Abstract
CD26 truncates several chemokines as well as neuropeptides and influences immune responses via modulation of cell adhesion and T cell activation, suggesting an involvement of CD26 in asthmatic and airway inflammation. Therefore, Fischer 344 (F344), Brown Norway (BN) and Lewis (LEW) rat strains, which differ in their CD26-like enzymatic activity, were compared using an asthma model. Additionally, two CD26-deficient mutant F344 rat substrains were included and compared to the wild-type F344 substrain. Immunization was performed twice with ovalbumin (OVA), and 2 weeks later the rats were challenged with OVA intratracheally Flow cytometry (FACS) analysis of different leucocyte subsets as well as enzyme-linked immunosorbent assay (ELISA) for IgE levels in the blood and bronchoalveolar lavage (BAL) were performed 24 h after challenge. LEW rats with the lowest CD26 activity among the rat strains investigated here displayed significantly reduced CD4+ T cell numbers in the BAL compared to wild-type F344 and BN rats. Moreover, in asthma, the ratio of CD26+ to CD26– T cell receptor (TCR)-positive cells increased significantly in F344 and LEW but not BN rats. Most intriguingly, in both CD26-deficient F344 rat substrains the number of CD4+ T lymphocytes was markedly reduced compared to wild-type F344. The decrease in T cell recruitment observed in the CD26-deficient rats was associated with significantly reduced OVA-specific IgE-titres. This is the first report to show a remarkably reduced T cell recruitment in rat strains that either lack or exhibit reduced CD26-like enzymatic activity, suggesting a role for CD26 in the pathogenesis of asthma via T cell-dependent processes such as antibody production.
Keywords: brown Norway rats, CD4+ T lymphocytes, eosinophils, Fischer F344 rats, Lewis rats
Introduction
CD26 exerts several biological functions, such as proteolytic activity (also named dipeptidyl-peptidase/DPP IV, EC.3.4.14.5), regulation of cell adhesion and the ability to act as a co-stimulatory protein [1]. With regard to its role in cell adhesion, it has been demonstrated that an expression of CD26 on the endothelium of lung capillaries accounts for arrest of blood-borne breast cancer cells in vitro/ex vivo via binding of extracellular matrix protein and that metastasis is reduced in a CD26-deficient F344 rat substrain [2,3]. CD26 is also expressed on a fraction of resting T cells at low density but is strongly up-regulated following T cell activation [4,5]. Despite its small cytoplasmatic region of only six amino acids, CD26 delivers potent co-stimulatory activation signals into T cells. It associates with CD45 [6] and with adenosine deaminase (ADA), the deficiency of which causes severe combined immunodeficiency disease (SCID) in humans. Moreover, CD26 signalling is dependent on the expression of the T cell receptor (TCR) complex [4,5].
In the pathogenesis of asthma the dual role of Th2 lymphocytes and eosinophils is now widely accepted to be critical for the onset and the progression of the pulmonary pathology [7–9]. There is considerable evidence that T lymphocyte subpopulations and eosinophils communicate both by means of direct cell–cell interactions and through the secretion of inflammatory signals [7]. For example, cytokines such as interleukin (IL)-5 are released upon Th2 cell activation, increasing the production of eosinophils in the bone marrow and their release into the circulation [10]. However, the role of CD26 as a co-stimulatory factor in T cell activation as adhesion molecule and ectopeptidase involved in the truncation of certain chemokines has not yet been investigated with regard to the pathogenesis of asthma.
The severity of the ovalbumin (OVA)-induced airway inflammation − an asthma model in rats − is strain-dependent, e.g. in genetically Th2-predisposed BN rats the allergic-like response is more pronounced compared to that in Sprague–Dawley (SD) [11], Fischer 344 (F344) or Lewis (LEW) rats [12]. Notably, the more susceptible strains exhibit higher CD26-like enzymatic activity. In previous work [13] we have described differences of CD26-like enzymatic activity in several rat strains, and the degree of enzymatic activity seems, a priori, to correlate with the degree of pathological changes in asthma models. Among 12 investigated rat strains, BN rats exhibited the highest enzymatic activity, F344 rats showed intermediate activity, whereas the lowest endogenous CD26-like enzymatic activity was found in LEW [13]. However, all rat strains exhibited significant CD26 protein expression. Importantly, we also characterized two CD26-deficient F344 substrains which, among other differences [13,14], not only lack endogenous CD26-like enzymatic activity due to a spontaneous point mutation [15], but also exhibit a dramatically reduced expression of the mutant CD26-like protein in the lungs [2]. These rats may therefore be regarded as a spontaneous CD26 knock-out rat model [2,3] and represent a valuable tool to investigate how CD26 may contribute to the pathogenesis of asthma. Consequently, we compared F344, LEW and BN rats directly and also included the two mutant CD26-deficient F344 rat substrains in order to differentiate between the effects of lacking and/or reduced CD26-like enzymatic activity, on one hand, and different CD26 protein expression on the other hand.
