHR-adeno model than in the TSHR-M12 model. However, in both models, TSHR antibody levels measured by TBI and ELISA were unchanged in the two cytokine KO strains. Consequently, IFN-γ and/or IL-4 appear to be required for progression to clinically overt hyperthyroidism, but not for initiation of autoimmunity against the TSHR. Another difference in cytokine requirements between two models is also evident from the previous studies; transient immune deviation away from Th1 at the time of antigen-priming was sufficient to suppress hyperthyroidism in the TSHR-adeno model [4,6], whereas transient polarization to either Th1 (by fms-like tyrosine kinase 3 ligand) or to Th2 (by granulocyte macrophage-colony stimulating factor) had no effect in the TSHR-M12 model [5].
Although the reason(s) for different IFN-γ requirements for development of hyperthyroidism in the two models is presently unclear but may be partly attributed to different immunization protocols. As shown by our studies [4] as well as those of Barrett et al. [10], coexpression of IL-4, but not IL-12, suppressed hyperthyroidism induced by intramuscular injection of adenovirus or intradermal injection of plasmid encoding the TSHR. These observations suggest a critical role for Th1 immune responses in models based on genetic immunization. On the other hand, Th2 immune responses appear to be more relevant for hyperthyroidism induced by injecting cell lines stably expressing the TSHR (full-length or truncated). In support of the observations of Dogan et al. [5], Th1 adjuvants delayed, while Th2 adjuvants accelerated, hyperthyroidism in AKR/N mice induced by injecting fibroblasts coexpressing the TSHR and MHC class II (‘Shimojo model’) [11]. Moreover, in another Graves’ model involving injection of 293 human embryonic kidney (HEK) cells expressing the TSHR ectodomain [3], hyperthyroidism was suppressed in mice deficient in Stat-6, a Th2 signalling molecule [12]. It is important to note that hyperthyroidism induced by injecting TSHR-expressing M12 B cells or 293HEK cells usually involved coadministration of cholera toxin B subunit, a Th2 adjuvant [5,12]. Clearly, this modification could contribute to the difference in Th1/Th2 balance observed in different BALB/c models.
It should be appreciated that the observations by Dogan et al. [5] do not necessarily imply that IFN-γ is a dispensable cytokine in the in TSHR-M12 model. As noted by Steinman [13] in regard to other induced models of autoimmunity, IFN-γ may be critical for the induction of hyperthyroidism in wt mice, yet hyperthyroidism still occurs in IFN-γ KO mice. The basis for this apparent paradox is that the absence of a particular cytokine following gene disruption may be compensated by other cytokines. That is, there is redundancy in cytokine actions. Moreover, it is unlikely that disease pathology can be attributed to a single cytokine/mediator.
The outcome of gene disruption of IFN-γ and IL-4, or their respective receptors, on other autoimmune diseases is variable and possible mechanisms have been extensively discussed elsewhere [14–30]. Focusing on autoantibody-mediated diseases such as lupus erythematosus [14,15] and myasthenia gravis [16–18], disruption of IFN-γ (or the IFN-γ receptor) generally ameliorated disease activity. Accordingly, three autoantibody-mediated autoimmune diseases (lupus erythematosus, myasthenia gravis and Graves’ hyperthyroidism) are all found to involve IFN-γ. These results are not surprising, at least for lupus and myasthenia, because Th2 deviation in the absence of a functional IFN-γ signalling pathway decreased the titre of Th1 subclass autoantibodies that mediate tissue inflammation/damage, namely IgG3 cryoglobulins and/or complement-activating IgG2a and IgG3. However, in cell-mediated autoimmune diseases, including type 1 diabetes [19–21], collagen-induced arthritis [22–24], experimental autoimmune encephalomyelitis (EAE) [25–27], uveitis [28] and thyroiditis [29,30], in which IFN-γ is considered indispensable for disease pathology, the absence of IFN-γ or IFN-γR does not necessarily ameliorate disease. Paradoxically, collagen-induced arthritis and EAE were exacerbated in IFN-γ deficient mice [23,24,26,27].
