Abstract
The cytochrome P450-dependent monooxygenase system catalyzes the metabolism of xenobiotics and endogenous compounds, including hormones and retinoic acid. In order to establish the role of these enzymes in embryogenesis, we have inactivated the system through the deletion of the gene for the electron donor to all microsomal P450 proteins, cytochrome P450 reductase (Cpr). Mouse embryos homozygous for this deletion died in early to middle gestation (∼9.5 days postcoitum [dpc]) and exhibited a number of novel phenotypes, including the severe inhibition of vasculogenesis and hematopoiesis. In addition, defects in the brain, limbs, and cell types where CPR was shown to be expressed were observed. Some of the observed abnormalities have been associated with perturbations in retinoic acid homeostasis in later embryogenesis. Consistent with this possibility, embryos at 9.5 dpc had significantly elevated levels of retinoic acid and reduced levels of retinol. Further, some of the observed phenotypes could be either reversed or exacerbated by decreasing or increasing maternal retinoic acid exposure, respectively. Detailed analysis demonstrated a close relationship between the observed phenotype and the expression of genes controlling vasculogenesis. These data demonstrate that the cytochrome P450 system plays a key role in early embryonic development; this process appears to be, at least in part, controlled by regional concentrations of retinoic acid and has profound effects on blood vessel formation.
Cytochrome P450 proteins, a diverse multigene family of hemoproteins with a wide range of overlapping substrate specificities, are responsible for the metabolism of many exogenous products (drugs and xenobiotics) and endogenous products, such as steroids, fatty acids, and prostaglandins (10, 29). Most studies have concentrated on the pharmacological and toxicological properties of these enzymes, and only recently has attention turned to the role of cytochrome P450 proteins in embryonic development (46, 47). The identification of cytochrome P450 CYP26, the only enzyme characterized to date as metabolizing retinoic acid in the embryo, has intensified developmental studies of the cytochrome P450 system (2).
Retinoic acid, the active metabolite of vitamin A, is a key determinant of vertebrate embryo patterning and organogenesis (30). Vitamin A (retinol) is converted to retinoic acid by two reactions: retinol dehydrogenases (ROLDH) convert retinol to retinaldehyde, which is then further converted to retinoic acid by retinal dehydrogenases (RALDH) (25). All-trans-retinoic acid (atRA) crosses the plasma membrane and is bound by cellular retinoic acid binding protein type I or II (CRABP-I or CRABP-II, respectively). atRA serves as a ligand to two nuclear receptor families (RAR α, β, and γ and RXR α, β, and γ) (27, 51). CYP26 catabolizes atRA to the metabolites 4-hydroxy-retinoic acid and 4-oxo-retinoic acid as part of an excretory pathway, although 4-oxo-retinoic acid remains a potent bioactive retinoid (38). The deletion of individual Rar and Rxr genes results in mild phenotypic changes, while compound deletions result in defects which resemble those found in fetal vitamin A deficiency (VAD) syndrome (19).
The tissue distribution and control of retinoic acid metabolism in embryos appear to be finely tuned; VAD causes embryonic malformations, including abnormal development of somites, limbs, the heart, and eyes (30). Excess retinoic acid perturbs the formation of the anteroposterior body axis and leads to craniofacial, heart, and limb defects (34). Targeted disruption of the gene encoding the major RALDH enzyme, Raldh2, resulted in the failure of axial rotation, incomplete neural tube closure, reduction of the trunk region, and heart defects, with embryonic lethality occurring at approximately 10.5 days postcoitum (dpc) (33). In contrast, embryos of a Cyp26 homozygous null line showed midgestational defects of neural tube closure, cardiac abnormalities, and tail truncations but survived up to 18.5 dpc (1). In a second Cyp26 null line, some mutants were born but died shortly after birth (41). Recent data obtained by crossing Raldh2+/− with a Cyp26-deficient mutant line suggested that CYP26 acts predominantly to protect tissues against inappropriate exposure to atRA (32). It has not been established whether other cytochrome P450 proteins are involved in embryonic retinoic acid metabolism or whether cytochrome P450 proteins play other essential roles in embryonic development.
Cytochrome P450 reductase (CPR) is an essential electron donor to cytochrome P450 proteins located in the endoplasmic reticulum (45). Deletion of Cpr would therefore be predicted to lead to a global inactivation of all microsomal P450 proteins. It was shown previously that homozygous disruption of part of the gene encoding the membrane-anchoring peptide of CPR leads to embryonic lethality at 11.5 to 13.5 dpc (44). However, conditional deletion of Cpr, specifically in the liver, the major organ for cytochrome P450 activity in an adult animal, although significantly interfering with hepatic metabolism, does not affect murine survival (12).
In this report, we have used a genetic approach to generate a murine model in which the microsomal cytochrome P450 system has been totally inactivated by the homozygous deletion of Cpr. We describe the phenotype of Cpr−/− mutants, which exhibit a very early embryonic lethal phenotype associated with altered retinoic acid homeostasis and severely compromised vasculogenesis. This phenotype can be partially reversed by the manipulation of maternal retinoic acid intake. These data provide evidence that other members of the cytochrome P450 system, in addition to CYP26, play a key role in both atRA homeostasis and early vasculogenesis.
MATERIALS AND METHODS
Targeting vector and generation of mutant mice.
The targeting vector and mutant mice were generated as described previously (12). In short, a DNA fragment of the human CPR gene was used as a probe to clone genomic DNA from an 129/Ola mouse genomic library. A replacement-type targeting vector was constructed from a 12-kb SalI fragment containing exons 3 to 16 of the mouse Cpr gene. A cassette flanked by loxP sites in the same orientation, containing a selectable marker (neomycin [neo]), and driven by the herpes simplex virus thymidine kinase promoter was inserted into intron 4. A third loxP site was cloned into intron 15.
After transfection of the construct into GK129/1 embryonic stem (ES) cells and G418 selection, eight correctly targeted ES cell clones were identified by Southern blot and PCR analyses with primers 1199 (5′-GCTCTCTGAATAAGTGGGTTCTGGC-3′) and 1185 (5′-GAATAGCCTCTCCACCCAAGCGGC-3′). To generate a null allele, five of the ES cell clones were subjected to electroporation with a Cre expression vector (pMC1Cre). Cre-mediated deletion was verified by Southern blot analysis, which identified nine clones (Cpr+/−), two of which were expanded to generate chimeric mice.
Male chimeric mice were bred with C57BL/6 female mice, yielding heterozygous F1 offspring. The F1 heterozygotes were mated with each other to produce Cpr−/− embryos. The genotype of yolk sacs was identified by either Southern blot or double-PCR analyses with primers 1105 (5′-GACCCTGAAGAGTATGACTTG-3′) and 1104 (5′-AGGCAGGCTGCTCAGGTCGGC-3′) and primers 1105 and 1215 (5′-CTAGCTCCATACATCCAGCGAGTA-3′). All animal work was carried out in accordance with the Annual Scientific Procedures Act (1986) and after local ethical committee review.
Analysis of Cpr−/− embryos.
Timed matings were performed with Cpr+/− mice in a mixed genetic background (C57BL/6J × 129/Ola). Over a period of 2.5 years, the mice were crossed consistently into a C57BL/6 background, reaching the F7 generation. This process had no noticeable effect on the phenotype of Cpr−/− embryos. Females with copulation plugs were considered to be at day 0.5 of gestation. Pregnant females were sacrificed at different times of gestation, and the embryos were dissected, examined, and photographed before being fixed in 4% paraformaldehyde in phosphate-buffered saline.
For whole-mount immunohistochemical analysis, fixed embryos were bleached with 5% H2O2 in methanol for 6 h, blocked with 3% instant skim milk powder-0.1% Triton X-100 in phosphate-buffered saline (blocking solution) for 2 h, and incubated overnight with rat monoclonal anti-PECAM-1, anti-Flk-1, and anti-erythropoietin (EPO) antibodies (Pharmingen; 1:50 dilution). Following extensive washes in blocking solution, the embryos were incubated with secondary biotinylated anti-rat immunoglobulin G antibody (Vector; 1:100). Embryos were incubated in a solution for binding avidin-conjugated peroxidase (Vector; 1:100) and washed extensively. Embryos were developed in 3,3′-diaminobenzidinetetrahydrochloride (Vector).
