Abstract
Heat-shock proteins are highly conserved and immunogenic proteins, which may be involved in the initiation and perpetuation of autoimmune diseases. Heat-shock protein 47 (HSP47) is expressed by collagen-secreting cells such as fibroblasts and serves as a collagen-specific molecular chaperone that plays a crucial role in collagen metabolism. Abnormal collagen accumulation and autoimmunity are characteristics of systemic sclerosis (SSc). We determined the presence and prevalence of autoantibodies to HSP47 in patients with SSc and also in tight-skin (TSK/+) mice, an animal model for SSc. Anti-HSP47 autoantibodies were present in SSc patients with a frequency of 26%, while patients with systemic lupus erythematosus, those with dermatomyositis, those with keloid and healthy subjects did not have anti-HSP47 antibodies. IgG1 and IgG2 were the major Ig isotypes of the autoantibodies. Patients positive for anti-HSP47 had a significantly shorter duration of disease than those who were negative. Anti-HSP47 autoantibodies were also positive in 79% of TSK/+ mice. Thus, autoantobodies to HSP47 were present in the sera from SSc patients as well as those from TSK mice, and may be associated with the pathogenesis of SSc.
Keywords: autoimmunity, scleroderma, stress protein, tight-skin mouse
INTRODUCTION
Systemic sclerosis (SSc) is a connective tissue disorder that is characterized by fibrosis in the skin, lung and other visceral organs. The central event in the pathogenesis of SSc is an excessive accumulation of extracellular matrix components, predominantly collagens [1]. Consistently, fibroblasts cultured from the affected skin of patients with SSc display a morphologically activated phenotype producing increased amounts of type I collagen and other various connective tissue components [1]. Cytokines or growth factors that are produced partly by inflammatory cells infiltrating the affected tissues may be involved in the initiation or development of the fibrotic process [2].
Heat shock proteins (HSPs) function as molecular chaperones facilitating protein folding, assembly and intracellular transport, and thus are essential for cellular functions [3]. Their synthesis is increased greatly in response to a variety of stressful stimuli. Among the HSP members, HSP47 (also designated as colligin) serves uniquely as a collagen-specific molecular chaperone [4]. HSP47 locates in the endoplasmic reticulum of collagen-producing cells, binds specifically to collagenous peptides and plays a crucial role during the folding, maturation and secretion of procollagen. The importance of HSP47 in vivo has been demonstrated by gene-disrupting of the molecule in the mouse, resulting in severe deficiency of the mature form of collagen and death in utero [5].
The role of HSP47 has been also implicated in the pathogenesis of fibrotic diseases. Increased expression of HSP47 has been demonstrated in the fibrosis of lung, kidney and liver [6–8]. Furthermore, HSP47 mRNA and protein levels were significantly higher in fibroblast cultures from SSc patient-involved skin samples than in fibroblasts from normal skin from healthy individuals [9]. Also, SSc skin had a higher number of fibroblasts with high HSP47 levels than normal by in situ hybridization [9]. Because the mRNA and protein levels of HSP47 can be up-regulated by transforming growth factor-β1 and interleukin-4, both of which are also implicated in playing important roles in the pathogenesis of SSc [10–13], these findings may indicate that HSP47 is involved in the development of SSc.
Although HSPs are well conserved through evolution, they are highly immunogenic and it has been postulated that they could activate antigen-stimulating cells, serving as a danger signal to the immune system [14]. Indeed, immune reactivity to various HSPs, such as HSP60, HSP70, HSP90 and ubiquitin, is detected in autoimmune diseases [15]. The presence of autoantibodies is a hallmark of SSc, as antinuclear antibody was detected in >90% of patients [16]. Patients with SSc have autoantibodies reacting with various intracellular components, such as DNA topoisomerase I, centromere and RNA polymerases. While the relationship between fibrosis and autoimmunity remains unclear, the two components may influence each other in the pathogenesis. In this study, we showed the presence of autoantibodies to HSP47 in patients with SSc as well as tight-skin (TSK) mice, an animal model for SSc.