Materials and methods
Animals and experiments
Wild-type F344 rat substrains from Charles River (CR) USA [F344/Crl(Por)] as well as BN and LEW rats were obtained from a breeding colony kept in barrier-reared conditions at the Central Animal Laboratory of the Medical School of Hannover, Germany. The CD26-deficient F344 rat substrains derived initially from CR colonies in Germany [F344/Crl(Ger/DPPIV-)] and Japan [F344/DuCrj(DPPIV-)] (for rat nomenclature see [13]). Rats were kept in a specific pathogen-free facility at 25°C under a 12-h light−12-h dark cycle (lights on at 0700 h), with ad libitum access to food and water. Two different experiments were conducted. First, wild-type F344, BN and LEW rats were immunized and compared with controls. In a second experiment, the two CD26-deficient F344 substrains were immunized and compared with the wild-type substrain. For the experiments age-matched 4-month-old male rats were used. Plasma of each individual F344 rat from the three F344 substrains was measured for CD26-like (DPPIV-like) enzymatic activity in order to confirm the phenotype, as described previously [13]. All research and animal care procedures were approved by the Review Board for the Care of Animal Subjects of the district government, Hannover, Germany, and performed according to international guidelines for the use of laboratory animals.
Sensitization and allergen challenge
The rats were sensitized as described previously [16]. In brief, sensitization was performed with 1 mg of OVA (Sigma, Deisenhofen, Germany) and 200 mg of Al(OH)3 (Sigma) in 1 ml 0·9% (sterile, pyrogen-free) NaCl applied subcutaneously. As the second adjuvant, concentrated preparations of 5 × 109 heat-killed Bordetella pertussis bacilli (kindly donated by the manufacturers, Chiron Behring, Marburg, Germany) in 0·4 ml 0·9% NaCl were given intraperitoneally at the same time. These injections were repeated 6–7 days after the first sensitization. After another 6–7 days the animals were challenged with 300 µl of a 0·5% OVA/saline solution. For this intratracheal instillation a short combined isoflurane oxygen inhalation anaesthesia was performed, and the rats were suspended in a hanging position by a rubber band fixed to the incisor teeth of the upper jaw [17]. The trachea was intubated via the oral cavity, and before OVA instillation the lungs were blown up with air to check the correct position of the tube. In each rat strain sham controls were included, i.e. half the rats received saline instead of OVA and B. pertussis.
Dissection of animals and isolation of BAL and lung tissue leucocytes
The animals were dissected under isoflurane anaesthesia 22 ± 2 h after intratracheal challenge, as described by Schuster et al. [16]. Briefly, the abdominal wall was opened and the animals were killed by aortic exsanguination. A cannula was inserted into the trachea in situ and the lungs were lavaged with portions of 5 ml cold (4°C) NaCl. The fluid was retrieved by gentle aspiration and this procedure was repeated 10 times. The recovery of fluid was over 90% in all animals. The BAL was pooled, centrifuged (400 g, 10 min) and the cell pellet was resuspended in 1 ml phosphate-buffered saline (PBS) [Seromed, Berlin, Germany; containing 1% bovine serum albumin (BSA), Merck, Darmstadt, Germany; and 0·1% sodium azide (NaN3), Sigma]. For lung cell extraction a mechanical disaggregation method was used. The trachea, main bronchi and hilar lymph nodes were removed from the rest of the lung tissue, and the left lungs were used for analysis of the lung cells. The complete lung tissue was disaggregated by passing it through a metal sieve with two rounded tweezers and rinsed with 40 ml PBS. Cells were separated from debris by passing through a 75 µm nylon mesh and centrifuged (400 g, 10 min). The cell pellet was finally resuspended in 1 ml PBS.
Cell counts, staining for eosinophils, immunostaining of lymphocyte subsets and flow cytometry (FACS) analysis
Leucocyte numbers were determined via staining with Tuerk's solution (Merck) in a Neubauer counting chamber. The eosinophil cell count was assessed on slides prepared by centrifuging 1 × 105 cells in anticoagulant [100 ml 0·9% NaCl; 100 mg ethylenediamine–tetra-acetic acid (EDTA); 5 g BSA; pH = 7·2] for 8 min at 800 g on a cytospin centrifuge (Shandon, Pittsburgh, PA, USA). After a Pappenheim (combined May–Grünwald–Giemsa) staining (Riedel de Haen, Seelze, Germany), eosinophils were identified under the light microscope at ×1000 magnification. At least 1000 cells were differentiated on each slide by two independent observers.
For FACS analysis, parts of the cell solution were transferred to a microtitre plate (Greiner, Solingen, Germany; with approximately 1 × 106 cells in each well) and washed twice with 100 µl PBS, including incubation with 50 µl human serum (1 : 10) for 5 min (also described in [17]). The following mouse antirat fluorescein isothiocyanate (FITC)-conjugated primary monoclonal antibodies (MoAb) (Serotec, Oxford, UK) were used for incubation at 4°C for 30 min in 50 µl portions: MoAb R73 for αβTCR+ cells, MoAb Ox12 for IgM+ B cells, MoAb 3.2.3bright for natural killer (NK) cells, MoAb W3/25high for CD4+ cells and MoAb Ox8 for CD8+ cells. Isotype-matched antibodies served as controls. As all cells were double-stained for CD26+, MoAb Ox61 (Serotec) was used as unconjugated additional primary antibody. For the detection of Ox61 a phycoerythrin (PE)-conjugated secondary antimouse antibody was used (kappa PE; Dianova, Hamburg, Germany). This incubation was performed for 30 min at 4°C after another two washing procedures. Finally, the cells were washed three times, resuspended in 200 µl PBS and analysed on a FACscan flow cytometer (Becton Dickinson, Mountain View, CA, USA) focusing on the lymphocyte cluster. Viability was determined by adding 25 µl propidium iodide (1 µl/ml; Sigma) to 200 µl unstained cell solution. At least 10 000 events were counted in the previously defined lymphocyte gate and the percentage was calculated for each lymphocyte subset.
