Abstract
Increased adhesion and diapedesis of monocytes appear to be primary initiating factors in the pathophysiology of occlusive vascular diseases, including atherosclerosis and restenosis. However, the underlying mechanisms of transendothelial migration and invasion of monocytes into the blood vessels are not known. Alterations in ion channels on the cell membrane are generally involved in induced changes in shape and volume. In the present study, we investigated the expression and functional role of chloride channels in freshly isolated human blood monocytes. The Cl− currents in whole-cells were measured by the patch-clamp technique. We observed whole cell Cl− currents, which were time-independent and outwardly rectifying. The chloride channel blockers 5-nitro-2-(3-phenylpropylamino) benzoic acid (NPPB) and R(+)-[(6,7-dichloro-2-cyclopentyl-2,3-dihydro-2-methyl-1-oxo-1H-inden-5yl)-oxy]acetic acid 94 (IAA94) attenuated the Cl− currents. NPPB and IAA94 also inhibited chemotaxis of monocytes, as measured in Boyden chemotactic chambers, with the same sensitivity. NPPB but not IAA94, increased the cell volume as measured by shape change, and decreased tumour necrosis factor (TNF)-α-induced monocyte adhesion to endothelial cells. These results suggest that monocytes contain Cl− channels which regulate transendothelial migration of monocytes, due presumably to an alteration in cell volume.
Keywords: atherosclerosis, chemotaxis, chloride channel, IAA94, migration, monocyte, NPPB
INTRODUCTION
Monocytes are immune effector cells for defence against microbial pathogens and tumour surveillance. These cells also play a critical initiating role in the pathogenesis of occlusive vascular diseases, including atherosclerosis and restenosis [1]. Monocytes migrate into tissues and differentiate to macrophages. Monocytes are conjugated with oxidized low-density lipoproteins and exert their cytotoxic effector functions by secreting proinflammatory cytokines, chemokines, nitric oxide, arachidonic acid and its metabolites, free radicals and other secretory products [2]. Several studies indicate that the secretory products generated by monocytes and monocyte-derived macrophages play a direct role in the onset and/or progression of degenerative disorders such as neurodegenerative and occlusive vascular diseases [3]. Successful invasion of monocytes into tissues, which is a critical determinant for the underlying pathology, involves change in cell shape and volume, which are brought about by alterations in ion channels at the cell membrane.
Most animal cells are capable of regulating their cell volume upon exposure to anisotonicity. When cells are exposed to hypotonicity, they initially swell and then restore their cell volume towards normal. This is termed regulatory volume decrease (RVD), and efflux of K+ and Cl– via K+ and Cl– channels, respectively with obligated loss of water is responsible for RVD [4–6]. Anion channels are related to cell volume regulation. Several lines of evidence suggest that volume-regulated ion channels regulate cell migration through changing cell shape. For example, Cl– channels were involved in invasion of glioma tumour cells into the brain [7]. A number of electrophysiological studies have identified the expression of voltage- and ligand-gated K+, Ca2+, Cl–, H+ and cation channels in inflammatory leucocytes [8]. The ion channels are expressed in monocytic cell lines and monocyte-derived macrophages, and their electrophysiological and pharmacological properties have been studied [9–14]. A few studies have examined the functional roles of these ion channels in monocytes and monocyte-derived macrophages and indicate that these ion channels are involved in differentiation, production of nitric oxide and migration to the brain [15–17]. However, there is no information about the Cl– channels expression in normal human blood monocytes.
Because migration of monocytes through changing cell shape and volume is critical for trafficking from/to vascular blood vessels and further progression of numerous degenerative disorders, we investigated the expression and functional role of Cl– channels in normal human blood monocytes. In the present study, we demonstrated that human monocytes expressed outwardly rectifying Cl– currents. The blockers of the Cl– channels inhibited the Cl– currents, chemotaxis and adhesion of human blood monocytes.
MATERIALS AND METHODS
Blood collection
Normal volunteers, male and female between 21 and 45 years of age, were recruited in the study. These subjects were healthy non-smokers, taking no medication, free of any respiratory infection and had no caffeine in the morning prior to the collection of venous blood in heparinized vials. The Institutional Review Board (IRB) of Creighton University approved the research protocol. All subjects were required to give informed consent as approved by the IRB of Creighton University.