METHODS
Patients
Serum samples were obtained from 70 Japanese patients with SSc (61 females and nine males). All patients fulfilled the criteria proposed by the American College of Rheumatology [17]. These patients were between 9 and 76 years old (mean age 45 years). They were grouped according to the classification system proposed by LeRoy et al. [18]; 37 patients (34 females and three males) had limited cutaneous SSc (lcSSc) and 33 patients (27 females and six males) had diffuse cutaneous SSc (dcSSc). The disease duration of patients with lcSSc and dcSSc was 8·3 ± 9·1 and 4·7 ± 7·2 years, respectively. The duration of the disease was calculated from time of onset of the first clinical event (other than Raynaud's phenomenon) that was a clear manifestation of SSc. Five patients had been treated with low-dose corticosteroids (prednisolone, 5–20 mg/day) and four patients with low-dose d-penicillamine (100–500 mg/day) at their first visit. None of the SSc patients had received immunosuppressive therapy or had a recent history of infection or other inflammatory diseases. Fifteen patients with systemic lupus erythematosus (SLE) who fulfilled the criteria proposed by the American College of Rheumatology [19], 16 patients with dermatomyositis (DM) who fulfilled Bohan and Peter criteria [20,21] and 14 with keloid were also examined as disease control in this study. Twenty age- and sex-matched Japanese healthy individuals were used as normal controls. Fresh venous blood samples were centrifuged shortly after clot formation. All samples were stored at −80°C prior to use.
Complete medical histories, physical examinations and laboratory tests were conducted for all patients. Organ system involvement was defined as described previously [22]; lung: bibasilar fibrosis on chest radiography; oesophagus: hypomotility shown by barium radiography; joint: inflammatory polyarthralgias or arthritis; heart: pericarditis, congestive heart failure or arrhythmias requiring treatment; kidney: malignant hypertension and rapidly progressive renal failure without any other explanation; and muscle: proximal muscle weakness and elevated serum creatine kinase. The protocol was approved by the Kanazawa University School of Medicine and Kanazawa University Hospital, and informed consent was obtained from all patients.
Mice
Heterozygous TSK (TSK/+) mice, C57BL/6 mice and MRL/MpJ-Faslpr (MRL/lpr) mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). TSK+ mice were on C57BL/6 background. All mice were housed in a specific pathogen-free barrier facility. Serum samples were obtained from 24 TSK/+ mice, 13 C57BL/6 mice and 10 MRL/lpr mice at the age of 6 months by retro-orbital venous puncture and stored at −80°C prior to use. All studies and procedure were approved by the Animal Committee of International Medical centre of Japan.
Enzyme-linked immunosorbent assay (ELISA)
Recombinant rat HSP47 was purchased from Stressgen (Victoria, BC, Canada). ELISAs were conducted as described previously [23]. Ninety-six-well plates (EIA/RIA plate, Costar, Cambridge, MA, USA) were coated with 1 µg/ml of recombinant HSP47 (Stressgen) at 4°C overnight. The wells were blocked with 2% bovine serum albumin (BSA) and 1% gelatin in Tris-buffered saline for 1 h at 37°C and the serum samples diluted to 1 : 100 were added to duplicate wells for 90 min at 20°C. After washing four times, the bound antibodies were detected with alkaline phosphatase-conjugated goat antihuman IgG or IgM antibody or alkaline phosphatase-conjugated goat antimouse IgG or IgM antibody (Cappel, Durham, NC, USA), using p-nitrophenyl phosphate (Sigma-Aldrich, St Louis, MO, USA) as substrate. To determine IgG subclass reactive for HSP47, alkaline phosphatase-conjugated antihuman IgG1, IgG2, IgG3 and IgG3 antibodies (Zymed Laboratories Inc., South San Francisco, CA, USA) were also used as secondary antibodies. The optical density (OD) of the wells was subsequently determined. Relative levels of autoantibodies were determined for each group of patients and normal controls using pooled serum samples as described previously [24]. Among each disease or control group, the same amounts of all the serum samples were pooled into a single tube. The pooled sera were then diluted at log intervals (1 : 10–1 : 105) and were subjected to ELISA assays to obtain OD values for each dilution. The results were plotted as OD versus dilution (log scale). The dilutions of sera giving half-maximal OD values were determined by linear regression analysis, thus generating arbitrary unit per millilitre values for comparison between sets of sera.