Detection of total IgE and OVA-specific IgE
Enzyme-linked immunosorbent assay (ELISA) plates were coated with anti-IgE (for total IgE) and 500 µg/ml OVA in PBS (for OVA-specific IgE) overnight at 4°C. After blocking with 3% BSA in PBS, serial dilutions of the plasma and BAL collected 24 h after challenge of additional F344/Crl(Por) (n = 6) and F344/Crl(Ger/DPPIV-) (n = 5) rats or PBS were added to the plates. Bound IgE was detected using HRP-conjugated anti-IgE MoAb. After addition of substrate reagents, antibody titres were estimated on the basis of dilutions/absorbance curves (450 nm). All antibodies were purchased from Serotec (Düsseldorf, Germany).
Detection of cytokines
The plasma concentrations of IL-4 and interferon (IFN)-γ were measured by ELISA according to the manufacturer's instructions (DuoSet ELISA Development Kit, R&D Systems, Wiesbaden, Germany). In brief, plates were coated with mouse antirat IL-4 antibodies or mouse antirat IFN-γ antibodies (each 2 µg/ml) overnight at room temperature. After blocking with 1% BSA/5% sucrose in PBS with 0·05% NaN3, 100 µl of the samples (diluted 1 : 1 in 1% BSA in PBS, pH 7·2–7·4) or the standards were added to each well. Bound cytokines were detected with a biotinylated goat antirat IL-4 antibody (50 ng/ml) or a biotinylated goat antirat IFN-γ antibody (150 ng/ml). After repeated washing steps, 100 µl of streptavidin-HRP (diluted 1 : 200 in 1% BSA in PBS) were added to each well. Subsequently, samples and standards were incubated with 100 µl of substrate solution (1 : 1 mixture of colour reagent A containing H2O2 and colour reagent B containing tetramethylbenzidine) (R&D Systems) followed by adding 50 µl of stop solution to each well (2 N H2SO4). The optical density of each well was determined using a microplate reader set at 450 nm.
Statistical analysis
Statistical analysis was performed using either two-way analysis of variance (anova) with the factors ‘strain (BN, LEW, or F344)’ and ‘asthma (yes or no)’ or one-way anova (factor: ‘asthma’) followed by the Fisher test for post hoc comparison, if appropriate. All data are given as arithmetic mean ± standard error of the mean (s.e.m.), the latter represented by error bars. P-values < 0·05 were considered to indicate a statistically significant difference versus sham controls or versus the control strain, as indicated in the the figure legends.
Results
Sensitized F344 and BN but not LEW rats show increased T cell numbers in the BAL compared to non-sensitized controls
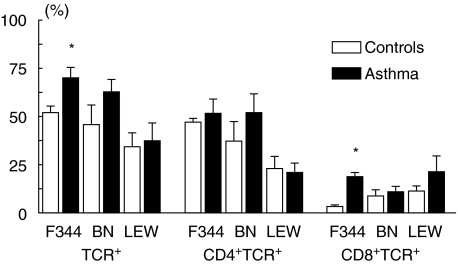
For the BAL, two-way anova revealed significant differences in the percentage of TCR+ lymphocytes for both factors analysed, rat ‘strain’ (P = 0·006) as well as ‘asthma’ (P = 0·04), but no significant interaction was found. The subsequent post hoc analysis for the factor ‘strain’ revealed significant differences between BN versus LEW (P = 0·02) as well as F344 versus LEW (P = 0·001), indicating that in LEW rats TCR+ cells are on a much lower level compared with BN and F344 rats. Separate one-way anova for each strain showed that this difference was due to an asthma-induced increase of BAL TCR+ cells mainly in BN and F344 rats. This differential effect became significant in the post hoc analysis only for the F344 strain (P = 0·04) (Fig. 1). In addition, it should be mentioned that in the lung parenchyma BN rats also showed significantly higher TCR+ cell numbers compared to the non-sensitized controls (P = 0·003).
Fig. 1.
T cell receptor positive (TCR+), CD4+TCR+ and CD8+TCR+ cells in the BAL of Fischer 344 (F), Brown Norway (BN) and Lewis (LEW) rats after sensitization and challenge with OVA (asthma group; black bars) or application of saline according to the same protocol (control group; open bars). All data derived from FACS analysis and represent relative cell counts in percentage of lymphocytes. Data represent mean ± s.e.m. of three to five rats per group. *P < 0·05.