Isolation of monocytes
Monocytes were isolated and purified using a method reported from our laboratory [18]. Briefly, 120 ml of blood was collected from each volunteer using heparin as anticoagulant. Blood was then diluted 1 : 1 with 0·9% saline followed by dextran sedimentation for 1 h at room temperature and hypotonic lysis to eliminate erythrocytes. The buffy coat of white blood cells was layered over Percoll density gradient of 1·077 g/ml to 1·085 g/ml (Atlanta Biologicals, GA, USA), and centrifuged to separate the peripheral blood mononuclear cells (PBMCs). The layer of the PBMCs with a density of 1·077 g/ml was collected, washed twice in phosphate-buffered saline (PBS, pH 7·4) and suspended in the desired buffer. Monocytes were separated from lymphocytes by the adherence technique by incubating PBMCs in RPMI-1640 medium for 2 h. Adherent cells were removed, washed and used for experiments. Purity of isolated monocytes was >95% as stained with Diff-Quick and viability was >98% by trypan blue dye exclusion. All culture reagents were screened for endotoxin (<0·01 ng/ml) and mycoplasma tests before and during use.
Electrophysiological recordings
The whole-cell patch-clamp technique was used to record Cl– currents in normal human blood monocyte. Whole-cell recordings were made using an Axopatch 200B patch-clamp amplifier, and pClamp 8 and Digidata 1322 A interface (Axon Instruments, Foster City, CA, USA) were used to control voltage and acquire data [19]. Whole-cell Cl– currents were measured using ruptured cell membrane. Pipettes were fabricated of borosilicate glass capillary tubing (A-M Systems, Carlsborg, WA, USA) with resistance of 5–7 MΩ for each of the pipette solutions used. Currents were filtered at 5 kHz with the eight-pole Bessel filter in the Axopatch amplifier.
The bathing medium was NMDGCl or CsCl saline consisting of (in m m) 140 CsCl (NMDGCl), 1 CaCl2, 1 MgCl2 and 10 HEPES (pH 7·4, 290 mOsm). The pipette solution was CsCl saline consisting of (in m m) 140 CsCl (NMDGCl), 0·1 EGTA, 1 CaCl2, 1 MgCl2 and 10 HEPES (pH 7·4, 280 mOsm). The bathing medium was exchanged by continuous perfusion.
Chloride channel blockers
We examined the effect of several chloride channel blockers in this study. These included: anthracene-9-carboxylic acid (9AC), 4,4′-diisothiocyanostilbene-2,2′-disulphonic acid (DIDS), gadolinium chloride, R(+)-[(6,7-dichloro-2-cyclopentyl-2,3-dihydro-2-methyl-1-oxo-1H-inden-5yl)-oxy] acetic acid 94 (IAA94) and 5-nitro-2-(3-phenylpropylamino)benzoic acid (NPPB). These channel blockers were prepared as 10−2m stock solutions in dimethylsulphoxide (DMSO), and diluted on the day of the experiment in fresh solution. All reagents were purchased from Sigma Chemical Company (St Louis, MO, USA).
Monocyte shape change assay using flow cytometry
Monocyte shape change was assayed as previously described [20,21]. Purified monocytes (5 × 106 cells) were suspended in assay buffer [comprising PBS without Ca2+/Mg2+ supplemented with 0·1% bovine serum albumin (BSA), 10 m m HEPES and 10 m m glucose, pH 7·4] for 30 min at 37°C. To stop the reaction, samples were transferred to ice and fixed with 250 µl of fixative solution. Samples were analysed immediately on a FACSCalibur flow cytometer (Becton Dickinson) and monocytes were identified by their forward-/side-scatter (FSC/SSC) characteristics. Chloride channel blocker-induced monocyte shape change was compared with unstimulated monocytes (control). A percentage change in forward-scatter (FSC) was used to estimate the extent of increase in the shape change from unstimulated cells. Ten thousand monocyte events were counted for each sample.