Immunoblotting
Recombinant HSP47 (0·1 µg/lane; Stressgen) was subjected to electrophoresis on 8·5% sodium dodecyl sulphate-polyacrylamide slab gels. The proteins were electrotransferred from the gels to nitrocellulose sheets for immunoblotting analysis. The nitrocellulose sheets were cut into strips and incubated overnight with serum samples diluted 1 : 50. The strips were then incubated for 1·5 h with horseradish peroxidase-conjugated goat antihuman IgG antibody (Cappel), and developed using an enhanced chemiluminescence kit (Pierce, Rockford, IL, USA). Ten SSc patients positive for IgG anti-HSP47 antibody by ELISA, nine SSc patients positive for either antitopoisomerase I antibody, anticentromere antibody or anti-U1RNP antibody, but not for IgG anti-HSP47 antibody by ELISA, and six healthy individuals were evaluated.
Statistical analysis
Statistical analysis was performed using the Mann–Whitney U-test for comparison of antibody levels, Fisher's exact probability test for comparison of frequencies and Bonferroni's test for multiple comparisons. Spearman's rank correlation coefficient was used to examine the relationship between two continuous variables. A P-value less than 0·05 was considered statistically significant.
RESULTS
Anti-HSP47 autoantibody levels in SSc patients by ELISA
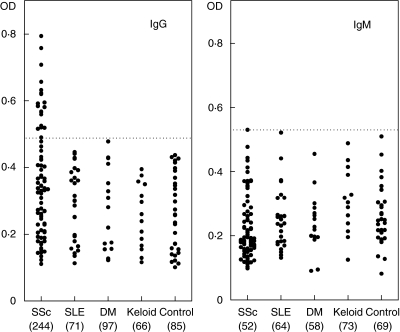
The presence and levels of anti-HSP47 autoantibodies in serum samples from patients with SSc were assessed by ELISA (Fig. 1). Other collagen diseases, including SLE and DM, keloid as a non-autoimmune skin fibrotic disease, as well as healthy individuals as normal controls were also examined. The dilution of sera giving half-maximal OD values in ELISAs was determined to generate arbitrary units per millilitre that could be compared directly between patients and normal controls. Pooled sera from patients with SSc had mean IgG anti-HSP47 antibody levels that were significantly 2·9-fold higher than those found in normal controls (P < 0·05), but had almost normal IgM levels of anti-HSP47 antibody (Fig. 1). By contrast, there were no significant differences in IgG anti-HSP47 antibody levels between patients with SLE, those with DM, those with keloid and healthy individuals. In patients with SSc, IgG anti-HSP47 antibody levels were significantly higher than those found in patients with SLE (P < 0·05), those with DM (P < 0·05) and those with keloid (P < 0·05) as well as normal controls (P < 0·05), while IgM anti-HSP47 antibody levels were similar for all these groups. In SSc patients, IgG anti-HSP47 antibody levels did not correlate with serum total IgG levels (data not shown). Thus, IgG anti-HSP47 antibody levels were increased in SSc but not in other collagen diseases, including SLE and DM, or in keloid.
Fig. 1.
Serum levels of anti-HSP47 antibody in patients with systemic sclerosis (SSc), systemic lupus erythematosus (SLE), dermatomyositis (DM), keloid and normal controls (Control). Anti-HSP47 levels were determined by a specific ELISA. A broken line indicates the cut-off value (mean ± 2 s.d. of the control samples). Values in parenthesis represent the dilutions of pooled sera giving half-maximal OD values in ELISA, which were determined by linear regression analysis to generate arbitrary units per millilitre that could be compared directly between each group of patients and normal controls.
Frequency of anti-HSP47 antibody positivity and clinical correlation in SSc
In anti-HSP47 ELISA, absorbance values higher than the mean ± 2 s.d. (0·492 for IgG anti-HSP47 antibody and 0·534 for IgM anti-HSP47 antibody) of the control serum samples were considered positive in this study (Fig. 1). In total patients with SSc, IgG anti-HSP47 antibody was found in 26% (18 patients), while no patients were positive for IgM anti-HSP47. IgG anti-HSP47 antibody was detected in nine patients with dcSSc (27%) and nine with lcSSc (24%). IgG anti-HSP47 antibody levels in patients with dcSSc were similar to those in patients with lcSSc (data not shown). Concerning clinical correlation, frequency of IgG anti-HSP47 antibody positivity was not associated with the total skin score or the presence or absence of organ involvement, including lung, joint, muscle, oesophagus, kidney and heart (Table 1). Also, the presence of anti-HSP47 antibody had no significant correlation with the presence of other autoantibody, including antitopoisomerase I antibody, anticentromere antibody, anti-U1RNP antibody and anti-RNA polymerase antibody. By contrast, patients positive for anti-HSP47 antibody had a significantly shorter duration from the onset of the disease than those negative for the anti-HSP47 antibody (Table 1). Thus, anti-HSP47 antibody may be present only at the early phase of the disease.