Transverse sections of embryos at 9.5 dpc were processed with anti-PECAM-1 antibody and counterstained with hematoxylin and eosin.
Whole-mount in situ hybridizations with digoxigenin-labeled probes was performed as previously described (3). Riboprobes were either produced in our laboratory or kindly provided by A. McMahon (Harvard University; Shh), C. Dickson (Cancer Research UK; Fgf8), P. Charnay (ENS; Krox20), and C. Bradfield (University of Wisconsin; ARNT and HIF-1α). I.M.A.G.E. Consortium clones 5317152, 6306291, and 3972003 (23) were used to generate in situ hybridization probes for CRABP-II, VEGFA, and EpoR, respectively.
Retinoic acid analysis was performed with frozen embryos by using automated online solid-phase extraction high-pressure liquid chromatography-electrochemical detection (42) and verified by using single-wave UV detection.
All mice were given a mouse No3 breeding diet (Special Diets Services, Witham, Essex, United Kingdom) containing 0.7 mg of retinol per kg. The VAD diet was also supplied by Special Diet Services. Dams were orally gavaged with atRA (Sigma, Poole, Dorset, United Kingdom) dissolved in corn oil at concentrations of 5 and 7.5 mg/kg. All animal procedures were carried out under UK Home Office license and after gaining local ethical committee approval.
Comparisons between wild-type and Cpr−/− embryos were performed by using the nonparametric Mann-Whitney U test. Statistical analysis was carried out by using SPSS version 11.
RESULTS
Generation of Cpr−/− mice.
Mice and embryos heterozygous for one Cpr allele (Cpr+/−) developed normally and were phenotypically indistinguishable from their wild-type littermates. Crossing of Cpr+/− mice failed to produce Cpr−/− pups. With timed matings, no viable Cpr−/− embryos were recovered at 10.5 dpc, and by 11.5 dpc, mutant embryos were resorbed. At 9.5 dpc, Cpr−/− embryos were still alive but were significantly smaller and severely malformed compared to Cpr+/+ or Cpr+/− embryos. In all Cpr−/− embryos, the embryonic trunk was markedly shortened, remained open ventrally, and had no visible limb buds (Fig. 1A to D); branchial arches were also absent, and the neural tube failed to close in many areas, most prominently in the head region.
FIG. 1.
Morphological abnormalities of Cpr−/− embryos. (A to D) Comparison of wild-type (A) and Cpr−/− (B to D) embryos at 9.5 dpc. At this stage, Cpr−/− embryos were still alive but were smaller, with fewer somites and an open neural tube (B to D). About two-thirds of mutants (70%) (P2 group) were severely malformed, with very few somites, and had not turned (B). About one-third of mutants (30%) (P1 group) had undergone axial rotation and had 12 to 16 somites (C and D). (E and F) At 8.75 to 9 dpc, Cpr+/+ (E) and Cpr−/− (F) embryos were similar in size, but mutants lacked branchial arches and had an underdeveloped forebrain. (G to I) Whole-mount immunostaining for CPR with a polyclonal rabbit antibody raised against the human FMN domain of CPR revealed high levels of staining for CPR in the posterior region, limb buds, branchial arches, and forebrain of Cpr+/− embryos at 9.5 dpc (G and H) but not in a Cpr−/− embryo at 9.5 dpc (I). Scale bars, 200 μm. b1 and b2, branchial arches 1 and 2, respectively; fl, forelimb bud; fn, frontonasal region; h, heart; nt, neural tube; op, optic vesicle; ot, otic vesicle; p, pericardium; so, somite; t, tail.
Two groups of Cpr−/− embryos could be distinguished. One group (P1), approximately 30% of mutants, underwent axial rotation and had 12 to 16 small somites (Fig. 1C and D). The second group (P2), 70% of mutants, did not turn, had only 8 to 12 somites, and had more severe growth retardation (Fig. 1B). Normal embryos at 9.5 dpc have completed axial rotation and display 20 to 25 somites (Fig. 1A). For all mutants, loss of CPR affected cardiac development. Hearts were enlarged and highly symmetrical in shape (Fig. 1B to D); additional malformations included a reduction in head size and a lack of development in the eye and frontonasal region. The forebrain and parts of the midbrain were also markedly underdeveloped, a feature already noticeable by 8.75 to 9 dpc (Fig. 1E and F). Cpr+/+ and Cpr−/− embryos at 8.5 dpc were comparable in size and had similar body structures, although the first branchial arch was absent from the null embryos (data not shown). Consistent with the above phenotypes, whole-mount immunohistochemical analysis with an antibody raised against the human FMN domain of CPR (45) revealed high levels of CPR protein in the frontonasal region, branchial arches, somites, limb buds, and tail of wild-type and Cpr+/− littermates at 9.5 dpc, while Cpr−/− mutants showed only background staining (Fig. 1G to I).
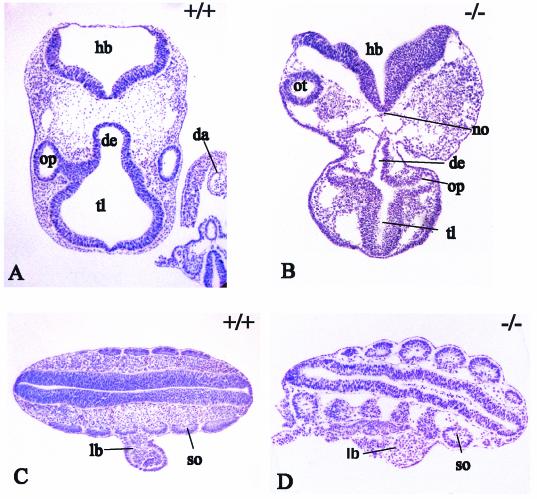
Histological examination of Cpr−/− embryos at 9.5 dpc showed the optic vesicles, clearly visible in wild-type littermates (Fig. 2A), to be underdeveloped and not separated from the diencephalon (Fig. 2B). In contrast, otic vesicles were well developed (Fig. 2B) and clearly visible in Cpr−/− embryos (Fig. 1). Branchial pouches formed in Cpr−/− embryos, but tissues of the developing branchial arches were absent. In the posterior body region, the small and irregularly shaped somites had a primitive epithelial organization and, although proper limb buds never formed, sections showed small outgrowths from the lateral plate mesoderm, reminiscent of early stages in limb bud formation (Fig. 2D). In comparison, in Cpr+/+ embryos of the same stage, somites were well organized and proper limb buds formed (Fig. 2C).
FIG. 2.
Transverse sections stained for PECAM-1 and counterstained with hematoxylin and eosin through the brain and somite region. (A and B) In Cpr−/− embryos, the neural tube failed to close, and optic vesicles, diencephalon, and telencephalon were underdeveloped (compare littermates in panels B and A); however, otic vesicles appeared to develop normally (B). Note the red blood cells in the dorsal aorta of the tail region in Cpr+/+ embryos (A). (C and D) Somites and limb buds formed normally in wild-type embryos (C) but were disorganized, with only a rudimentary limb bud visible, in Cpr−/− embryos (D). da, dorsal aorta; de, diencephalon; hb, hindbrain; lb, limb bud; no, notochord; op, optic vesicle; ot, otic vesicle; so, somite; tl, telencephalon.
Defective vascularization in Cpr−/− embryos.
Yolk sacs from Cpr+/+ embryos at 9.5 dpc displayed a hierarchically organized vessel architecture with a number of vitelline vessels with circulating red blood cells (Fig. 3A and B). Cpr−/− mutants of the same stage had very pale, transparent yolk sacs and no network of interconnecting vessels or red blood. This pale color was also characteristic of freshly dissected embryos, which showed no signs of any red blood cells. To characterize these findings further, embryos were immunostained for platelet endothelial cell adhesion molecule 1 (PECAM-1). PECAM-1 staining showed normal vascular development in whole Cpr+/+ or Cpr+/− embryos (Fig. 3C), while the vasculature of Cpr−/− mutants exhibited multiple major defects and was poorly developed in all regions of the embryos (Fig. 3D and E). Whereas the heads of control embryos displayed a well-developed, hierarchically organized vascular system of larger vessels branching into smaller vessels (Fig. 3F), in Cpr−/− mutants, a primitive capillary plexus did not form. Only a few very small vessels were noted, and abundant dots of stain suggested the presence of either blood islands or scattered clusters of endothelial cells (Fig. 3G). Likewise, major vessels, such as the dorsal aorta, aortic arches, and anterior cardinal vein, were absent from Cpr−/− mutants, and PECAM-1-positive cells were seen only sparsely in many regions of the trunk (Fig. 3I versus 3H). Intersomitic vessels were visible in better-developed Cpr−/− mutants (data not shown).