Another intriguing finding is the difference in the effects of exogenous versus endogenous IL-4 on TSHR-adeno-induced hyperthyroidism. Hyperthyroidism is suppressed by both transient over-expression of exogenous IL-4 (our previous study [4]); and by the lack of endogenous IL-4 by gene disruption (present study). As expected, we found previously that pharmacological amounts of exogenous IL-4 (provided by coinjecting IL-4 expressing adenovirus) polarized TSHR-specific immune response towards Th2, as demonstrated by increased IgG1 to IgG2a ratios of TSHR antibodies and impaired antigen-induced secretion of IFN-γ by splenocytes. In contrast, in IL-4 KO mice, the absence of endogenous IL-4 had a mixed outcome, namely decreased IgG1 to IgG2a ratios (implying deviation towards Th1) and blunted IFN-γ responses to TSHR289 protein by splenocytes (suggesting deviation away from Th1). How can these apparently contradictory findings be explained? Besides its Th2-promoting function, IL-4 is also reported to have a profound effect on Th1 immune responses [31,32]. Consequently, the lack of endogenous IL-4 simultaneously dampens the pathogenically critical Th1 immune response, thereby preventing Graves’ hyperthyroidism. That is, the IL-4 KO mouse has two ‘defects’, not a single defect. Incidentally, although IFN-γ is the prototype Th1 cytokine, it also promotes the development of pathological autoantibodies via its influence on B cell maturation and antibody secretion [33,34].
In conclusion, we have shown that disruption of IFN-γ or IL-4 in BALB/c mice suppresses the induction of Graves’ hyperthyroidism by TSHR-adenovirus immunization, probably by inhibiting the pathogenic TSHR specific Th1 immune response. Combined with previous observations [5], our present data indicate different cytokine requirements in two murine Graves’ models. IFN-γ is more important in the TSHR-adenovirus model than in the TSHR-M12 model. Therefore, one should be cautious in drawing conclusions from a single animal model of disease. Multiple animal models appear to be necessary to provide insight into Graves’ hyperthyroidism in humans.
REFERENCES
- 1.Rapoport B, Chazenbalk GD, Jaume JC, McLachlan SM. The thyrotropin (TSH) receptor. interaction with TSH and autoantibodies. Endocr Rev. 1998;19:673–716. doi: 10.1210/edrv.19.6.0352. [DOI] [PubMed] [Google Scholar]
- 2.Nagayama Y, Kita-Furuyama M, Nakao K, Ando T, Mizuguchi H, Hayakawa T, Eguchi K, Niwa M. A novel murine model of Graves’ hyperthyroidism with intramuscular injection of adenovirus expressing thyrotropin receptor. J Immunol. 2002;168:2789–94. doi: 10.4049/jimmunol.168.6.2789. [DOI] [PubMed] [Google Scholar]
- 3.Kaithamana S, Fan J, Osuga Y, Liang S-G, Prabhakar BS. Induction of experimental autoimmune Graves’ disease in BALB/c mice. J Immunol. 1999;163:5157–64. [PubMed] [Google Scholar]
- 4.Nagayama Y, Mizuguchi H, Hayakawa T, Niwa M, McLachlan SM, Rapoport B. Prevention of autoantibody-mediated Graves’-like hyperthyroidism in mice by IL-4, a Th2 cytokine. J Immunol. 2003;170:3522–7. doi: 10.4049/jimmunol.170.7.3522. [DOI] [PubMed] [Google Scholar]
- 5.Dogan R-NE, Vasu C, Holterman MJ, Prabhakar BS. Absence of IL-4, and not suppression of the Th2 response, prevents development of experimental autoimmune Graves’ disease. J Immunol. 2003;170:2195–204. doi: 10.4049/jimmunol.170.4.2195. [DOI] [PubMed] [Google Scholar]