Table 1.
Clinical and laboratory data of patients with SSc positive for anti-HSP47 antibody*
| Anti-HSP47 positive (n = 18) | Negative (n = 52) | |
|---|---|---|
| Age at onset, year, mean ± s.d. | 47 ± 15 | 44 ± 17 |
| Sex, male : female | 1 : 17 | 3 : 49 |
| Duration, year, mean ± s.d. | 2·5 ± 2·1** | 6·9 ± 8·7 |
| Clinical features | ||
| Pitting scars | 39 | 44 |
| Contracture of phalanges | 44 | 58 |
| Diffuse pigmentation | 44 | 51 |
| Organ involvement | ||
| Lung | 55 | 52 |
| Decreased percentageVC | 55 | 53 |
| Decreased percentageDlco | 77 | 68 |
| Oesophagus | 72 | 72 |
| Heart | 11 | 18 |
| Kidney | 0 | 2 |
| Joint | 17 | 30 |
| Muscle | 11 | 14 |
| Laboratory findings | ||
| Anti-topoisomerase I antibody | 33 | 42 |
| Anti-centromere antibody | 33 | 28 |
| Anti-U1RNP antibody | 6 | 5 |
| Anti-RNA polymerase antibody | 18 | 8 |
| Elevated ESR | 33 | 33 |
| Elevated CRP | 11 | 29 |
| Increased IgG | 44 | 43 |
Values are percentages unless indicated. ESR = erythrocyte sedimentation rates and CRP = C-reactive protein.
P < 0·05 versus SSc patients with normal anti-HSP47 levels.
Immunoblotting analysis for anti-HSP47 antibody
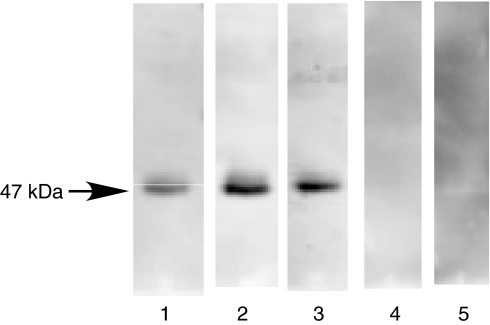
The presence of anti-HSP47 antibody was evaluated by immunoblotting analysis using recombinant rat HSP47 (Fig. 2). Serum samples from SSc patients positive for IgG anti-HSP47 antibody by ELISA all exhibited reactivity with HSP47 (47 kDa) by immunoblotting (lanes 1–3, Fig. 2). By contrast, serum samples from the SSc patients positive for antitopoisomerase I antibody but not for IgG anti-HSP47 antibody by ELISA did not react with HSP47 (lane 4). Similarly, no reactivity with HSP47 was observed using serum samples with either anticentromere antibody or anti-U1RNP antibody but without IgG anti-HSP47 antibody by ELISA (data not shown). Furthermore, serum samples from healthy individuals did not react with HSP47 (lane 5). Thus, the presence of anti-HSP47 autoantibody in patients with SSc was confirmed by immunoblotting analysis.
Fig. 2.
Representative immunoblotting of recombinant HSP47 with sera from patients with SSc positive for IgG anti-HSP47 antibody by ELISA. Lanes 1–3, serum samples from patients with SSc positive for IgG anti-HSP47 antibody by ELISA; lane 4, a serum sample from the SSc patient positive for antitopoisomerase I antibody, but not for IgG anti-HSP47 antibody by ELISA; and lane 6, a normal human serum. The results represent those obtained with 18 SSc positive for IgG anti-HSP47 antibody by ELISA, nine SSc patients positive for either antitopoisomerase I antibody, anticentromere antibody or anti-U1RNP antibody, but not for IgG anti-HSP47 antibody by ELISA, and 10 healthy individuals.
IgG subclass profile of anti-HSP47 antibody
To further characterize anti-HSP47 antibody in SSc, the isotypes of IgG autoantibodies binding to HSP47 were then assessed (Table 1). Eleven (61%) of the 18 sera reactive with HSP47 contained IgG1 autoantibodies. Twelve (67%) were found to have IgG2 autoantibodies binding to HSP47. One patient who was positive for both IgG1 and IgG2 also had IgG3 antibodies, while IgG4 was not detectable in any patients. Thus, IgG1 and IgG2 were the predominant subclasses of IgG anti-HSP47 antibody.