FIG. 3.
Vascular defects in Cpr−/− embryos. (A) Freshly dissected yolk sacs of Cpr+/+ embryos at 9.5 dpc displayed numerous vessels, in which blood was circulating. (B) Vessels and blood circulation appeared completely absent in Cpr−/− yolk sacs. (C to M) Whole-mount immunohistochemical analysis for PECAM-1 revealed in Cpr+/− embryos at 9.5 dpc (C) a well-organized primitive vascular system, which was severely disrupted in Cpr−/− mutants (D and E). At higher magnifications, the heads showed hierarchically organized blood vessels of different diameters (arrowheads) in Cpr+/− embryos (F), whereas Cpr−/− mutants lacked the formation of a primitive capillary plexus but showed small sprouting vessels (arrowheads) (G). In trunk regions, major vessels, such as the anterior cardinal vein, were visible in Cpr+/− embryos (arrow) (H) but were absent from Cpr−/− embryos (arrow) (I). In the hearts of Cpr+/− and Cpr+/+ embryos (J and L, respectively), extensive looping and trabeculation in the ventricle were visible (arrowheads); these features were confirmed in the sections. Looping and ventricle trabeculation were absent from Cpr−/− embryos (arrowheads) (K and M). v, ventricle.
The heart of Cpr+/− embryos developed normally, showing extensive looping and trabeculation in the ventricle(s); these features were confirmed in transverse sections (Fig. 3J and L). In contrast, in Cpr−/− embryos, the heart did not show looping, and sections showed that trabeculation also had not occurred (Fig. 3K and M). In addition, atrial and bulbus chordis chambers were either absent or severely malformed. The enlarged hearts of Cpr−/− mutants were probably the result of functional compensation for the failure of looping and trabeculation.
PECAM-1 staining of Cpr−/− embryos revealed endothelial cells, either incorporated into small branching vessels or still in blood islands, which precede the formation of a primary vascular plexus (49). However, none of the sections of Cpr−/− embryos showed endothelial cavities containing any blood cells, although these were easily visible in wild-type littermates (Fig. 2A). The apparent lack of red blood cells coincides with the pale color of Cpr−/− yolk sacs and embryos and indicates a defect in hematopoiesis.
Retinoid levels in Cpr−/− and Cpr+/+ embryos.
Cytochrome P450 proteins are responsible for the metabolism of atRA into polar metabolites and thus regulate the concentration of retinoic acid. One hypothesis for explaining the Cpr−/− phenotype could be perturbations in retinoic acid homeostasis. Therefore, we measured the concentrations of all-trans retinol (atROH) and atRA in wild-type littermates and Cpr−/− mutants at 9.5 dpc (Table 1). The concentrations of atRA were markedly increased in Cpr−/− mutants, on average 40 to 70% higher than those in wild-type littermates. In contrast, the levels of atROH were decreased in the mutants, leading to significant changes in atRA/atROH ratios, which were two- to threefold higher in mutant embryos than in wild-type embryos (Table 1). It is interesting that the perturbations in retinoic acid homeostasis were greatest in embryos of group P2, which exhibited the more severe phenotype, in which axial rotation had failed to occur.
TABLE 1.
atRA and atROH concentrations in wild-type and Cpr−/− embryos at 9.5 dpca
| Embryo (n) | Mean concn (pg/embryo) of:
|
atRA/atROH ratio | |
|---|---|---|---|
| atRA | atROH | ||
| WT (8) | 74.0 | 176.2 | 0.64 |
| P1 (6) | 106.2 | 71.8 | 1.49* |
| P2 (8) | 126.1 | 58.9 | 1.87** |
Retinoids were measured in wild-type (WT) embryos, less severe Cpr−/− embryos, which turned (P1), and very severe mutants, which did not turn (P2). Retinoids were measured in single embryos (four to seven in each group) or pooled samples (one to four in each group). There were large individual variations in the retinoid content per embryo in all groups, probably due to variations in embryonic staging. Single and double asterisks indicate results that were significantly different from those for wild-type littermates at P values of 0.059 and 0.028, respectively.
Partial recovery of the phenotype in Cpr−/− embryos from mice given a VAD diet.
To investigate whether changes in atRA metabolism were responsible for the defects in Cpr−/− mutants, dams were fed a VAD diet either 1 week prior to mating or at mating. No detectable differences between the two feeding regimens of the VAD diet were detected. Cpr+/+ and Cpr+/− embryos from mothers on a VAD diet still developed normally (Fig. 4A), but Cpr−/− embryos partially recovered from the severely malformed phenotype. From six litters from mothers which received the VAD diet, we obtained 10 Cpr−/− embryos, 9 of which had turned. In these nine embryos, the anteroposterior axis was markedly elongated and an increased number of somites had formed; head development, in particular, the forebrain and frontonasal region, had improved, and heart trabeculation and looping were ameliorated (Fig. 4B and C). However, the VAD diet was not sufficient for full rescue; branchial arches and limb buds were still absent, and the neural tube remained open.
FIG. 4.
Partial rescue of the 9.5 dpc Cpr−/− phenotype by feeding dams a VAD diet. (A) Depletion of maternal retinoic acid supply did not affect Cpr+/+ embryos. (B and C) However, feeding dams with the VAD diet either at mating (B) or 1 week prior to mating (C) resulted in a marked recovery in Cpr−/− embryos. Mutants turned and had more tissue, improved forebrain and frontonasal regions, and an elongation of the anteroposterior axis. VAD diet-exposed Cpr−/− embryos at this stage had 16 to 19 somites. Scale bars, 200 μm. fn, frontonasal region; h, heart; nt, neural tube; so, somite.
In contrast to the partial rescue of Cpr−/− embryos obtained with the VAD diet, supplementation of mothers with atRA during early postimplantation resulted in either a more severe phenotype or the complete loss of Cpr−/− mutant embryos at 9.5 dpc. Maternal supplementation with atRA at 7.5 mg/kg led to abnormalities in the heart, head, and somites, an open neural tube, and the loss of branchial arches in Cpr+/+ and Cpr+/− littermates, while atRA at 5 mg/kg resulted predominantly in heart defects (Table 2 and Fig. 5A).
TABLE 2.
Comparison of wild-type and Cpr−/− embryos at 9.5 dpc from dams with altered RA supplya
| Genotype | Treatment (mg/kg) | Axial rotation | Pairs of somites | Branchial arches | Heart formation | Neural tube | Presence of limb buds | No. of examined embryos |
|---|---|---|---|---|---|---|---|---|
| Cpr+/+/Cpr+/− | None | Yes | 18-26 | 3 | Asymmetric looped | Closed | Yes | >300 |
| RA (5) | Yes | 18-26 | 2 or 3 | Looped, enlarged | Closed | Yes | 28 | |
| RA (7.5) | Yes | 16-21 | 0 or 1 | Asymmetric enlarged | Open | 0-2 forelimb buds | 27 | |
| VAD | Yes | 18-26 | 3 | Asymmetric looped | Closed | Yes | 42 | |
| Cpr−/− | None (P1) | Yes | 12-16 | 0 | Symmetric, enlarged | Open | No | >60 |
| None (P2) | No | 8-12 | 0 | Symmetric enlarged | Open | No | >140 | |
| RA (5) | No | <10 | 0 | Symmetric enlarged | Open | No | 3 | |
| RA (7.5) | 3 (resorptions) | |||||||
| VAD | Yes | 16-19 | 0 | Asymmetric | Open | No | 9 | |
| VAD | No | 16 | 0 | Asymmetric | Open | No | 1 |
Dams were gavaged with 7.5 or 5 mg of atRA/kg (n = 3 for each concentration) at 7.5 and 8.5 dpc or fed a normal diet or a VAD diet (n = 6), and embryos were harvested at 9.5 dpc. Note that no Cpr−/− embryos of mothers given a high dose of RA were identified, but three placentas found in the three litters contained resorbed material with a Cpr−/− genotype.