For CD4+TCR+ cells, significant strain-dependent differences were also found (P = 0·005). In the subsequent post hoc analysis for the factor ‘strain’ BN and F344 rats both showed significantly higher percentages of CD4+TCR+ cells compared to LEW (P = 0·01, P = 0·001). For CD8+TCR+ the factor ‘asthma’ showed a significant increase of cells in F344 rat BAL (P = 0·0007).
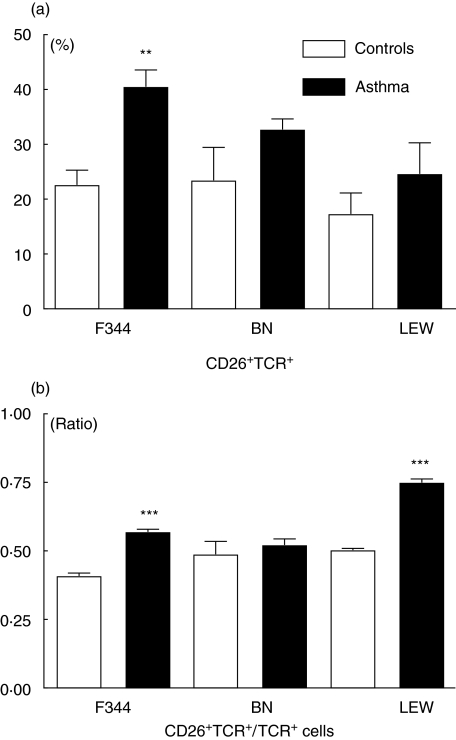
Double-staining shows a higher CD26+TCR+/TCR+ cell ratio in sensitized compared to non-sensitized F344 and LEW but not BN rats
In Fig. 2a the percentage of CD26+TCR+ positive cells is shown comparing challenged rats with sham controls. These cell numbers were related to TCR single positive cells (Fig. 2b): one-way anova revealed a significant difference in F344 and LEW (F344: P = 0·0001; LEW: P = 0·0007), indicating an up-regulation of CD26 in sensitized rats. However, in the BN rat strain there was no difference in the ratio of CD26+TCR+ cells to TCR+ cells.
Fig. 2.
Percentage (a) of CD26+TCR+ cells and relative ratio (b) of CD26+TCR+ divided by single TCR+ cells in the BAL of Fischer 344 (F), Brown Norway (BN) and Lewis (LEW) rats after sensitization and challenge with OVA (asthma group; black bars) or application of saline according to the same protocol (control group; open bars). **P < 0·01; ***P < 0·001.
Reduced CD4+ T cell counts in the BAL of sensitized CD26-deficient F344 rats
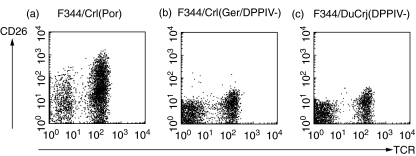
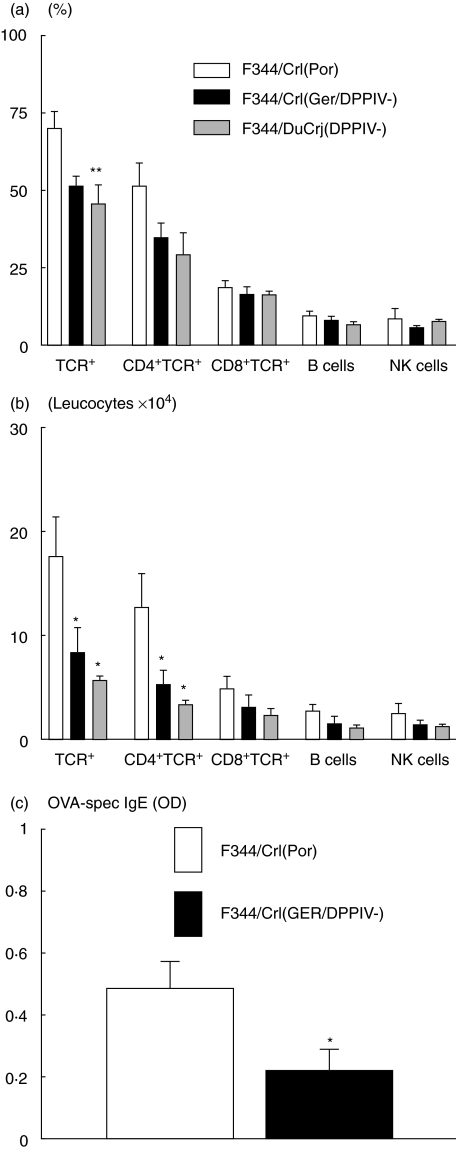
All cells in the BAL of the F344/Crl(Ger/DPPIV-) and F344/DuCrj(DPPIV-) rat substrains were CD26– (see representative CD26/TCR dot plots in Fig. 3). Along with the lack of enzymatic activity (data not shown), these results confirm the phenotype of both CD26-deficient rat substrains, i.e. illustrating the lack of enzymatic activity as well as protein expression. Furthermore, in sensitized rats FACS analysis showed reduced TCR+ cell numbers in both CD26-deficient F344 rat substrains compared to the wild-type F344 rats. There was a significant difference of about one-third in the F344/DuCrj(DPPIV-) substrain (P = 0·008), as the relative count of TCR+ cells was reduced from about 70% to 45% of all lymphocytes (Fig. 4a). For the other leucocyte subsets investigated, including CD4+TCR+, CD8+TCR+ cells, B and NK cells, no significant result was found. However, comparing the absolute cell numbers (Fig. 4b), not only the amount of TCR+ but also CD4+TCR+ cells was only half the cell count in the CD26-deficient rats compared to the wild-type substrain {TCR+: P = 0·03 [F344/Crl(Ger/DPPIV-)], P = 0·01 [F344/DuCrj(DPPIV-)]; CD4+TCR+: P = 0·05 [F344/Crl(Ger/DPPIV-)], P = 0·01 [F344/DuCrj(DPPIV-)]}. In the cytospins there was no statistically significant difference of eosinophil numbers between the three groups at this early time-point after challenge (data not shown).