Chemotaxis of monocytes
Chemotaxis was tested in 48-well Boyden microchambers [22]. Briefly, 26 µl of macrophage chemotactic protein-1 (MCP-1) solution (50 ng/ml) was added to the bottom wells of a chemotaxis chamber (Neuroprobe, Gaithersburg, MD, USA). A PVP-free polycarbonate filter (5 µm pore size) was layered onto the wells and covered with both a silicon gasket and the top plate. Then 50 µl of the cell suspension (1–1·5 × 106 cells/ml) was loaded into the top chamber. This was incubated at 37°C in 5% CO2 atmosphere for 1·5 h. After the incubation, filters were removed and the topside of the membrane scratched to remove non-migrated cells followed by staining with Diff-Quik. The cells are counted in five high-power fields (× 400). Experiments were performed in triplicate and data were pooled. When NPPB or IAA94 was applied, it was added to both bottom and top chambers.
Assay of monocytes adherence to human umbilical vein endothelial cells (HUVEC)
HUVECs were purchased from Clonetics (San Diego, CA, USA) and grown in minimum essential medium (MEM) with 2% fetal bovine serum (FBS; Clonetics, San Diego, CA, USA). Cells were used for experiments when they were 85–100% confluent. HUVECs were plated in 96-well tissue culture plates for the adherence assays. Monocytes were washed twice with PBS and resuspended in RPMI-1640 without serum at 1 × 106 cells/ml. The cells were labelled with calcein AM (Molecular Probes, Eugene, OR, USA) at a final concentration of 7·5 µm for 30 min at 37°C and 5% CO2. Labelling was stopped by the addition of RPMI-1640 medium. The cells were washed twice by centrifugation followed by resuspension to 2 × 105 cells/ml in RPMI-1640 medium.
It has been reported that adherent human monocytes to HUVECs could be increased substantially when HUVECs are stimulated with TNF-α (10 ng/ml) [23]. In the present study, HUVECs were pre-incubated with 10 ng/ml TNF-α for 24 h. Immediately before the assay, the HUVEC cultures were washed twice with RPMI-1640 medium. Calcein AM-labelled monocytes were added to each well and co-incubated with HUVECs at 37°C for 1 h in the presence of 200 µm NPPB or IAA94. To remove non-adherent cells, each well was washed carefully by the addition of prewarmed RPMI-1640 followed by gentle swirling and inversion of the plate and blotting of the excess liquid onto paper towels. This was repeated twice and then 1 ml of RPMI-1640 medium was added to each well. Relative fluorescence was read using the fluorescence plate reader (FL × 800, Bio-Tek) set at excitation 485 nm and emission at 530 nm. Absolute cell numbers were determined by comparison of fluorescence values determined after several dilutions of calcein AM-labelled cells in RPMI-1640 medium.
Data analyses
Data are expressed as mean ± s.e.m. (n = number of individual experiments) and statistical significance was determined with Student's paired t-test; P < 0·05 was considered significant.
RESULTS
Whole-cell Cl– currents in monocytes
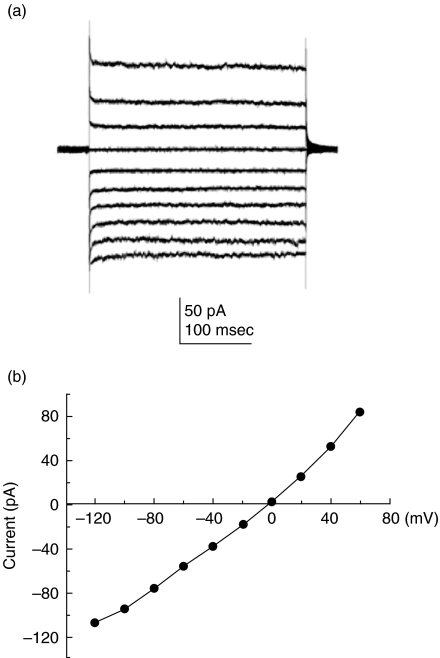
We investigated the presence of Cl– channel expression in freshly isolated human blood monocytes. Whole-cell Cl– currents and current versus voltage relationships are shown in Fig. 1. Cl– currents were isolated by adding Cs+ in the pipette and bathing medium to block K+ currents. The whole-cell Cl– currents were time-independent and outwardly rectifying (Fig. 1a). When Cs+ was replaced with NMDG+, a bulky cation that prevents contamination of cationic currents, the same electrophysiological properties of the whole-cell Cl– currents were observed (data not shown), indicating that the currents were carried by Cl– ions. The current amplitudes after establishment of the capacity transients were plotted as a function of voltage and exhibited outward rectification (Fig. 1b).