FIG.5.
PECAM-1 staining in 9.5 dpc embryos with altered maternal retinoic acid supply. (A) Cpr+/+ embryos collected after maternal retinoic acid (RA) supplementation (7.5 mg/kg) displayed severe structural and vascular malformations, with a loss of major blood vessels. (B) For comparison, a well-formed Cpr−/− mutant (P1 group) is shown. (C) Cpr−/− mutants from a VAD diet-treated mother did not recover major vessels, despite the improved phenotype. All embryos in panels A to C had open neural tubes. (D to G) Intersomitic vessels in the trunk of retinoic acid-manipulated embryos. A Cpr+/− embryo showed a segmented pattern and intersomitic vessels (arrows) (D), while Cpr+/+ embryos from a dam receiving retinoic acid (RA) supplementation maintained good somite segmentation but had small intersomitic vessels (arrows) (E). Better-developed Cpr−/− embryos (P1 group) exhibited markedly reduced somite count and size but still formed very small intersomitic vessels (arrows) (F). Cpr−/− mutants from VAD diet-fed dams had medium-sized somites and maintained discernible intersomitic vessels (arrows) (G). h, heart; so, somite.
Table 2 summarizes the major developmental changes induced by alterations in maternal retinoic acid exposure. Retinoic acid homeostasis in the mothers appeared to be directly linked to embryo phenotype. Interestingly, maternal administration of atRA at 7.5 mg/kg resulted in a vascular phenotype in wild-type embryos comparable to that in Cpr−/− (P1) embryos from untreated mothers. The severe disruption of vasculogenesis, with the loss of major vessels and the lack of a primitive vascular plexus in the head (Fig. 5A), appears to be comparable to the vascular defects in Cpr−/− (P1) mutants (Fig. 5B). On the other hand, the maternal VAD diet had no effect on the defective vessel formation in Cpr−/− embryos, although the formation of the heart (Fig. 5C) and intersomitic vessels, barely visible in nonmanipulated Cpr−/− embryos, was markedly improved (Fig. 5G). Intersomitic vessels of wild-type embryos exposed to increased retinoic acid levels were not well formed (Fig. 5E and F). Thus, an alteration of maternal atRA status affected blood vessel formation in both wild-type and Cpr−/− embryos.
Molecular characterization of the Cpr−/− phenotype in relation to vasculogenesis.
Hypoxia-inducible factor 1α (HIF-1α) and its transcription factor partner, the aryl hydrocarbon receptor nuclear translocator (ARNT, also known as HIF-1β), as well as downstream target genes encoding vascular endothelial growth factor A (VEGFA), EPO, the VEGF receptor Flk-1 (VEGFR2), and the EPO receptor (EpoR), are critically required for the development of the vascular system and erythropoiesis (9). The expression of genes encoding these factors was significantly changed in Cpr−/− embryos compared to wild-type littermates (Fig. 6). Whereas ARNT and HIF-1α were widely coexpressed in midbrain, forebrain, neuroepithelium, branchial arches, primitive gut, hepatic primordium, and limb buds of wild-type littermates at 9.5 dpc (Fig. 6A and C), ARNT was expressed only in reduced amounts in neuroepithelium and hepatic primordium and was not expressed in the posterior region of Cpr−/− mutants (Fig. 6B). HIF-1α expression was almost completely absent in Cpr−/− mutants at 9.5 dpc, with only a low level of expression in the gut region (Fig. 6D). Furthermore, although VEGFA mRNA was already detectable at sites of blood vessel morphogenesis in Cpr+/+ and Cpr+/− embryos at 9.5 dpc, expression was reduced in the head and tail of Cpr−/− mutants. For example, VEGFA highlighted the vicinity of the dorsal aorta in the tail, but this expression was absent from Cpr−/− mutants (Fig. 6E and F). Also, Flk-1 protein, which was highly expressed in blood islands and the primitive vasculature of control embryos at 8.5 dpc, showed severely reduced expression in Cpr−/− embryos (Fig. 6G and H). Staining for Flk-1 in Cpr−/− mutants at 9.5 dpc revealed disrupted blood vessel formation similar to that revealed by PECAM-1 immunohistochemical analysis (data not shown).
FIG. 6.
Expression of genes associated with vasculogenesis in Cpr−/− mutants. (A and B) ARNT mRNA was found in multiple tissues, including forebrain, midbrain, neuroepithelium, branchial arches, hepatic primordium, limb buds, and primitive gut, of wild-type embryos at 9.5 dpc (A) but was present at a reduced level in neuroepithelium and hepatic primordium and absent from the posterior region of Cpr−/− embryos (B). (C and D) Transcripts for HIF-1α were distributed in the same tissues as ARNT transcripts in wild-type embryos (C) but were almost completely absent in Cpr−/− embryos (D). (E and F) VEGFA transcripts were widely expressed in Cpr+/− (E) and Cpr−/− (F) embryos at 9.5 dpc. VEGFA mRNA was strongly expressed at sites of blood vessel formation (arrow), especially at the site of the dorsal aorta in the tail, but also throughout posterior somites of Cpr+/− embryos (E). VEGFA mRNA was found ventrally in Cpr−/− mutants but was absent from the tail, somites, midbrain, and forebrain (F). (G) Flk-1 protein was expressed in the developing blood islands of control embryos at 8.5 dpc, which were starting to form a primary capillary plexus (arrow). (H) Flk-1 was barely expressed in the heads of Cpr−/− mutants, consistent with the absence of capillary plexus formation (arrow). (I and J) EPO was detected in the aorto-gonado-mesonephros area at 8.5 dpc in control embryos (I) but was found only in small amounts in this region in Cpr−/− embryos (J). EPO was distributed throughout the brain tissues, branchial arches, dorsal aorta, heart, somites, and tail of Cpr+/− embryos at 9.5 dpc (inset in I) but was expressed only in markedly reduced amounts in the heart and somites of Cpr−/− embryos (inset in J). EpoR mRNA was expressed at high levels at sites of developing vasculature (arrows) in wild-type (K) and Cpr−/− (L) embryos. agm, aorto-gonado-mesonephros area; b1, first branchial arch; da, dorsal aorta; fb, forebrain; fl, forelimb bud; hg, hindgut; hp, hepatic primordium; ne, neuroepithelium; so, somite; t, tail.
EPO protein was markedly expressed in the aorto-gonado-mesonephros area of control embryos (Fig. 6I) and was also detectable in Cpr−/− embryos at 8.5 dpc (Fig. 6J). EPO protein was highly expressed in the forebrain, midbrain, hindbrain, branchial arches, major blood vessels, heart, somites, limb buds, and tail of Cpr+/− embryos at 9.5 dpc (Fig. 6I, inset) but was expressed only at markedly reduced levels in the heart and somites of Cpr−/− embryos (Fig. 6J, inset). Interestingly, the EPO protein expression pattern of Cpr+/+ embryos (Fig. 6I) resembled that of CPR protein (Fig. 1G). EpoR mRNA was detected in the developing vasculature of wild-type littermates at 9.5 dpc and was as widely expressed at such sites in Cpr−/− mutants (Fig. 6K and L).
The severe head abnormalities of Cpr−/− embryos, such as an open neural fold and anterior truncation, prompted us to examine the pattern of expression of Krox20, a gene which is pivotal in early hindbrain patterning. Krox20 is functionally important in segmental processes in the developing hindbrain and is normally expressed in two alternate rhombomeres, rhombomere 3 (r3) and r5 (26) (Fig. 7A). In Cpr−/− embryos at 8.5 dpc, this rhombomere-restricted pattern of expression was fully established (Fig. 7B), indicating that, at least to some extent, rhombomere formation proceeds normally in these mutants. Later on, Krox20 transcripts were weakly expressed in a single domain in Cpr−/− mutants at 9.5 dpc, while Krox20 expression in r3 and r5 was maintained and intensified in wild-type littermates (data not shown).
FIG. 7.