Anti-HSP47 antibody in TSK/+ mice
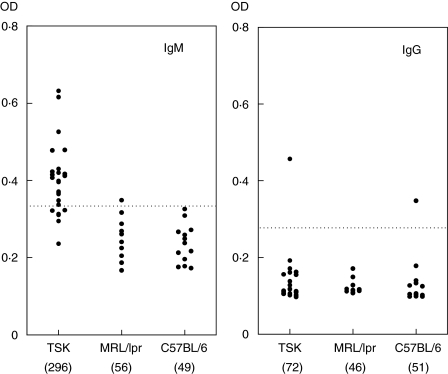
TSK+ mice develop cutaneous fibrosis resembling human SSc [25]. Although homozygous TSK mice die in utero, heterozygous TSK/+ mice survive and develop the syndrome. The presence and levels of anti-HSP47 autoantibodies in 24 serum samples from TSK/+ mice were assessed by ELISA (Fig. 2). TSK/+ mice exhibited significantly higher mean IgM anti-HSP47 antibody levels than those in wild-type C57BL/6 mice as normal controls (604% of wild-type; P < 0·01), but had similar levels of IgG anti-HSP47 antibody to wild-type mice. Both IgM and IgG anti-HSP47 antibody levels in lupus-prone MRL/lpr mice were similar to those in C57BL/6 mice. When absorbance values higher than the mean ± 2 s.d. (0·336 for IgM anti-HSP47 antibody and 0·262 for IgG anti-HSP47 antibody) of the control serum samples were considered positive, IgM anti-HSP47 antibody was found in 79% in TSK/+ mice, while IgG anti-HSP47 antibody was detected in only one TSK/+ mouse and also a wild-type mouse. In MRL/lpr mice, one was positive for IgG anti-HSP47 antibody and none was positive for IgM anti-HSP47 antibody. The presence of IgM antihsp47 antibody in sera from TSK/+ mice was also confirmed by immunoblotting analysis (data not shown). These results suggest that anti-HSP47 antibody is detected specifically in TSK/+ mice.
DISCUSSION
In the current study, IgG anti-HSP47 autoantibody levels were elevated significantly in the sera from patients with SSc relative to normal controls by ELISA (Fig. 1). The presence of anti-HSP47 antibody in the sera from SSc patients was also confirmed by immunoblotting analysis (Fig. 2). Anti-HSP47 antibody was also detected in TSK/+ mice, an animal model for SSc (Fig. 3). These results suggest that the induction of anti-HSP47 antibody is associated closely with skin fibrosing process.
Fig. 3.
Serum levels of anti-HSP47 antibody in TSK/+ mice, MRL/lpr mice and C57BL/6 mice. Anti-HSP47 levels were determined by a specific ELISA. A broken line indicates the cut-off value (mean ± 2 s.d. of the control samples). Values in parenthesis represent the dilutions of pooled sera giving half-maximal OD values in ELISA, which were determined by linear regression analysis to generate arbitrary units per millilitre that could be compared directly between each group of mice.
The presence of anti-HSP47 autoantibody was specific for patients with SSc, as anti-HSP47 antibody levels in patients with SLE or DM were similar to those in normal controls (Fig. 1). This may be consistent with a recent study, which has described that the sera from patients with mixed connective-tissue disease (MCTD) showed particularly high levels of anti-HSP47 antibody compared with rheumatoid arthritis, SLE, Sjogren's syndrome and idiopathic pulmonary fibrosis as well as healthy individuals [26]. They have not examined SSc patients but have found that patients with rheumatoid arthritis, SLE or Sjogren's syndrome had slightly higher levels of anti-HSP47 than healthy controls [26]. Their findings are not completely consistent with ours because our study failed to find a significant increase in SLE or DM sera compared with normal subjects. None the less, anti-HSP47 may be highly associated with SSc and SSc-related diseases such as MCTD. While anti-HSP47 antibody was specific for SSc patients, the frequency of anti-HSP47 antibody positivity was less than 30%. Notably, however, anti-HSP47 autoantibody was detected mainly in early cases of SSc, in whom fibrosis may be developing actively. Among three patients positive for anti-HSP47 antibody whose longitudinal sera were available, two patients showed declining antibody titres (data not shown). Therefore, the presence of anti-HSP47 antibody in SSc patients may be transient and may disappear once fibrosis is completed. That anti-HSP47 did not correlate with total skin score also suggests that anti-HSP47 may have an association with disease activity but not with disease severity.