Fig. 1.
Expression of Cl– channels in human monocytes. (a) Families of whole-cell Cl– currents were recorded in freshly isolated human monocytes. The bathing medium and the pipette solution contained CsCl saline. From a holding potential (VH) of 0 mV, command voltage (VC) stepped from −100 mV to 60 mV applied every 1 s in 20 mV increments. (b) Current versus voltage relation was determined by measuring currents at 10 ms after the onset of the command voltages (n = 14).
Pharmacology of Cl– currents
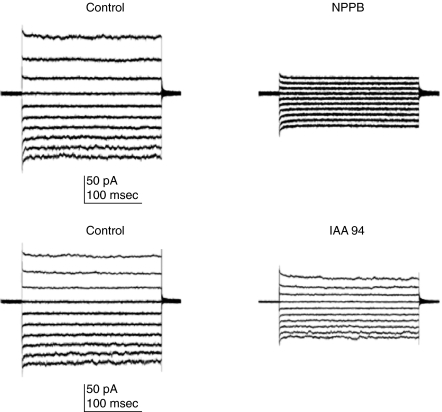
Pharmacological properties of Cl– currents were characterized (Fig. 2). NPPB and IAA94 are known to selectively block volume-sensitive Cl– currents in leucocytes [24]. Whole-cell recordings were established and Cl– currents were recorded (Fig. 2, control, left panel). After perfusing the bathing medium with 200 µm NPPB or IAA94, Cl– currents were recorded (Fig. 2, NPPB, IAA94, right panel). The representative traces of the current are shown and indicate that Cl– currents were inhibited by NPPB and IAA94. We also examined the effect of additional Cl– channel blockers such as DIDS, 9AC and gadolinium chloride. However, these Cl– channel blockers inhibited partially but not significantly Cl– currents (data not shown). The most probable explanation for this finding is the contribution of more than one type of Cl– channel to Cl– currents of monocytes.
Fig. 2.
Cl– currents are blocked by volume-sensitive channel blockers. The bathing medium and pipette solution contained CsCl saline. Families of whole-cell Cl– currents were recorded in human monocytes from a holding potential (VH) of 0 mV at command voltage (VC) steps from −100 mV to 60 mv applied every 1 s in 20 mV increments. Control whole-cell Cl– current were recorded before (left panel) and after perfusion of 200 µm NPPB (n = 7) or IAA94 (n = 6, right panel).
Cl– channel blocker NPPB induces monocytes shape change
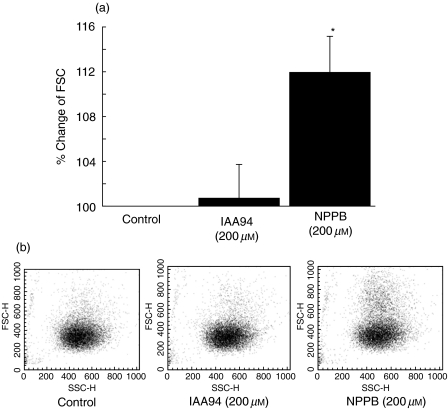
We used the FSC assay to measure the effect of NPPB and IAA94 on shape change of monocytes. The representative dot-plots are shown in Fig. 3, which depicts the shape change of monocytes in response to stimulation with chloride channel blocker, detected as an increase in mean FSC of monocytes. Following stimulation with NPPB (200 µm), the mean FSC of monocytes increased significantly. In comparison, IAA94 (200 µm) treatment did not affect the shape change of monocytes (Fig. 3a).
Fig. 3.
Effect of Cl– channel blockers on monocyte shape change. The resulting shape change of the monocyte was measured simultaneously, as described in the text. Results are expressed as percentage increase in FSC induced by each Cl– channel blocker compared with that of unstimulated cells (a). (b) Representative dot-plot of FSC versus SSC of 10 000 monocytes. The data are shown as the mean ± s.e.m. (n = 4–6).