Gene expression in postimplantation embryos. (A and B) Whole-mount in situ hybridizations were performed to show the distribution of gene transcripts encoding the zinc finger transcription factor Krox20, which was expressed in r3 and r5, respectively of wild-type embryos at 8.5 dpc (A); the same pattern was seen in Cpr−/− embryos (B). (C) Shh was strongly expressed along the anteroposterior axis in the notochord or floor plate of wild-type littermates at 8.75 dpc. (D) This expression pattern was continuous but markedly reduced in Cpr−/− embryos. Note that anterior Shh expression was lost in the mutants. Fgf8 was expressed in the tail of Cpr+/− and Cpr−/− embryos at 8.5 dpc (data not shown). (E and F) This caudal expression was lost in Cpr−/− mutants at 9.5 dpc (F), while it was strongly enhanced in wild-type littermates (E). Fgf8 transcripts were still noted in the isthmus and around the branchial pouch in Cpr−/− mutants at 9.5 dpc (F). Wild-type littermates at 9.5 dpc displayed marked Fgf8 expression in the first and second branchial arches, the apical ectodermal ridge of the forelimb buds, and the frontonasal region (E). None of these expression patterns was seen in the mutants (F). b1, first branchial arch; bp, branchial pouch; fl, forelimb bud; fn, frontonasal region; hg, hindgut; is, isthmus; t, tail.
We also investigated the expression of Sonic Hedgehog (Shh) along the anteroposterior body axis. Sonic Hedgehog is a signaling molecule, controlling the patterning of diverse tissues, and signaling by Hedgehog proteins is highly dependent on cholesterol, the biosynthesis of which involves a number of P450 proteins. Shh expression was weak but continuous along the notochord or neural tube and in the hindgut of Cpr−/− mutants (Fig. 7C and D). Shh transcripts in the developing brain were well defined in Cpr+/+ embryos but were absent from anterior regions of Cpr−/− embryos (Fig. 7D).
It has been proposed that, apart from retinoids, fibroblast growth factors (Fgfs), in particular, Fgf8, are involved in early caudalization and patterning along the anteroposterior embryonic axis (50). In Cpr−/− embryos at 8.5 dpc, as in wild-type and Cpr+/− littermates, Fgf8 was expressed caudally (data not shown). Cpr+/+ and Cpr+/− embryos at 9.5 dpc showed strong expression of Fgf8 in the tail, apical ridge of the forelimb bud, branchial arches, and frontonasal region (Fig. 7E). Caudal expression was lost in Cpr−/− embryos at 9.5 dpc, there was no sign of apical ridge formation in limb buds, but there was still notable Fgf8 expression in the isthmus and branchial pouch (Fig. 7F).
Finally, we examined the expression of CRABP-I and CRABP-II. CRABP-I, a retinoid binding protein, is thought to function as a cytosolic sink for atRA, while CRABP-II is thought to make atRA available for its receptors (35, 39). CRABP-I was expressed at high levels throughout the entire head region of Cpr−/− embryos at 9.5 dpc (Fig. 8B). In Cpr+/+ littermates, CRABP-I was expressed at high levels in the frontonasal mass, hindbrain, midbrain, and branchial arches (Fig. 8A) and along the dorsal neural tube (data not shown). In Cpr+/+ embryos exposed to atRA, CRABP-I transcript patterns similar to those in Cpr−/− embryos were observed, with high levels of expression being seen throughout the head (Fig. 8C). At 8.5 dpc, CRABP-I showed a distinct expression pattern in the hindbrain. CRABP-I levels are normally low in r2, absent in r3, high in r4 to r6, and low again in r7 (Fig. 8C). In Cpr−/− mutants, CRABP-I transcripts were expressed throughout the region of r1 and r7 and also extended anteriorly and posteriorly (Fig. 8E). CRABP-I transcripts were overexpressed in a similar manner in Cpr−/− embryos from dams treated with atRA at 7.5 mg/kg (Fig. 8F). These changes in CRABP-I expression were almost fully reversed in Cpr−/− embryos from VAD diet-fed dams, and zones of low expression in r2 and r3, high expression in r4 to r6, and low expression in r7 were reinstated in embryos at 9 dpc (Fig. 8H). Thus, substantial portions of the forebrain and midbrain, which do not express CRABP-I, appeared to have been recovered (Fig. 8H).
FIG.8.
CRABP-I and CRABP-II transcript distributions in Cpr−/− mutants. Cpr−/− embryos (9.5 dpc) were stained by whole-mount in situ hybridization with respective antisense probes. (A to C) Neural crest cells expressed high levels of CRABP-I. Note zones where gene transcripts were absent from the forebrain, midbrain, and hindbrain regions in the head of a wild-type littermate (A), while CRABP-I appeared uniformly expressed in the heads of Cpr−/− embryos (B) and Cpr+/+ embryos after maternal atRA administration (7.5 mg/kg) (C). (D) Transcript distributions of CRABP-I revealed a strict pattern of expression in specific rhombomeres in the hindbrain of control embryos at 8.5 dpc, with staining in r2, strong staining in r4 to r6, and weak staining in r7. (E) In comparison, all putative rhombomeres (r1 to r7) were uniformly and strongly stained in Cpr−/− mutants, with marked CRABP-I expression also being seen in the midbrain (E) and in cells along the neural tube and anterior somites (data not shown). (F) CRABP-I was also expressed in r1 to r7 and in the midbrain of retinoic acid (RA)-exposed Cpr+/+ embryos (F). (G) CRABP-I expression in a 9 dpc control embryo from a VAD diet-fed dam, showing hindbrain patterning and migration of neural crest cells into branchial arches. (H) Much of the specific distribution pattern could be recovered in 9 dpc Cpr−/− embryos from a VAD diet-fed dam, with weak staining in r2 and r3, strong staining in r4 to r6, and weak staining in r7. (I and J) CRABP-II transcripts were uniformly distributed throughout the neuroepithelium of Cpr+/+ (I) and Cpr−/− (J) embryos at 9.5 dpc, with a high level of expression in the forebrain of wild-type littermates (I). Exposure to atRA had no visible effect on CRABP-II expression in Cpr+/+ embryos (data not shown). Maternal feeding with a VAD diet resulted in a lower level of expression of CRABP-II in Cpr+/− embryos (K) than in Cpr−/− embryos (L). b1, first branchial arch; fn, frontonasal region; nt, neural tube.
There were only minimal differences in CRABP-II expression patterns between Cpr−/− and control embryos, with strong expression in neural epithelia and high levels of transcripts in the forebrain of Cpr+/+ embryos, which were lacking in the mutants (Fig. 8I and J). The administration of atRA to mothers had no visible effects on CRABP-II expression in Cpr+/+ embryos (data not shown). In 8.5 dpc embryos from mothers fed the VAD diet for 2 weeks prior to and during mating, CRABP-II transcript levels were significantly lower in wild-type littermates than in Cpr−/− mutants (Fig. 8K and L), while no difference was observed in any of the embryos from mothers fed a normal diet (data not shown).
DISCUSSION
Functional inactivation of the microsomal cytochrome P450 system through genetic deletion of CPR results in embryonic lethality in an early postimplantation stage prior to 10.5 dpc. Despite sparse knowledge about function and expression of cytochrome P450 proteins in early development, our Cpr−/− mice indicate a vital role for P450 proteins not only as previously demonstrated in mid-late gestation (1, 41) but already in early midgestation.
Embryonic defects in Cpr−/− mutants.
CPR is known to function as the primary electron donor to microsomal cytochrome P450 proteins. It has been shown that the cytochrome b5/NADH cytochrome b5 reductase system is sufficient to support cytochrome P450 metabolism in yeast (22). However, results from Shen et al. (44) and our data indicate that electron transport via CPR is essential to maintain adequate cytochrome P450 functioning in the early stages of mammalian development. Interestingly, deletion of the CPR membrane binding domain, which is thought to be vital for interaction of CPR with P450 enzymes, generated a distinct phenotype to that described here. Most of these Cpr−/− embryos survived until 11.5 to 13.5 dpc (44) with abnormalities of neural tube and heart, but axial rotation occurs and limb buds and branchial arches form. Development may proceed further due to the residual presence of a truncated 66-kDa CPR protein, which could still have certain metabolic activities. In our model, CPR function was completely deleted resulting in a more severe phenotype and death before 10.5 dpc. Our mutants did not turn, had a shortened anteroposterior axis, open neural tube, severe heart and vascular defects, no signs of branchial arches, or development of limb buds and lacked anterior head structures (Fig. 1). Many of the defects occur in regions where CPR protein is expressed at high levels, in particular forebrain, tail, limb buds and branchial arches. CPR mRNA has previously been reported to be expressed in mouse embryos as early as 7.5 dpc and transcripts were found to be markedly concentrated in limb buds and tail of 10.5 dpc embryos (20).