In the physiological condition, HSP47 locates intracellularly within the endoplasmic reticulum and is not secreted. However, the levels of serum HSP47 antigen are reported to be elevated in MCTD and rheumatic diseases [26]. HSP47 production in fibroblasts is up-regulated in SSc [9]. Therefore, it is likely that HSP47 is secreted in these inflammatory and fibrotic conditions or leaks from apototic cells, resulting in the induction of anti-HSP47 antibody. Interestingly, although HSP47 expression is also increased in the fibroblasts from keloid, we did not find the presence of anti-HSP47 antibody in the sera from patients with keloid. While this may reflect the extent of the lesions, this finding suggests that specific autoimmune backgrounds are also required for the induction of anti-HSP47 antibody. Almost universally, autoantibodies in SSc exhibit blocking activities to the function of the targeted molecules. Although the immunodominant epitopes on HSP47 and the functional blocking activities have yet to be determined, anti-HSP47 might promote collagen secretion in SSc if secreted HSP47 play a role in the disorder.
In mice, anti-HSP47 antibody was also detected in TSK/+ mice, but not in either lupus-prone MRL/lpr mice or wild-type C57BL/6 mice. TSK mice have a spontaneous mutation within the gene encoding fibrillin 1 (Fbn-1) [27], an extracellular matrix glycoprotein crucial for microfibril assembly, which results in increased synthesis and excessive accumulation of collagen and other extracellular matrix proteins in the skin. TSK mice exhibit not only skin fibrosis but also abnormal humoral responses, also resembling SSc [28]. TSK mice produce anti-Fbn-1 antibody spontaneously [29], which is also detected in human SSc patients [30]. On the other hand, highly disease-specific antitopoisomerase I antibody is also present in TSK/+ mice. TSK mice and SSc patients showed different Ig subclass profiles for anti-HSP47 antibody: TSK mice exhibited IgM-dominant autoantibody profiles, while SSc patients showed IgG-dominant anti-HSP47 antibody, especially IgG1 and IgG2. None the less, these results suggest that anti-HSP47 autoantibody is induced specifically by the development of fibrosis in SSc and similar conditions. Notably, it takes several months for autoantobodies to be detected in TSK mice, whereas skin thickening is apparent much earlier.
HSPs are highly conserved and immunogenic proteins, which may be involved in the initiation and perpetuation of autoimmune diseases. Overexpression of HSP47 in SSc results in abnormal collagen accumulation but may also become a trigger for the induction or acceleration of autoimmunity in SSc. To date, there is no evidence that anti-HSP47 antibody directly contributes to the development of fibrosis, although HSP47 may be expressed on the cell surface of SSc fibroblasts and become a target of the autoantibody, as in the case of calreticulin, another HSP, in SLE [31]. If HSP47 is expressed on the surface of fibroblasts in SSc, anti-HSP47 may block the functions of HSP47, which would result in collagen deposition. Alternatively, secreted HSP47 may play unknown roles in the pathogenesis, as aminoacyl-tRNA synthetases, major autoantigens in polymyositis/dermatomyositis, have been shown recently to induce leucocyte migration [32]. Further investigations are necessary to clarify the roles of HSP47 and anti-HSP47 antibody in SSc. While the presence of autoantibodies is a central feature of SSc, the pathogenic relationship between systemic autoimmunity and clinical manifestations of SSc, including skin and visceral fibrosis, remains unknown. However, several lines of evidence indicate a link between the two phenomena. We have reported recently that antimatrix metalloproteinase-1 autoantibodies detected in patients with SSc can inhibit the collagenase activity of matrix metalloproteinase-1 directly and thus can reduce the extracellular matrix turnover [33]. Furthermore, a recent study has shown that the presence of anti-Fbn 1 autoantibodies in SSc may be the link between fibrosis and systemic autoimmunity, as normal fibroblasts treated with anti-Fbn 1 antibodies display an activated phenotype overexpressing Fbn 1 as well as some other extracellular matirx components [34]. The current study suggests that systemic autoimmunity may be linked to the development of fibrosis through a collagen-specific molecular chaperone, HSP47.