Fig. 3.
Flow cytometry dot plot examples of double stainings for CD26 (MoAb Ox61) and T cell receptor (TCR; MoAb R73) in the BAL of F344 CD26-positive wild-type control rats (F344/Crl(Por) (a), and the CD26-deficient substrains F344/Crl(Ger/DPPIV-) (b) and F344/DuCrj(DPPIV-) (c).
Fig. 4.
Different leucocyte subsets in the BAL of F344/Crl(Por) (controls) versus F344/Crl(Ger/DPPIV-) and F344/DuCrj(DPPIV-) rats after sensitization and challenge with OVA. Cells were detected in FACS analysis, and bars show relative (a) and absolute (b) cell counts. OVA-specific IgE-levels in wild-type versus CD26-deficient F344 rats (c). Data represent mean ± s.e.m. of four to five rats per group. *P < 0·05; **P < 0·01.
The CD26-deficient F344 rats show lower IgE-levels compared with the wild-type F344 strain
As shown in Fig. 4c, wild-type F344/Crl(Por) show significantly higher OVA-specific IgE-levels in the blood serum 24 h after challenge (P = 0·03). The different cytokines measured did not show detectable levels of either IL-4 or IFN-γ (data not shown).
Discussion
As reported previously, several rat strains and substrains exhibit dramatic differences in CD26-like enzymatic activity and protein expression [13]. Furthermore, these strains also differ in the degree of airway inflammation prevalent in rat asthma models [11,12]. In the present study, these observations were linked in a way that LEW rats expressing the lowest endogenous CD26 enzymatic activity as well as two mutant CD26-deficient F344 rat substrains showed the lowest asthmatic-like reaction with regard to T cell recruitment into the BAL and IgE-levels. Moreover, in wild-type F344 and LEW rats these T cell changes involved mainly CD26-positive T lymphocytes. It is widely accepted that changes in T lymphocyte numbers play an important role in the pathogenesis of asthma [7]. In the present study, the main finding of a reduced CD4+ T cell increase in challenged CD26-deficient F344 rats suggests a blunted inflammatory status. This is, per se, a novel and remarkable finding, indicating that CD26 [1, 4, 5] is a necessary prerequisite for constituting a full-blown T cell component in response to the challenge with antigens such as OVA. It is necessary to mention that all results in LEW and BN rats that are not clearly CD26+ or CD26– remain on the level of an association-like observation and lack the possibility of providing a causal connection.
In order to explain the results of the present study, the role of T lymphocytes and cytokines in the pathogenesis of asthma can be summarized as follows [8]: Th2 CD4+ cells secrete type 2 cytokines that promote airway eosinophilia (IL-5) and immunoglobulin isotype switching to IgE (IL-4, IL-13) [18,19]. IL-4, IL-13 and transforming growth factor β (TGF-β) are secreted additionally by eosinophils as well as IgE-activated mast cells and exert direct effects on fibroblasts, epithelial cells and airway smooth muscle. This leads in turn to the release of growth factors and fibrogenic factors involved in remodelling, airway hyperresponsiveness (AHR) and airway narrowing. For example, in vitro, both T cell-derived IL-5 as well as IL-13 have been shown to have the ability to increase smooth muscle contractility to acetylcholine [20,21]. Under these circumstances, it is clear that functionally impaired T lymphocytes or an imbalance of type 2 cytokines might influence the orchestra of asthmatic airway inflammation. As it has been shown that CD26 is an important activation marker for T lymphocytes [22], it can be speculated that CD26– T cells might be unable to contribute to asthmatic hypersensitivity reactions in a normal way, as has been described for other disease models. In multiple sclerosis patients, a change in the percentage of T cells expressing the activation marker CD26 correlated significantly with a change in lesion volume or in the number of lesions detected on MRI [23]. Patients with psoriasis vulgaris and atopic dermatitis showed a significant reduction of CD26 expression on CD8+ T cells [24]. Therefore, CD26– T cells might represent an inactive state with decreased cytokine production and, moreover, impaired adhesion properties. Taken together, this may result in impaired extravasation of T cells towards the bronchoalveolar space and explain the lower T cell numbers in the CD26-deficient rat substrains of the present study.