- 6.Nagayama Y, Watanabe K, Niwa M, McLachlan SM, Rapoport B. Schistosoma mansoni and α-galactosylceramide: prophylactic effect of Th1 suppression in a mouse model of Graves’ hyperthyroidism. J Immunol. 2004;173:2167–73. doi: 10.4049/jimmunol.173.3.2167. [DOI] [PubMed] [Google Scholar]
- 7.Chen C-R, Pichurin P, Nagayama Y, Latrofa F, Rapoport B, McLachlan SM. The thyrotropin receptor autoantigen in Graves’ disease is the culprit as well as the victim. J Clin Invest. 2003;111:1897–904. doi: 10.1172/JCI17069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andoh A, Masuda A, Kumazawa Y, Kasajima T. Serum antibody response and nasal lymphoid tissue (NALT) structure in the absence of IL-4 or IFN-γ. Cytokine. 2002;20:107–12. doi: 10.1006/cyto.2002.1980. [DOI] [PubMed] [Google Scholar]
- 9.Nagayama Y, McLachlan SM, Rapoport B, Niwa M. A major role for non-major histocompatibility complex genes but not for microorganisms in a novel murine model of Graves’ hyperthyroidism. Thyroid. 2003;13:233–8. doi: 10.1089/105072503321582024. [DOI] [PubMed] [Google Scholar]
- 10.Barrett K, Liakata E, Rao PV, Watson PF, Weetman AP, Lymberi P, Banga JP, Carayanniotis G. Induction of hyperthyroidism in mice by intradermal immunization with DNA encoding the thyrotropin receptor. Clin Exp Immunol. 2004;136:413–22. doi: 10.1111/j.1365-2249.2004.02483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shimojo N, Kohno Y, Yamaguchi K, et al. Induction of Graves-like disease in mice by immunization with fibroblasts transfected with the thyrotropin receptor and a class II molecule. Proc Natl Acad Sci USA. 1996;93:11074–9. doi: 10.1073/pnas.93.20.11074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Land KJ, Moll JS, Kaplan MH, Seetharamaiah GS. Stat6- but not Stat4-dependent immunity is required for the development of autoimmunity in Graves’ hyperthyroidism. Endocrinology. 2004;145:3724–30. doi: 10.1210/en.2004-0352. [DOI] [PubMed] [Google Scholar]
- 13.Steinman L. Some misconceptions about understanding autoimmunity through experiments with knockouts. J Exp Med. 1997;185:1039–41. doi: 10.1084/jem.185.12.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peng SL, Moslehi J, Craft J. Roles of interferon-γ and interleukin-4 in murine lupus. J Clin Invest. 1997;99:1936–46. doi: 10.1172/JCI119361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haas C, Ryffel B, Hir ML. IFN-γ receptor deletion prevents autoantibody production and glomerulonephritis in lupus-prone (NZB × NZW) F1 mice. J Immunol. 1998;160:3713–8. [PubMed] [Google Scholar]
- 16.Balasa B, Deng C, Lee J, Bradley LM, Dalton DL, Christadoss P, Sarvetnick N. Interferon γ (IFN-γ) is necessary for the genesis of acetylcholine receptor-induced clinical experimental autoimmune myasthenia gravis in mice. J Exp Med. 1997;186:385–91. doi: 10.1084/jem.186.3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang G-X, Xiao B-G, Bai X-F, van der Meide PH, Orn A, Link H. Mice with IFN-γ receptor deficiency are less susceptible to experimental autoimmune myasthenia gravis. J Immunol. 1999;162:3775–81. [PubMed] [Google Scholar]
- 18.Ostlie N, Milani M, Wang W, Okita D, Conti-Fine BM. Absence of IL-4 facilitates the development of chronic autoimmune myasthenia gravis in C57BL/6 mice. J Immunol. 2003;170:604–12. doi: 10.4049/jimmunol.170.1.604. [DOI] [PubMed] [Google Scholar]