Acknowledgments
This study was funded by a grant from the Ministry of Education, Science, and Culture of Japan. We thank Ms S. Yoshitake for technical assistance.
REFERENCES
- 1.Jimenez SA, Hitraya E, Varga J. Pathogenesis of scleroderma. Collagen. Rheum Dis Clin North Am. 1996;22:647–74. doi: 10.1016/s0889-857x(05)70294-5. [DOI] [PubMed] [Google Scholar]
- 2.Sato S. Abnormalities of adhesion molecules and chemokines in scleroderma. Curr Opin Rheumatol. 1999;11:503–7. [PubMed] [Google Scholar]
- 3.Hightower LE. Heat shock, stress proteins, chaperones, and proteotoxicity. Cell. 1991;66:191–7. doi: 10.1016/0092-8674(91)90611-2. [DOI] [PubMed] [Google Scholar]
- 4.Nagata K. Hsp47: a collagen-specific molecular chaperone. Trends Biochem Sci. 1996;21:22–6. doi: 10.1016/0968-0004(96)80881-4. [DOI] [PubMed] [Google Scholar]
- 5.Nagai N, Hosokawa M, Itohara S, et al. Embryonic lethality of molecular chaperone hsp47 knockout mice is associated with defects in collagen biosynthesis. J Cell Biol. 2000;150:1499–506. doi: 10.1083/jcb.150.6.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawada N, Kuroki T, Kobayashi K, et al. Expression of heat-shock protein 47 in mouse liver. Cell Tissue Res. 1996;284:341–6. doi: 10.1007/s004410050594. [DOI] [PubMed] [Google Scholar]
- 7.Razzaque MS, Hossain MA, Kohno S, Taguchi T. Bleomycin-induced pulmonary fibrosis in rat is associated with increased expression of collagen-binding heat shock protein (HSP) 47. Virchows Arch. 1998;432:455–60. doi: 10.1007/s004280050191. [DOI] [PubMed] [Google Scholar]
- 8.Razzaque MS, Nazneen A, Taguchi T. Immunolocalization of collagen and collagen-binding heat shock protein 47 in fibrotic lung diseases. Mod Pathol. 1998;11:1183–8. [PubMed] [Google Scholar]
- 9.Kuroda K, Tsukifuji R, Shinkai H. Increased expression of heat-shock protein 47 is associated with overproduction of type I procollagen in systemic sclerosis skin fibroblasts. J Invest Dermatol. 1998;111:1023–8. doi: 10.1046/j.1523-1747.1998.00437.x. [DOI] [PubMed] [Google Scholar]
- 10.Atamas SP, White B. Interleukin 4 in systemic sclerosis: not just an increase. Clin Diagn Lab Immunol. 1999;6:658–9. doi: 10.1128/cdli.6.5.658-659.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Denton CP, Abraham DJ. Transforming growth factor-beta and connective tissue growth factor: key cytokines in scleroderma pathogenesis. Curr Opin Rheumatol. 2001;13:505–11. doi: 10.1097/00002281-200111000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Simms RW, Korn JH. Cytokine directed therapy in scleroderma: rationale, current status, and the future. Curr Opin Rheumatol. 2002;14:717–22. doi: 10.1097/00002281-200211000-00015. [DOI] [PubMed] [Google Scholar]
- 13.Takehara K. Hypothesis: pathogenesis of systemic sclerosis. J Rheumatol. 2003;30:755–9. [PubMed] [Google Scholar]
- 14.Gallucci S, Matzinger P. Danger signals: SOS to the immune system. Curr Opin Immunol. 2001;13:114–9. doi: 10.1016/s0952-7915(00)00191-6. [DOI] [PubMed] [Google Scholar]
- 15.Winfield J, Jarjour W. Stress proteins in autoimmunity. Adv Exp Med Biol. 1994;347:99–113. doi: 10.1007/978-1-4615-2427-4_11. [DOI] [PubMed] [Google Scholar]
- 16.Okano Y. Antinuclear antibody in systemic sclerosis (scleroderma) Rheum Dis Clin North Am. 1996;22:709–35. doi: 10.1016/s0889-857x(05)70297-0. [DOI] [PubMed] [Google Scholar]
- 17.Subcommittee for Scleroderma Criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Preliminary criteria for the classification of systemic sclerosis (scleroderma) Arthritis Rheum. 1980;23:581–90. doi: 10.1002/art.1780230510. [DOI] [PubMed] [Google Scholar]