In the case of asthmatic reactions this finding might be advantageous and offer new therapeutic strategies, for example with specific inhibitors of CD26. Using such inhibitors, T cell proliferation can be suppressed in vitro, and antibody production in mice immunized with BSA decreases [25]. It has been shown in other disease models that potent inhibitors of CD26 are able to cause similar effects to those shown in the spontaneously mutant substrains used in this study. In experimental autoimmune encephalomyelitis pharmacological inhibition of CD26 resulted in the suppression of autoreactive T cell proliferation, and clinical and neuropathological signs of the disease were delayed [26]. CD26-inhibitors also caused a marked suppression of paw swelling in rats with adjuvant arthritis due to antiproliferative effects on T cells [27]. Despite these promising findings towards a general immunomodulatory role of DC26 inhibitors, it is important to mention that so far the subpopulation of CD26bright T cells has been shown to be responsible for most of the IL-2 production, and in vivo observations support the concept that CD26 expression is a marker for Th1 cells (for review see [28]). It is, however, also possible that CD26 exerts special function on subsets of T cells including T regulatory cells. This latter hypothesis might also explain the differential production of IgE described in the present study. It is known that human B lymphocytes activated by CD40 cross-linking in the presence of IL-4 or IL-13 switch from IgM to IgE [29]. As these cytokines are secreted by Th2 CD4+ cells, the possible role of CD26+ T cells as activated subtypes of T lymphocytes might contribute to this IgM–IgE switch to a greater and more effective extent. So far, those CD4+ T cells have not been characterized with regard to CD26 and might be the same CD4+TCR+ cells that we show in the BAL of our CD26+ F344/Crl(Por) rats.
There is convincing evidence that mediators such as the CC chemokines eotaxin and RANTES also act in synergy with type 2 cytokines to recruit eosinophils into asthmatic airways [30]. Furthermore, macrophage-derived cytokines such as tumour necrosis factor alpha (TNF-α) and IL-1β facilitate extravasation of eosinophils and other leucocytes in part by up-regulating cellular adhesion molecules such as intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) [31]. Some of these mediators, such as eotaxin, RANTES and IL-1β, are actually substrates of the peptidase CD26 (for review see [1]). If there is a lack of CD26 enzymatic activity, as has been demonstrated for the two deficient rat substrains of the present study, the truncation of such mediators is most probably altered, also possibly resulting in differential distribution patterns of leucocyte subsets.
Surprisingly, the substantial changes in T cell recruitment did not yield corresponding changes in eosinophil numbers. In several previous reports on the clinical course of asthma in rat models, the 24-h time-point after challenge was chosen for analysis of inflammatory changes in the BAL and lung tissue (e.g. [16]), which is consistent with the protocol of the present investigation. In a kinetic study in a BN rat asthma model, lung tissue eosinophils increased significantly after 24 h, but with time the differences became increasingly pronounced until 72 h [32]. Schneider et al. [12] also described an accumulation peak of eosinophil numbers in the BAL of BN rats at 72 h. As there were no differences in eosinophil numbers in the F344 rat substrains of the present study, this might be due to the fact that the BAL was taken only 24 h after challenge. However, Schneider et al. [12] reported that in F344 and LEW rats essentially no pulmonary inflammation was observed. In contrast to these findings, increased eosinophil numbers of up to 10% are detectable in the BAL of F344 rats in our hands, being indicative of an allergic-like effect of immunization. Apart from this, the pattern of leucocyte distribution completely changes in the BAL of sensitized F344 rats compared to saline-treated controls despite all the well-known reasons arguing against rodent asthma models in general [33]. Moreover, different physiological parameters showed increased airway smooth muscle responsiveness in F344 compared to LEW rats [34]. We therefore believe that the lack of significant differences in eosinophil numbers in the present study is due to the early time-point and that not only BN but also other rat strains exhibit allergic-like cell compositions within the BAL including significant eosinophil numbers. Supporting the presence of allergic-like reactions, we found that IgE-levels were significantly correlated in a way that CD26-deficient rats did not only show less inflammatory effects represented by lower T cell counts but also lower serum-specific IgE-levels. The significance of IgE levels under allergic conditions is further underlined by the demonstration that atopic patients show a correlation between IgE serum levels and the prevalence or severity of asthmatic symptoms [35].
In conclusion, we have demonstrated for the first time that CD26 is directly associated with T cell recruitment and IgE production in a model of airway inflammation, suggesting that the use of CD26 inhibitors might prove a new therapeutic approach to the escalating burden of asthma and airway inflammation.
Acknowledgments
The authors thank M. Heuer, K. Westermann, S. Kuhlmann, and S. Faßbender for excellent technical assistance, F. Bühling for helpful comments and S. Fryk for correction of the English. Grants from the Deutsche Forschungsgemeinschaft (SFB 587/B1) (to R. P.) and the Medical School of Hannover (HILFII; to S. v. H.) are gratefully acknowledged.