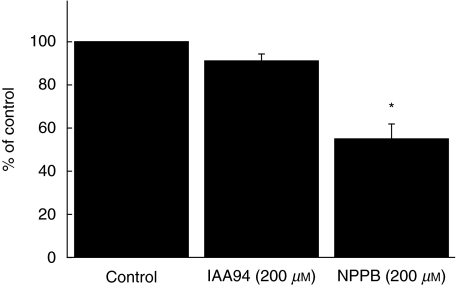
Cl– channel blockers inhibited chemotactic migration of monocytes
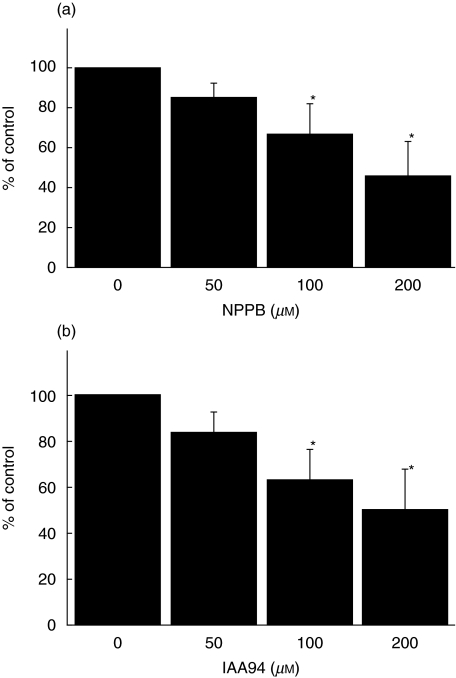
We investigated whether monocyte Cl– currents are functionally involved in migration. We used the Cl– channel blockers, NPPB and IAA94, to test functional role of Cl– channels in migration of monocytes by using a Boyden chemotaxis chamber (Fig. 4). MCP-1 was a potent chemokine to induce migration of monocytes. Both NPPB and IAA94 significantly inhibited MCP-1-induced chemotactic migration of monocytes in a dose-dependent fashion (Fig. 4).
Fig. 4.
Cl– channel blockers inhibit the chemotactic migration of monocytes. Freshly isolated human monocytes were used for chemotactic migration in 48-well Boyden microchambers. Twenty-six µl of MCP-1 solution (50 ng/ml) was added to the bottom wells of a chemotaxis chamber and then 50 µl of the cell suspension (1–1·5 × 106 cells/ml) was loaded into the top chamber. NPPB (a) or IAA94 (b) in several doses were applied to both bottom and top wells. Experiments were performed in triplicate and the data are shown as mean ± s.e.m. (n = 4). *P < 0·05.
Cl– channel blocker, NPPB, suppressed monocytes adhesion to HUVECs
Prior incubation of the cells with TNF-α (10 ng/ml) significantly increased the adhesion of monocytes to HUVECs. IAA94 did not affect monocyte adhesion to HUVECs. In comparison, NPPB significantly suppressed monocyte adhesion to HUVECs (Fig. 5).
Fig. 5.
Effect of Cl– channel blockers on monocyte adhesion to HUVEC. HUVECs were pre-incubated with 10 ng/ml TNF-α for 2 h. Adhesion of calcein AM-labelled monocytes after 60 min co-incubation with Cl– channel blockers was determined as described in the Methods section. The data are shown as mean ± s.e.m. (n = 6). *P < 0·05.
DISCUSSION
In the present study we investigated whether Cl– channels are expressed in normal human blood monocytes and examined whether Cl– channels play a role in the adhesion and migration of these cells. We observed that time-independent and outwardly rectifying Cl– currents were expressed in human blood monocytes and these currents were inhibited significantly by NPPB and IAA94, the Cl– channel blockers. Both NPPB and IAA94 also inhibited monocyte migration through artificial membranes of the Boyden chemotaxis chamber with similar potency. We also observed that NPPB changed the shape of monocytes and inhibited monocytes adhesion to HUVECs. To our knowledge this is the first report on the presence and functional role of Cl– channels in human blood monocytes.