Genes encoding several cytochrome P450 proteins involved in xenobiotic metabolism (Cyp1A1, Cyp1A2, Cyp2E1, and Cyp1B1) have been deleted in mice. These mice generally develop normally and only exhibit abnormalities either postnatally or in adult life following drug or chemical treatment (4). Deletion of certain P450 proteins considered to be involved in “housekeeping” reactions, such as bile acid synthesis (cholesterol 7α-hydroxylase; Cyp7A1) or estrogen biosynthesis (aromatase; Cyp19), also do not affect embryonic development (15, 18). On the other hand, genetic deletion of the atRA-metabolizing enzyme, Cyp26, results in embryonic lethality between 12.5 to 18.5 dpc, with some pups being born but dying postnatally (1, 41). Embryos deficient for squalene synthase, a protein involved in cholesterol biosynthesis, only survive until 10.5 dpc and display neural tube defects (48). Changes in cholesterol homeostasis by altered cytochrome P450 metabolism may interfere with signaling of Hedgehog proteins, which normally occurs in neural tube development. It has been shown that overall cholesterol homeostasis is affected by a disrupted, hepatic cytochrome P450 system in adult Cpr hepatic null mice (12). Lack of Shh expression in the anterior brain region of Cpr−/− mutants could be the result of the absence of midbrain and forebrain tissue, but could also be caused by abnormal Shh signaling (14).
Cytochrome P450 proteins and vascular development.
This is the first report suggesting that cytochrome P450 plays an essential role in vasculogenesis, although some links between cytochrome P450 proteins and vascular homeostasis in adult mammals have been suggested (7). The identification of a porcine equivalent of CYP2C9 as endothelial derived polarization factor (6) substantiated a link between arachidonic acid metabolites, generated by cytochrome P450 activities, and vasculature, while a recent report linked epoxyeicosatrienoic acid generation by CYP2C9 to endothelial cell proliferation and angiogenesis (28). The arachidonic acid-metabolizing P450 proteins involved in vascular regulation are members encoded by the CYP2 gene family (CYP2B, 2C8, 2C9, 2C10, and 2J2 in humans) and ω-hydroxylases encoded by the CYP4A family (7, 17). Other P450 proteins such as thromboxane synthase and prostacyclin synthase are known to mediate vasoconstriction and vasodilation, respectively (5). However, the expression and functions of the genes encoding these P450 proteins in developing embryos has not been established.
Recently, a link between CPR and HIF-1α has been reported (36). A CPR-deficient cell line was shown to be unresponsive to HIF-1α and resulted in a lack of EPO induction under hypoxia, while CPR overexpression enhanced the DNA binding activity of HIF-1 to hypoxia response element (36). These results are interesting given that Hif1α null mice are developmentally lethal, with embryos dying at 11 dpc (16, 40). It has been proposed that a hemoprotein functions as oxygen sensor and is thus fundamental in regulation of HIF-1α and as a result target genes such as EPO and VEGF (8). There is still expression of VEGFA in several tissues of Cpr−/− embryos, but its low expression in the midbrain and forebrain as well as tail could also be a result of the severe malformations in these tissues. However, the severe reduction of EPO and Flk-1 in Cpr−/− mutants are in concordance with the low mRNA expression of ARNT and HIF-1α, suggesting that the lack of CPR already intereferes with the transcriptional regulation of HIF-1α. Our results thus support previous reports (8, 36) by demonstrating that the absence of CPR/P450 proteins markedly impacts on the expression pattern of ARNT, HIF-1α, EPO, VEGF and their respective receptors. Furthermore, the distribution of EPO protein appears to coincide with those of CPR. Our data now provide direct in vivo evidence that CPR/P450 proteins play a fundamental role in the earliest steps of embryonic vasculogenesis.
The absence of red blood cells and circulating blood, which was observed in histological sections and in freshly dissected yolk sacs and embryos, indicate early defects in hematopoiesis. In normal development, primitive red blood cells are already numerous at the time of amalgamation of embryonic and yolk sac vasculature and their origin can be traced back to hemangioblasts, which aggregate to form blood islands (49). Flk-1 is fundamental for hematopoietic and endothelial development (43), and a reduction in Flk-1 expression in Cpr−/− mutants may be largely responsible for the severe vascular defects, which may reflect the failure to form a primitive vascular plexus and major vessels. These defects remind of Flk1 null embryos which show an early defect in development of hematopoietic and endothelial cells (43). Interestingly, EpoR transcripts were increased in Cpr−/− mutants. One possibility is that a significant decrease in EPO leads to overexpression of EpoR and that EpoR may not be directly regulated downstream of CPR, HIF-1α and EPO.
Cytochrome P450 proteins and embryonic retinoic acid homeostasis.
Our finding that retinoid levels in the diet interact with the genetic deletion of Cpr suggests that the developmental defects observed in Cpr−/− mutants occur at least in part through imbalances in retinoic acid homeostasis. Retinoic acid is a key molecule in vertebrate embryonic development (30) and spatio-temporal regulation of retinoic acid concentration is of vital importance. This is achieved through a balance between retinoic acid synthesis (through ROLDH and RALDH) and catabolism (involving enzymes such as CYP26). Expression patterns of these enzymes are tightly controlled in development (2, 25). Interestingly, our Cpr−/− mutants display a significantly different phenotype from Cyp26−/− mutants, which have multiple defects such as truncation of the tail and a multitude of vertebral and organ abnormalities (1, 41). Some of the defects seen in Cpr−/− embryos could be either entirely or partially due to a lack of CYP26 activity, such as caudal truncation, neural tube defects and heart abnormalities (1). However, many of the defects in Cyp26−/− mutants occur at much later embryonic stages and Cpr−/− embryos do not develop that far. However, the truncated heads and disrupted vasculogenesis are defects that occur early in Cpr−/− mutants and do not occur in Cyp26−/− mutants. Thus, the magnitude of defects is greater in Cpr−/− than in Cyp26−/− mutants, consistent with expression pattern of CPR (Fig. 1) (20).
Cpr−/− embryos display a markedly similar phenotype to Raldh2−/− mutants, which have a short anteroposterior axis, severe heart defects and lack body turning (33). This appears paradoxical, as Raldh2−/− mutants can be rescued by maternal supplementation of atRA (33), while the phenotype of Cpr−/− embryos worsens with atRA exposure. It has previously been noted that vitamin A deficiency and hypervitaminosis A result in comparable malformed phenotypes. However, perturbations in retinoid metabolism are not fully reversed by maternal dietary alterations, as both Raldh2−/− (33, 34) and Cpr−/− mutants cannot be completely rescued.
It has been suggested that the primary function of atRA metabolism by cytochrome P450 proteins is to protect tissues from inappropriate exposures to atRA (32). This has been shown by crossing genetically reduced atRA (Raldh2 haploinsufficient) mice with Cyp26 mutant mice. These Cyp26−/− Raldh2+/− mutant mice survive to adulthood with diminished atRA concentrations. Thus, the teratotogenic effects of atRA are reversed, but sufficient amounts of atRA are still supplied for survival (32).
Our results confirm that P450 metabolism is vital for atRA inactivation in early development, as Cpr−/− mutants accumulate much higher amounts of atRA, leading to substantial alterations of patterns of gene expression, severe malformations and growth retardation. Furthermore, several P450 proteins apart from CYP26 must play a role in retinoic acid degradation not only in adults (27) but also in embryos. These various P450 proteins may produce other retinoid metabolites, such as didehydroretinoids, which are known to play a role in embryo development (37).