References
- 1.Mentlein R. Dipeptidyl-peptidase IV (CD26)-role in the inactivation of regulatory peptides. Regul Pept. 1999;85:9–24. doi: 10.1016/s0167-0115(99)00089-0. [DOI] [PubMed] [Google Scholar]
- 2.Shingu K, Helfritz A, Zielinska-Skowronek M, et al. CD26 expression determines lung metastasis in mutant F344 rats: involvement of NK cell function and soluble CD26. Cancer Immunol Immunother. 2003;52:546–54. doi: 10.1007/s00262-003-0392-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng HC, Abdel-Ghany M, Zhang S, et al. Is the Fischer 344/CRJ rat a protein-knock-out model for dipeptidyl peptidase IV-mediated lung metastasis of breast cancer? Clin Exp Metastasis. 1999;17:609–15. doi: 10.1023/a:1006757525190. [DOI] [PubMed] [Google Scholar]
- 4.von Bonin A, Huhn J, Fleischer B. Dipeptidyl-peptidase IV/CD26 on T-cells: analysis of an activated T-cell activation pathway. Immunol Rev. 1998;161:43–53. doi: 10.1111/j.1600-065x.1998.tb01570.x. [DOI] [PubMed] [Google Scholar]
- 5.Morimoto C, Schlossman SF. The structure and function of CD26 in the T-cell immune response. Immunol Rev. 1998;161:55–70. doi: 10.1111/j.1600-065x.1998.tb01571.x. [DOI] [PubMed] [Google Scholar]
- 6.Torimoto Y, Dang NH, Vivier E, et al. Coassociation of CD26 (dipeptidyl peptidase IV) with CD45 on the surface of human T lymphocytes. J Immunol. 1991;147:2514–7. [PubMed] [Google Scholar]
- 7.Lee NA, Gelfand EW, Lee JJ. Pulmonary T cells and eosinophils: coconspirators or independent triggers of allergic respiratory pathology? J Allergy Clin Immun. 2001;107:945–57. doi: 10.1067/mai.2001.116002. [DOI] [PubMed] [Google Scholar]
- 8.Larche M, Robinson DS, Kay AB. The role of T lymphocytes in the pathogenesis of asthma. J Allergy Clin Immun. 2003;111:450–63. doi: 10.1067/mai.2003.169. [DOI] [PubMed] [Google Scholar]
- 9.Herrick CA, Bottomly K. To respond or not to respond: T cells in allergic asthma. Nat Rev Immunol. 2003;3:405–12. doi: 10.1038/nri1084. [DOI] [PubMed] [Google Scholar]
- 10.Palframan RT, Collins PD, Williams TJ, et al. Eotaxin induces a rapid release of eosinophils and their progenitors from the bone marrow. Blood. 1998;91:2240–8. [PubMed] [Google Scholar]
- 11.Hylkema MN, Hoekstra MO, Luinge M, et al. The strength of the OVA-induced airway inflammation in rats is strain dependent. Clin Exp Immunol. 2002;129:390–6. doi: 10.1046/j.1365-2249.2002.01938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schneider T, van Velzen D, Moqbel R, et al. Kinetics and quantitation of eosinophil and neutrophil recruitment to allergic lung inflammation in a brown Norway rat model. Am J Respir Cell Mol Biol. 1997;17:702–12. doi: 10.1165/ajrcmb.17.6.2849. [DOI] [PubMed] [Google Scholar]
- 13.Karl T, Chwalisz WT, Wedekind D, et al. Localization, transmission, spontaneous mutations, and variation of function of the Dppiv (Dipeptidyl-peptidase IV; CD26) gene in rats. Regul Pept. 2003;115:81–90. doi: 10.1016/s0167-0115(03)00149-6. [DOI] [PubMed] [Google Scholar]
- 14.Karl T, Hoffmann T, Pabst R, et al. Extreme reduction of dipeptidyl-peptidase IV activity in F344 rat substrains results in major behavioral differences. Physiol Behav. 2003;80:123–34. doi: 10.1016/s0031-9384(03)00229-4. [DOI] [PubMed] [Google Scholar]
- 15.Tsuji E, Misumi Y, Fujiwara T, et al. An active-site mutation (Gly633-]Arg) of dipeptidyl peptidase-IV causes its retention and rapid degradation in the endoplasmic-reticulum. Biochemistry. 1992;31:11921–7. doi: 10.1021/bi00162a035. [DOI] [PubMed] [Google Scholar]
- 16.Schuster M, Tschernig T, Krug N, et al. Lymphocytes migrate from the blood into the bronchoalveolar lavage and lung parenchyma in the asthma model of the brown Norway rat. Am J Respir Crit Care Med. 2000;161:558–66. doi: 10.1164/ajrccm.161.2.9812021. [DOI] [PubMed] [Google Scholar]
- 17.Pabst R, Lührmann A, Steinmetz I, et al. A single intratracheal dose of the growth factor Fms-like tyrosine kinase receptor-3 ligand induces a rapid differential increase of dendritic cells and lymphocyte subsets in lung tissue and bronchoalveolar lavage, resulting in an increased local antibody production. J Immunol. 2003;171:325–30. doi: 10.4049/jimmunol.171.1.325. [DOI] [PubMed] [Google Scholar]