Several studies have indicated that ionic leucocytes affect its mobility and influence transendothelial migration. Ransom and colleagues [25] reported that volume-activated chloride currents contributed to the invasive migration of human glioma cells. The migration of human glioma cells was modulated by chloride channel blocker such as NPPB [25,26]. Electrophysiological studies demonstrated that several types of voltage- and ligand-gated ion channels are expressed in leucocytes. An outwardly rectifying swelling-activated Cl-current has been well characterized in many cell types, including the T84 human adenocarcinoma cell line [27]. A few studies have examined the functional role of these ion channels in monocytes and monocyte-derived macrophages and indicate that these ion channels are involved in differentiation, production of nitric oxide and migration [28,29]. Migration of monocytes through changing cell shape and volume is critical for trafficking from/to vascular blood vessels and furthering progression of necrosis and apoptosis of vascular smooth muscle cells. Our data support the thesis and establish a functional role of Cl– channels in normal human blood monocytes.
Molecular mechanisms for Cl– channel-mediated migration of monocytes are still unclear and specific Cl– channel blockers have not been identified as yet. However, a sustained increase in intracellular Ca2+ is required for cell functions, such as cytoskeletal change for migration and gene expression for proliferation of leucocytes. K+ channels are up-regulated during activation, maturation or differentiation in monocyte-derived macrophages and human endothelium [13,30–32]. K+ channels may play a role in maintaining cell membrane potential for Ca2+ influx required for their physiological functions, including proliferation, differentiation and migration. The present study indicates that the outwardly rectifying Cl– channels may be involved in monocyte migration, presumably by establishing the driving force for Ca2+ influx required for migration.
We observed that NPPB but not IAA94 exhibited significant effect on monocyte shape change and adhesion to HUVECs. However, both NPPB and IAA94 suppressed monocyte migration induced by MCP-1. These results suggest a heterogeneity in Cl– channels in human blood monocytes and potentially more than one type of channel population might be involved in cell volume regulation and adhesion of human blood monocytes. It has been reported that NPPB can block the CIC2 type of Cl– channels. However, in view of the non-specificity of NPPB and IAA94, the exact mechanism of action of these channel blockers is still controversial. Further studies are warranted to clarify the molecular identity of volume-sensitive Cl– channels expressed in monocytes. Interestingly, Cl– channel blockers other than NPPB and IAA94 did not show any significant effect in attenuating Cl– currents in human blood monocytes.
Transendothelial migration of monocytes is a critical event in the initiation of occlusive vascular diseases. However, there is limited information available on the precise molecular and biochemical events that affect monocyte entry into the blood vessels during advanced stage of these diseases. What is known is that monocytes enter the blood vessels, take up ox-LDL and become foam cells, and activate the immune system. Thus, foam cells are the primary target of ox-LDL in occlusive vascular diseases. Ultimately an expanded reservoir of foam cells in the diseased arteries is produced by the large number of monocytes that cross vascular endothelial cells into the tissues. Cigarette smoke and immune-activated monocytes also serve as perpetrators of disease through their production of toxins, leading to endothelial cell injury. As monocytes differentiate into mature, non-dividing macrophages they can elicit a multitude of immune responses including chemotaxis, phagocytosis, antigen presentation, secretion of numerous cytokines, chemokines and other factors. The expression of membrane proteins during cell differentiation and activation is significant. We propose here that monocytes can adjust their cell shape and cell volume to facilitate invasion into blood vessels. These changes may require secretion of Cl– ions along with K+ to allow water loss and cell shrinkage. Clearly, the recognition of Cl– channels and their role in monocyte migration is the beginning of our understanding of mechanisms that permit the transendothelial migration of monocytes into the blood vessels.
In summary, for the first time we report the presence of volume-sensitive Cl– channels in human blood monocytes that regulate migration of these cells. Cell migration is an orchestrated process requiring adhesion of cells into tissue, adjustment of cell shape and generation of locomotion into the narrow space. Migration of inflammatory cells can provide valuable information for therapeutic interventions to target various diseases, including atherosclerosis and restenosis, where onset is due to their recruitment and activation.
Acknowledgments
This work was supported by National Institutes of Health grants R01HL070885 and R01HL073349.