Although we have not measured the activity of the RAR/RXR transcription factor system, the significant overexpression of CRABP-I we observed in Cpr−/− mutants is consistent with the accumulation of atRA. Treatment of embryos with high atRA concentrations before 7.5 dpc in embryogenesis has been reported to result in significant CRABP-I overexpression in mouse brain, with CRABP-I being expressed in the midbrain region and leading to abnormal neural crest migration (24). The almost normal CRABP-I expression in Cpr null mutants from VAD diet-fed mothers is again consistent with the idea that this procedure helps to normalize retinoid levels in these embryos. Excess retinoic acid appears responsible for many of the defects seen in Cpr−/− embryos, because the mutant phenotype resembles that of retinoic acid-exposed wild-type embryos. Excessive retinoic acid concentrations have also been reported to result in vascular defects (13) and genes encoding molecules crucial for vascular development have been reported to be retinoic acid responsive, such as Flk-1, von Willebrand Factor and thrombomodulin (11). It remains to be determined which of the vascular defects seen in Cpr−/− are dependent on retinoic acid accumulation and/or directly on absence of CPR. Moreover, heart development is known to be very sensitive to changes in retinoic acid concentration (31) and the absence of retinoic acid is required for the anterior patterning of the head (21).
Finally, it should be noted that only a partial rescue of the Cpr−/− phenotype could be achieved with the VAD diet. This could be due to failure to optimise the retinoid balance. Others have reported that finding appropriate retinoic acid treatment regimes that will support normal development, especially for mutants sensitive to retinoic acid concentrations, can be very difficult. Thus, Niederreither et al. (34) extended the viability of Raldh2 mutants by 1 day by switching atRA delivery from gavage to food supply. Another possibility is that not all of the defects in Cpr−/− mutants are based on retinoid imbalance. Indeed, given the multiple functions of cytochrome P450 proteins this cannot be ruled out. Nevertheless our results suggest that retinoic acid homeostasis in embryonic development is critically dependent on a functional cytochrome P450 system, mediated by CPR activity.
Acknowledgments
We thank I. Rosewell, S. Wilson, and M. A. Haskins (Cancer Research UK, Transgenic Services, Hertfordshire, United Kingdom) for transfection of ES cells and generation of chimeric mice. We also thank S. Adams for sequencing the I.M.A.G.E. Consortium clones, A. Campagni (Cancer Research UK, Vascular Development Laboratory) and M. Maden (King's College London) for valuable suggestions and discussions, and G. Elia (Cancer Research UK, Histopathology Unit, London, United Kingdom) for staining the transverse sections.
M.D. was supported by a studentship of the British Heart Foundation. This work was supported by Cancer Research UK.
REFERENCES
- 1.Abu-Abed, S., P. Dolle, D. Metzger, B. Beckett, P. Chambon, and M. Petkovich. 2001. The retinoic acid-metabolizing enzyme, CYP26A1, is essential for normal hindbrain patterning, vertebral identity, and development of posterior structures. Genes Dev. 15:226-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abu-Abed, S. S., B. R. Beckett, H. Chiba, J. V. Chithalen, G. Jones, D. Metzger, P. Chambon, and M. Petkovich. 1998. Mouse P450RAI (CYP26) expression and retinoic acid-inducible retinoic acid metabolism in F9 cells are regulated by retinoic acid receptor gamma and retinoid X receptor alpha. J. Biol. Chem. 273:2409-2415. [DOI] [PubMed] [Google Scholar]
- 3.Adams, R. H., G. A. Wilkinson, C. Weiss, F. Diella, N. W. Gale, U. Deutsch, W. Risau, and R. Klein. 1999. Roles of ephrinB ligands and EphB receptors in cardiovascular development: demarcation of arterial/venous domains, vascular morphogenesis, and sprouting angiogenesis. Genes Dev. 13:295-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buters, J. T., J. Doehmer, and F. J. Gonzalez. 1999. Cytochrome P450-null mice. Drug Metab. Rev. 31:437-447. [DOI] [PubMed] [Google Scholar]
- 5.Cheng, Y., S. C. Austin, B. Rocca, B. H. Koller, T. M. Coffman, T. Grosser, J. A. Lawson, and G. A. FitzGerald. 2002. Role of prostacyclin in the cardiovascular response to thromboxane A2. Science 296:539-541. [DOI] [PubMed] [Google Scholar]
- 6.Fisslthaler, B., R. Popp, L. Kiss, M. Potente, D. R. Harder, I. Fleming, and R. Busse. 1999. Cytochrome P450 2C is an EDHF synthase in coronary arteries. Nature 401:493-497. [DOI] [PubMed] [Google Scholar]
- 7.Fleming, I. 2001. Cytochrome p450 and vascular homeostasis. Circ. Res. 89:753-762. [DOI] [PubMed] [Google Scholar]
- 8.Goldberg, M. A., S. P. Dunning, and H. F. Bunn. 1988. Regulation of the erythropoietin gene: evidence that the oxygen sensor is a heme protein. Science 242:1412-1415. [DOI] [PubMed] [Google Scholar]
- 9.Harris, A. L. 2002. Hypoxia—a key regulatory factor in tumour growth. Nat. Rev. Cancer 2:38-47. [DOI] [PubMed] [Google Scholar]
- 10.Hasler, J. A. 1999. Pharmacogenetics of cytochromes P450. Mol. Aspects Med. 20:12-24, 25-137. [DOI] [PubMed] [Google Scholar]
- 11.Hatzopoulos, A. K., J. Folkman, E. Vasile, G. K. Eiselen, and R. D. Rosenberg. 1998. Isolation and characterization of endothelial progenitor cells from mouse embryos. Development 125:1457-1468. [DOI] [PubMed] [Google Scholar]
- 12.Henderson, C. J., D. M. E. Otto, D. Carrie, M. A. Magnuson, A. W. McLaren, I. Rosewell, and C. R. Wolf. 2003. Inactivation of the hepatic cytochrome P450 system by conditional deletion of hepatic cytochrome P450 reductase. J. Biol. Chem. 13480-13486. 278: [DOI] [PubMed]
- 13.Hogers, B., M. C. DeRuiter, A. C. Gittenberger-de Groot, and R. E. Poelmann. 1997. Unilateral vitelline vein ligation alters intracardiac blood flow patterns and morphogenesis in the chick embryo. Circ Res. 80:473-481. [DOI] [PubMed] [Google Scholar]
- 14.Ishibashi, M., and A. P. McMahon. 2002. A sonic hedgehog-dependent signaling relay regulates growth of diencephalic and mesencephalic primordia in the early mouse embryo. Development 129:4807-4819. [DOI] [PubMed] [Google Scholar]
- 15.Ishibashi, S., M. Schwarz, P. K. Frykman, J. Herz, and D. W. Russell. 1996. Disruption of cholesterol 7alpha-hydroxylase gene in mice. I. Postnatal lethality reversed by bile acid and vitamin supplementation. J. Biol. Chem. 271:18017-18023. [DOI] [PubMed] [Google Scholar]
- 16.Iyer, N. V., L. E. Kotch, F. Agani, S. W. Leung, E. Laughner, R. H. Wenger, M. Gassmann, J. D. Gearhart, A. M. Lawler, A. Y. Yu, and G. L. Semenza. 1998. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev. 12:149-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janssen, L. J. 2002. Are endothelium-derived hyperpolarizing and contracting factors isoprostanes? Trends Pharmacol. Sci. 23:59-62. [DOI] [PubMed] [Google Scholar]
- 18.Jones, M. E., A. W. Thorburn, K. L. Britt, K. N. Hewitt, N. G. Wreford, J. Proietto, O. K. Oz, B. J. Leury, K. M. Robertson, S. Yao, and E. R. Simpson. 2000. Aromatase-deficient (ArKO) mice have a phenotype of increased adiposity. Proc. Natl. Acad. Sci. USA 97:12735-12740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kastner, P., M. Mark, N. Ghyselinck, W. Krezel, V. Dupe, J. M. Grondona, and P. Chambon. 1997. Genetic evidence that the retinoid signal is transduced by heterodimeric RXR/RAR functional units during mouse development. Development 124:313-326. [DOI] [PubMed] [Google Scholar]