- 18.LeRoy EC, Black C, Fleischmajer R, et al. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol. 1988;15:202–5. [PubMed] [Google Scholar]
- 19.Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–7. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 20.Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts) N Engl J Med. 1975;292:344–7. doi: 10.1056/NEJM197502132920706. [DOI] [PubMed] [Google Scholar]
- 21.Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts) N Engl J Med. 1975;292:403–7. doi: 10.1056/NEJM197502202920807. [DOI] [PubMed] [Google Scholar]
- 22.Steen VD, Powell DL, Medsger TAJ. Clinical correlations and prognosis based on serum autoantibodies in patients with systemic sclerosis. Arthritis Rheum. 1988;31:196–203. doi: 10.1002/art.1780310207. [DOI] [PubMed] [Google Scholar]
- 23.Fujimoto M, Poe JC, Satterthwaite AB, Wahl MI, Witte ON, Tedder TF. Complementary roles for CD19 and Bruton's tyrosine kinase in B lymphocyte signal transduction. J Immunol. 2002;168:5465–76. doi: 10.4049/jimmunol.168.11.5465. [DOI] [PubMed] [Google Scholar]
- 24.Sato S, Hasegawa M, Fujimoto M, Tedder TF, Takehara K. Quantitative genetic variation in CD19 expression correlates with autoimmunity. J Immunol. 2000;165:6635–43. doi: 10.4049/jimmunol.165.11.6635. [DOI] [PubMed] [Google Scholar]
- 25.Green MC, Sweet HO, Bunker LE. Tight-skin, a new mutation of the mouse causing excessive growth of connective tissue and skeleton. Am J Pathol. 1976;82:493–512. [PMC free article] [PubMed] [Google Scholar]
- 26.Yokota S, Kubota H, Matsuoka Y, et al. Prevalence of HSP47 antigen and autoantibodies to HSP47 in the sera of patients with mixed connective tissue disease. Biochem Biophys Res Commun. 2003;303:413–8. doi: 10.1016/s0006-291x(03)00352-8. [DOI] [PubMed] [Google Scholar]
- 27.Kielty CM, Raghunath M, Siracusa LD, et al. The tight skin mouse: demonstration of mutant fibrillin-1 production and assembly into abnormal microfibrils. J Cell Biol. 1998;140:1159–66. doi: 10.1083/jcb.140.5.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bona C, Rothfield N. Autoantibodies in scleroderma and tightskin mice. Curr Opin Immunol. 1994;6:931–7. doi: 10.1016/0952-7915(94)90016-7. [DOI] [PubMed] [Google Scholar]
- 29.Murai C, Saito S, Kasturi KN, Bona CA. Spontaneous occurrence of anti-fibrillin-1 autoantibodies in tight-skin mice. Autoimmunity. 1998;28:151–5. doi: 10.3109/08916939808996283. [DOI] [PubMed] [Google Scholar]
- 30.Tan FK, Arnett FC, Antohi S, et al. Autoantibodies to the extracellular matrix microfibrillar protein, fibrillin-1, in patients with scleroderma and other connective tissue diseases. J Immunol. 1999;163:1066–72. [PubMed] [Google Scholar]
- 31.Eggleton P, Llewellyn DH. Pathophysiological roles of calreticulin in autoimmune disease. Scand J Immunol. 1999;49:466–73. doi: 10.1046/j.1365-3083.1999.00542.x. [DOI] [PubMed] [Google Scholar]
- 32.Howard ONZ, Dong HF, Yang D, et al. Histidyl-tRNA synthetase and asparaginyl-tRNA synthetase, autoantigens in myositis, activate chemokine receptors on T lymphocytes and immature dendritic cells. J Exp Med. 2002;196:781–91. doi: 10.1084/jem.20020186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sato S, Hayakawa I, Hasegawa M, Fujimoto M, Takehara K. Function blocking autoantibodies against matrix metalloproteinase-1 in patients with systemic sclerosis. J Invest Dermatol. 2003;120:542–7. doi: 10.1046/j.1523-1747.2003.12097.x. [DOI] [PubMed] [Google Scholar]
- 34.Zhou X, Tan FK, Bona CA, Wallis D, Milewicz DM, Arnett FC. Anti-fibrillin-1 autoantibodies induce increased gene expression of FBN1 in normal dermal fibroblasts. Arthritis Rheum. 2001;44:S194. [Google Scholar]