REFERENCES
- 1.Osterud B, Bjorklid E. Role of monocytes in atherogenesis. Physiol Rev. 2003;83:1069–112. doi: 10.1152/physrev.00005.2003. [DOI] [PubMed] [Google Scholar]
- 2.Niessen HW, Krijnen PA, Visser CA, et al. Type II secretory phospholipase A2 in cardiovascular disease: a mediator in atherosclerosis and ischemic damage to cardiomyocytes? Cardiovasc Res. 2003;60:68–77. doi: 10.1016/s0008-6363(03)00324-9. [DOI] [PubMed] [Google Scholar]
- 3.Becker RC, Eisenberg P, Turpie AG. Pathobiologic features and prevention of thrombotic complications associated with prosthetic heart valves: fundamental principles and the contribution of platelets and thrombin. Am Heart J. 2001;141:1025–37. doi: 10.1067/mhj.2001.115492. [DOI] [PubMed] [Google Scholar]
- 4.Grinstein S, Foskett JK. Ionic mechanisms of cell volume regulation in leukocytes. Annu Rev Physiol. 1990;52:399–414. doi: 10.1146/annurev.ph.52.030190.002151. [DOI] [PubMed] [Google Scholar]
- 5.McManus ML, Churchwell KB, Strange K. Regulation of cell volume in health and disease. N Engl J Med. 1995;333:1260–6. doi: 10.1056/NEJM199511093331906. [DOI] [PubMed] [Google Scholar]
- 6.Lang F, Lepple-Wienhues A, Paulmichl M, Szabo I, Siemen D, Gulbins E. Ion channels, cell volume, and apoptotic cell death. Cell Physiol Biochem. 1998;8:285–92. doi: 10.1159/000016290. [DOI] [PubMed] [Google Scholar]
- 7.Soroceanu L, Manning TJ, Sontheimer H. Modulation of glioma cell migration and invasion using Cl(−) and K(+) ion channel blockers. J Neurosci. 1999;19:5942–53. doi: 10.1523/JNEUROSCI.19-14-05942.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gallin EK. Ion channels in leukocytes. Physiol Rev. 1991;71:775–811. doi: 10.1152/physrev.1991.71.3.775. [DOI] [PubMed] [Google Scholar]
- 9.Kanno T, Takishima T. Chloride and potassium channels in U937 human monocytes. J Membr Biol. 1990;116:149–61. doi: 10.1007/BF01868673. [DOI] [PubMed] [Google Scholar]
- 10.Holevinsky KO, Jow F, Nelson DJ. Elevation in intracellular calcium activates both chloride and proton currents in human macrophages. J Membr Biol. 1994;140:13–30. doi: 10.1007/BF00234482. [DOI] [PubMed] [Google Scholar]
- 11.Kim SY, Silver MR, DeCoursey TE. Ion channels in human THP-1 monocytes. J Membr Biol. 1996;152:117–30. doi: 10.1007/s002329900091. [DOI] [PubMed] [Google Scholar]
- 12.Fierro L, Parekh AB. On the characterisation of the mechanism underlying passive activation of the Ca2+release-activated Ca2+ current ICRAC in rat basophilic leukaemia cells. J Physiol. 1999;520:407–16. doi: 10.1111/j.1469-7793.1999.00407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colden-Stanfield M, Gallin EK. Modulation of K+ currents in monocytes by VCAM-1 and E-selectin on activated human endothelium. Am J Physiol. 1998;275:C267–77. doi: 10.1152/ajpcell.1998.275.1.C267. [DOI] [PubMed] [Google Scholar]
- 14.DeCoursey TE, Kim SY, Silver MR, et al. Ion channel expression in PMA-differentiated human THP-1 macrophages. J Membr Biol. 1996;152:141–57. doi: 10.1007/s002329900093. [DOI] [PubMed] [Google Scholar]
- 15.Brown H, Kozlowski R, Perry H. The importance of ion channels for macrophage and microglial activation in vitro. Glia. 1998;22:94–7. [PubMed] [Google Scholar]
- 16.Floto RA, Mahaut-Smith MP, Allen LM, et al. Differentiation of the human monocytic cell line U937 results in an upregulation of the calcium release-activated current, ICRAC. J Physiol. 1996;495:331–8. doi: 10.1113/jphysiol.1996.sp021597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chung I, Zelivanskaya M, Gendelman HE. Molecular phagocyte biophysiology influences brain transendothelial and tissue migration: implication for HIV-1-associated dementia. J Neuroimmunol. 2002;122:40–51. doi: 10.1016/s0165-5728(01)00462-3. [DOI] [PubMed] [Google Scholar]