- 20.Keeney, D. S., and M. R. Waterman. 1999. Two novel sites of expression of NADPH cytochrome P450 reductase during murine embryogenesis: limb mesenchyme and developing olfactory neuroepithelia. Dev. Dyn. 216:511-517. [DOI] [PubMed] [Google Scholar]
- 21.Koide, T., M. Downes, R. A. Chandraratna, B. Blumberg, and K. Umesono. 2001. Active repression of RAR signaling is required for head formation. Genes Dev. 15:2111-2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lamb, D. C., D. E. Kelly, N. J. Manning, M. A. Kaderbhai, and S. L. Kelly. 1999. Biodiversity of the P450 catalytic cycle: yeast cytochrome b5/NADH cytochrome b5 reductase complex efficiently drives the entire sterol 14- demethylation (CYP51) reaction. FEBS Lett. 462:283-288. [DOI] [PubMed] [Google Scholar]
- 23.Lennon, G., C. Auffray, M. Polymeropoulos, and M. B. Soares. 1996. The I. M. A. G. E. Consortium: an integrated molecular analysis of genomes and their expression. Genomics 33:151-152. [DOI] [PubMed] [Google Scholar]
- 24.Leonard, L., C. Horton, M. Maden, and J. A. Pizzey. 1995. Anteriorization of CRABP-I expression by retinoic acid in the developing mouse central nervous system and its relationship to teratogenesis. Dev. Biol. 168:514-528. [DOI] [PubMed] [Google Scholar]
- 25.Maden, M. 2000. The role of retinoic acid in embryonic and post-embryonic development. Proc. Nutr. Soc. 59:65-73. [DOI] [PubMed] [Google Scholar]
- 26.Manzanares, M., J. Nardelli, P. Gilardi-Hebenstreit, H. Marshall, F. Giudicelli, M. T. Martinez-Pastor, R. Krumlauf, and P. Charnay. 2002. Krox20 and kreisler co-operate in the transcriptional control of segmental expression of Hoxb3 in the developing hindbrain. EMBO J. 21:365-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marill, J., T. Cresteil, M. Lanotte, and G. G. Chabot. 2000. Identification of human cytochrome P450s involved in the formation of all-trans-retinoic acid principal metabolites. Mol. Pharmacol. 58:1341-1348. [DOI] [PubMed] [Google Scholar]
- 28.Michaelis, U. R., B. Fisslthaler, M. Medhora, D. Harder, I. Fleming, and R. Busse. 2003. Cytochrome P450 2C9-derived epoxyeicosatrienoic acids induce angiogenesis via cross-talk with the epidermal growth factor receptor (EGFR). FASEB J. 17:770-772. [DOI] [PubMed] [Google Scholar]
- 29.Miller, W. L. 1988. Molecular biology of steroid hormone synthesis. Endocrinol. Rev. 9:295-318. [DOI] [PubMed] [Google Scholar]
- 30.Morriss-Kay, G. M., and S. J. Ward. 1999. Retinoids and mammalian development. Int. Rev. Cytol. 188:73-131. [DOI] [PubMed] [Google Scholar]
- 31.Moss, J. B., J. Xavier-Neto, M. D. Shapiro, S. M. Nayeem, P. McCaffery, U. C. Drager, and N. Rosenthal. 1998. Dynamic patterns of retinoic acid synthesis and response in the developing mammalian heart. Dev. Biol. 199:55-71. [DOI] [PubMed] [Google Scholar]
- 32.Niederreither, K., S. Abu-Abed, B. Schuhbaur, M. Petkovich, P. Chambon, and P. Dolle. 2002. Genetic evidence that oxidative derivatives of retinoic acid are not involved in retinoid signaling during mouse development. Nat. Genet. 31:84-88. [DOI] [PubMed] [Google Scholar]
- 33.Niederreither, K., V. Subbarayan, P. Dolle, and P. Chambon. 1999. Embryonic retinoic acid synthesis is essential for early mouse post-implantation development. Nat. Genet. 21:444-448. [DOI] [PubMed] [Google Scholar]
- 34.Niederreither, K., J. Vermot, N. Messaddeq, B. Schuhbaur, P. Chambon, and P. Dolle. 2001. Embryonic retinoic acid synthesis is essential for heart morphogenesis in the mouse. Development 128:1019-1031. [DOI] [PubMed] [Google Scholar]
- 35.Niederreither, K., J. Vermot, B. Schuhbaur, P. Chambon, and P. Dolle. 2000. Retinoic acid synthesis and hindbrain patterning in the mouse embryo. Development 127:75-85. [DOI] [PubMed] [Google Scholar]
- 36.Osada, M., S. Imaoka, T. Sugimoto, T. Hiroi, and Y. Funae. 2002. NADPH-cytochrome P-450 reductase in the plasma membrane modulates the activation of hypoxia-inducible factor 1. J. Biol. Chem. 277:23367-23373. [DOI] [PubMed] [Google Scholar]
- 37.Perlmann, T. 2002. Retinoid metabolism: a balancing act. Nat. Genet. 31:7-8. [DOI] [PubMed] [Google Scholar]
- 38.Pijnappel, W. W., H. F. Hendriks, G. E. Folkers, C. E. van den Brink, E. J. Dekker, C. Edelenbosch, P. T. van der Saag, and A. J. Durston. 1993. The retinoid ligand 4-oxo-retinoic acid is a highly active modulator of positional specification. Nature 366:340-344. [DOI] [PubMed] [Google Scholar]
- 39.Ruberte, E., V. Friederich, P. Chambon, and G. Morriss-Kay. 1993. Retinoic acid receptors and cellular retinoid binding proteins. III. Their differential transcript distribution during mouse nervous system development. Development 118:267-282. [DOI] [PubMed] [Google Scholar]
- 40.Ryan, H. E., J. Lo, and R. S. Johnson. 1998. HIF-1 alpha is required for solid tumor formation and embryonic vascularization. EMBO J. 17:3005-3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sakai, Y., C. Meno, H. Fujii, J. Nishino, H. Shiratori, Y. Saijoh, J. Rossant, and H. Hamada. 2001. The retinoic acid-inactivating enzyme CYP26 is essential for establishing an uneven distribution of retinoic acid along the anterior-posterior axis within the mouse embryo. Genes Dev. 15:213-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sakhi, A. K., T. E. Gundersen, S. M. Ulven, R. Blomhoff, and E. Lundanes. 1998. Quantitative determination of endogenous retinoids in mouse embryos by high-performance liquid chromatography with on-line solid-phase extraction, column switching and electrochemical detection. J. Chromatogr. A 828:451-460. [DOI] [PubMed] [Google Scholar]
- 43.Shalaby, F., J. Rossant, T. P. Yamaguchi, M. Gertsenstein, X. F. Wu, M. L. Breitman, and A. C. Schuh. 1995. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature 376:62-66. [DOI] [PubMed] [Google Scholar]
- 44.Shen, A. L., K. A. O'Leary, and C. B. Kasper. 2002. Association of multiple developmental defects and embryonic lethality with loss of microsomal NADPH-cytochrome P450 oxidoreductase. J. Biol. Chem. 277:6536-6541. [DOI] [PubMed] [Google Scholar]
- 45.Smith, G. C., D. G. Tew, and C. R. Wolf. 1994. Dissection of NADPH-cytochrome P450 oxidoreductase into distinct functional domains. Proc. Natl. Acad. Sci. USA 91:8710-8714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stoilov, I. 2001. Cytochrome P450s: coupling development and environment. Trends Genet. 17:629-632. [DOI] [PubMed] [Google Scholar]
- 47.Stoilov, I., I. Jansson, M. Sarfarazi, and J. B. Schenkman. 2001. Roles of cytochrome p450 in development. Drug Metab. Drug Interact. 18:33-55. [DOI] [PubMed] [Google Scholar]
- 48.Tozawa, R., S. Ishibashi, J. Osuga, H. Yagyu, T. Oka, Z. Chen, K. Ohashi, S. Perrey, F. Shionoiri, N. Yahagi, K. Harada, T. Gotoda, Y. Yazaki, and N. Yamada. 1999. Embryonic lethality and defective neural tube closure in mice lacking squalene synthase. J. Biol. Chem. 274:30843-30848. [DOI] [PubMed] [Google Scholar]
- 49.Traver, D., and L. I. Zon. 2002. Walking the walk: migration and other common themes in blood and vascular development. Cell 108:731-734. [DOI] [PubMed] [Google Scholar]
- 50.Vasiliauskas, D., and C. D. Stern. 2001. Patterning the embryonic axis: FGF signaling and how vertebrate embryos measure time. Cell 106:133-136. [DOI] [PubMed] [Google Scholar]
- 51.Wendling, O., N. B. Ghyselinck, P. Chambon, and M. Mark. 2001. Roles of retinoic acid receptors in early embryonic morphogenesis and hindbrain patterning. Development 128:2031-2038. [DOI] [PubMed] [Google Scholar]