- 19.Hultgren B, Huang X, Dybdal N, Stewart TA. Genetic absence of γ-interferon delays but does not prevent diabetes in NOD mice. Diabetes. 1996;45:812–7. doi: 10.2337/diab.45.6.812. [DOI] [PubMed] [Google Scholar]
- 20.von Herrath MG, Oldstone MBA. Interferon-γ is essential for destruction of β cells and development of insulin-dependent diabetes mellitus. J Exp Med. 1997;185:531–9. doi: 10.1084/jem.185.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Radu D, Noben-Trauth N, Hu-Li J, Paul WE, Bona CA. A targeted mutation in the IL-4Rα gene protects mice against autoimmune diabetes. Proc Natl Acad Sci USA. 2000;97:12700–4. doi: 10.1073/pnas.230431397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Myers LK, Tang B, Stuart JM, Kang AH. The role of IL-4 in regulation of murine collagen-induced arthritis. Clin Immunol. 2002;102:185–91. doi: 10.1006/clim.2001.5162. [DOI] [PubMed] [Google Scholar]
- 23.Manoury-Schwartz B, Chiocchia G, Bessis N, et al. High susceptibility to collagen-induced arthritis in mice lacking IFN-γ receptors. J Immunol. 1997;158:5501–6. [PubMed] [Google Scholar]
- 24.Vermeire K, Heremans H, Vandeputte M, Huang S, Billiau A, Matthys P. Accelerated collagen-induced arthritis in IFN-γ receptor-deficient mice. J Immunol. 1997;158:5507–13. [PubMed] [Google Scholar]
- 25.Liblau R, Steinman L, Brocke S. Experimental autoimmune encephalomyelitis in IL-4-deficient mice. Int Immunol. 1997;9:799–803. doi: 10.1093/intimm/9.5.799. [DOI] [PubMed] [Google Scholar]
- 26.Krakowski M, Owens T. Interferon-γ confers resistance to experimental allergic encephalomyelitis. Eur J Immunol. 1996;26:1641–6. doi: 10.1002/eji.1830260735. [DOI] [PubMed] [Google Scholar]
- 27.Ferber IA, Brocke S, Taylor-Edwards C, Ridgway W, Dinisco C, Steinman L, Dalton D, Fathman CG. Mice with a disrupted IFN-γ gene are susceptible to the induction of experimental autoimmune encephalitis. J Immunol. 1996;156:5–7. [PubMed] [Google Scholar]
- 28.Jones LS, Rizzo LV, Agarwal RK, Tarrant TK, Chan C-C, Wiggert B, Caspi R. IFN-γ-deficient mice develop experimental autoimmune uveitis in the context of deviant effector response. J Immnuol. 1997;158:5997–6005. [PubMed] [Google Scholar]
- 29.Alimi E, Huang S, Brazillet M-R, Charreire J. Experimental autoimmune thyroiditis (EAT) in mice lacking the IFN-γ receptor gene. Eur J Immunol. 1998;28:201–8. doi: 10.1002/(SICI)1521-4141(199801)28:01<201::AID-IMMU201>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 30.Tang H, Sharp GC, Peterson KE, Braley-Mullen H. Induction of granulomatous experimental autoimmune thyroiditis in IL-4 gene-disrupted mice. J Immunol. 1998;160:155–62. [PubMed] [Google Scholar]
- 31.Biedermann T, Zimmermann S, Himmelrich H, et al. IL-4 instructs TH1 responses and resistance to Leishmania major in susceptible BALB/c mice. Nature Immunol. 2001;2:1054–60. doi: 10.1038/ni725. [DOI] [PubMed] [Google Scholar]
- 32.Rhodes SG, Graham S. Is timing important for cytokine polarization? Trend Immunol. 2002;23:246–9. doi: 10.1016/s1471-4906(02)02200-7. [DOI] [PubMed] [Google Scholar]
- 33.Snapper CM, Mond JJ. Towards a comprehensive view of immunoglobulin class switching. Immunol Today. 1993;14:15–7. doi: 10.1016/0167-5699(93)90318-F. [DOI] [PubMed] [Google Scholar]
- 34.Sidman CL, Marshall JD, Shultz LD, Gray PW, Johnson HM. γ-interferon is one of several direct B cell-maturing lymphokines. Nature. 1984;309:801–4. doi: 10.1038/309801a0. [DOI] [PubMed] [Google Scholar]