- 18.Balaram SK, Agrawal DK, Edwards JD. Insulin like growth factor-1 activates nuclear factor-kappaB and increases transcription of the intercellular adhesion molecule-1 gene in endothelial cells. Cardiovasc Surg. 1999;7:91–9. doi: 10.1016/s0967-2109(98)00044-1. [DOI] [PubMed] [Google Scholar]
- 19.Hamill OP, Marty A, Neher E, et al. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 20.Sabroe I, Conroy DM, Gerard NP, et al. Cloning and characterization of the guinea pig eosinophil eotaxin receptor, C-C chemokine receptor-3: blockade using a monoclonal antibody in vivo. J Immunol. 1998;161:6139–47. [PubMed] [Google Scholar]
- 21.Phillips RM, Stubb VEL, Henson MR, et al. Variations in eosinophil chemokine responses: an investigation of CCR1 and CCR3 function, expression in atopy, and identification of a functional CCR1 promoter. J Immunol. 2003;170:6190–201. doi: 10.4049/jimmunol.170.12.6190. [DOI] [PubMed] [Google Scholar]
- 22.Sozzani SM, Molinon M, Locati W, et al. Receptor-activated calcium influx in human monocytes exposed to monocyte chemotactic protein-1 and related cytokines. J Immunol. 1993;150:1544–53. [PubMed] [Google Scholar]
- 23.Kang JS, Park SK, Yang KH, et al. Silymarin inhibits TNF-alpha-induced expression of adhesion molecules in human umbilical vein endothelial cells. FEBS Lett. 2003;550:89–93. doi: 10.1016/s0014-5793(03)00827-5. [DOI] [PubMed] [Google Scholar]
- 24.Schlichter LC, Sakellaropoulos G, Ballyk B, et al. Properties of K+ and Cl– channels and their involvement in proliferation of rat microglial cells. Glia. 1996;17:225–36. doi: 10.1002/(SICI)1098-1136(199607)17:3<225::AID-GLIA5>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 25.Ransom CB, O'Neal JT, Sontheimer H. Volume-activated chloride currents contribute to the resting conductance and invasive migration of human glioma cells. J Neurosci. 2001;21:7674–83. doi: 10.1523/JNEUROSCI.21-19-07674.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soroceanu L, Manning TJ, Jr, Sontheimer H. Modulation of glioma cell migration and invasion using Cl(−) and K(+) ion channel. J Neurosci. 1999;19:5942–54. doi: 10.1523/JNEUROSCI.19-14-05942.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Valverde MA, Mintenig GM, Sepulveda FV. Cl- currents of unstimulated T84 intestinal epithelial cells studied by intracellular recording. J Membr Biol. 1993;137:237–47. doi: 10.1007/BF00232592. [DOI] [PubMed] [Google Scholar]
- 28.Strange K, Emma F, Jackson PS. Cellular and molecular physiology of volume-sensitive anion channels. Am J Physiol. 1996;270:C711–30. doi: 10.1152/ajpcell.1996.270.3.C711. [DOI] [PubMed] [Google Scholar]
- 29.Sheppard DN, Valverde MA. Therapeutic potentials of chloride channel modulator. ID Res Alert. 1996;2:111–23. [Google Scholar]
- 30.Lin TA, Lustig KD, Sportiello MG, et al. Signal transduction pathways coupled to a P2U receptor in neuroblastoma × glioma (NG108-15) cells. J Neurochem. 1993;60:1115–25. doi: 10.1111/j.1471-4159.1993.tb03262.x. [DOI] [PubMed] [Google Scholar]
- 31.Lewis RS, Cahalan MD. Potassium and calcium channels in lymphocytes. Annu Rev Immunol. 1995;13:623–53. doi: 10.1146/annurev.iy.13.040195.003203. [DOI] [PubMed] [Google Scholar]
- 32.Nelson DJ, Jow B, Jow F. Lipopolysaccharide induction of outward potassium current expression in human monocyte-derived macrophages: lack of correlation with secretion. J Membr Biol. 1992;125:207–18. doi: 10.1007/BF00236434. [DOI] [PubMed] [Google Scholar]