- 18.Kon OM, Kay AB. T cells and chronic asthma. Int Arch Allergy Immunol. 1999;118:133–5. doi: 10.1159/000024049. [DOI] [PubMed] [Google Scholar]
- 19.Wardlaw AJ. Molecular basis for selective eosinophil trafficking in asthma: a multistep paradigm. J Allergy Clin Immunol. 1999;104:917–26. doi: 10.1016/s0091-6749(99)70069-2. [DOI] [PubMed] [Google Scholar]
- 20.Hakonarson H, Maskeri N, Carter C, et al. Autocrine interaction between IL-5 and IL-1 beta mediates altered responsiveness of atopic asthmatic sensitized airway smooth muscle. J Clin Invest. 1999;104:657–67. doi: 10.1172/JCI7137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laporte JC, Moore PE, Baraldo S, et al. Direct effects of interleukin-13 on signaling pathways for physiological responses in cultured human airway smooth muscle cells. Am J Respir Crit Care Med. 2001;164:141–8. doi: 10.1164/ajrccm.164.1.2008060. [DOI] [PubMed] [Google Scholar]
- 22.Simeoni L, Rufini A, Moretti T, et al. Human CD26 expression in transgenic mice affects murine T-cell populations and modifies their subset distribution. Hum Immunol. 2002;63:719–30. doi: 10.1016/s0198-8859(02)00433-0. [DOI] [PubMed] [Google Scholar]
- 23.Khoury SJ, Guttmann CRG, Orav EJ, et al. Changes in activated T cells in the blood correlate with disease activity in multiple sclerosis. Arch Neurol. 2000;57:1183–9. doi: 10.1001/archneur.57.8.1183. [DOI] [PubMed] [Google Scholar]
- 24.Bock O, Kreiselmeyer I, Mrowietz U. Expression of dipeptidyl-peptidase IV(CD26) on CD8(+) T cells is significantly decreased in patients with psoriasis vulgaris and atopic dermatitis. Exp Dermatol. 2001;10:414–9. doi: 10.1034/j.1600-0625.2001.100604.x. [DOI] [PubMed] [Google Scholar]
- 25.Kubota T, Flentke GR, Bachovchin WW, et al. Involvement of dipeptidyl peptidase-IV in an in vivo immune-response. Clin Exp Immunol. 1992;89:192–7. doi: 10.1111/j.1365-2249.1992.tb06931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steinbrecher A, Reinhold D, Quigley L, et al. Targeting dipeptidyl peptidase IV (CD26) suppresses autoimmune encephalomyelitis and up-regulates TGF-beta 1 secretion in vivo. J Immunol. 2001;166:2041–8. doi: 10.4049/jimmunol.166.3.2041. [DOI] [PubMed] [Google Scholar]
- 27.Williams YN, Baba H, Hayashi S, et al. Dipeptidyl peptidase IV on activated T cells as a target molecule for therapy of rheumatoid arthritis. Clin Exp Immunol. 2003;131:68–74. doi: 10.1046/j.1365-2249.2003.02020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Meester I, Korom S, van Damme J, et al. CD26, let it cut or cut it down. Immunol Today. 1999;20:367–75. doi: 10.1016/s0167-5699(99)01486-3. [DOI] [PubMed] [Google Scholar]
- 29.Vercelli D. Immunoglobuline E and ist regulators. Curr Opin All Clin Immunol. 2001;1:61–5. doi: 10.1097/01.all.0000010986.58020.8d. [DOI] [PubMed] [Google Scholar]
- 30.Bertrand CP, Ponath PD. CCR3 blockade as a new therapy for asthma. Expert Opin Invest Drugs. 2000;9:43–52. doi: 10.1517/13543784.9.1.43. [DOI] [PubMed] [Google Scholar]
- 31.Bjornsdottir US, Cypcar DM. Asthma: an inflammatory mediator soup. Allergy. 1999;54:55–61. doi: 10.1111/j.1398-9995.1999.tb04389.x. [DOI] [PubMed] [Google Scholar]
- 32.Underwood SL, Haddad E-B, Birrell MA, et al. Functional characterization and biomarker identification in the brown Norway model of allergic airway inflammation. Br J Pharmacol. 2002;137:263–75. doi: 10.1038/sj.bjp.0704865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pabst R. Animal models for asthma: controversial aspects and unsolved problems. Pathobiology. 2002;70:252–4. doi: 10.1159/000070737. [DOI] [PubMed] [Google Scholar]
- 34.Wang CG, Almirall JJ, Dolman CS, et al. In vitro bronchial responsiveness in two highly inbred rat strains. J Appl Physiol. 1997;82:1445–52. doi: 10.1152/jappl.1997.82.5.1445. [DOI] [PubMed] [Google Scholar]
- 35.Sears MR, Burrows B, Flannery EM, et al. Relation between airway responsiveness and serum IgE in children with asthma and in apparently normal children. N Engl J Med. 1991;325:1067–71. doi: 10.1056/NEJM199110103251504. [DOI] [PubMed] [Google Scholar]