Abstract
Over the last two decades there has been a significant increase in the number and types of immunosuppressive agents that have been available to clinicians. The protocols for immunosuppression used in liver transplantation have been derived historically from those in renal transplantation. During the last decade there has been a shift in the use of immunosuppression, with the introduction of interleukin (IL)-2 receptor antagonists in place of anti-lymphocyte preparations, substitution of tacrolimus for cyclosporin and mycophenolate for azathioprine. The use of corticosteroids has been reduced. For a variety of reasons, these changes have not always been made on the basis of properly randomized studies. The place of newer agents, such as sirolimus and leflunomide derivatives and of the microbiological agents, is unclear. In this review, we outline briefly the mechanism of action of drugs and suggest possible approaches to the management of the liver allograft recipient, suggesting how treatment could be adjusted according to the indication for transplantation as well as the individual's comorbidities.
Keywords: immunosuppression, liver, transplantation, synergy
Introduction
Immunosuppression following transplantation is key to the survival of both allograft and patient. Initially, drug regimens were limited by the lack of effective, specific and safe agents. However, the availability of an array of effective therapeutic agents now raises new challenges, namely to find the most effective regimen which is associated with least toxicity. Here, we review those agents that have gained an established place in transplant immunosuppression and explore the current regimens and strategies that have been evaluated in clinical practice. We also review some of the more novel agents and strategies that are at early stages of development. Finally, we speculate upon future strategies, proposing a more logical approach to immunosuppression following liver transplantation.
Rejection: the need for immunosuppression
The liver is a relatively privileged organ with regard to transplantation and is subject to less aggressive immunological attack than other organs [1]. Hyper-acute rejection is rare and is due to presensitization to donor antigens. It occurs within minutes to hours after reinstitution of hepatic circulation and is mediated by complement fixation resulting in intravascular thrombosis [2]. Cellular or acute rejection is more common, and is characterized by activated cytotoxic T cells orchestrating a generalized immune response within the liver [2]. This is initiated by the presentation of donor HLA-antigens to host T cells within the graft which, via the secretion of interleukin (IL)-2, recruit activated T cells into the graft, the accumulation of which result in tissue damage. Acute cellular rejection requiring treatment occurs in up to 60% of patients and, unlike acute rejection in kidney transplantation, acute rejection developing within 3 months of transplantation is not associated with reduced graft or patient survival [3,4]. Early acute rejection is readily treatable with increased immunosuppression. In contrast, chronic (ductopaenic) cellular rejection, which develops in approximately 5% grafts, responds poorly to treatment and frequently results in graft failure [5]. The need for immunosuppression has to be balanced against the side-effects of the drugs used: side-effects may be related to immunosuppression itself (manifest as increased risk of sepsis and malignancy) or drug-specific side-effects (such as calcineurin inhibitor associated renal failure or diabetes).
Mechanism of action of immunosuppressants
Immunosuppressants may be divided broadly into the following classes: general immunosuppressants, calcineurin inhibitors (CNI), anti-metabolites, inhibitors of TOR (target of rapamycin), antibodies and a growing array of novel agents (see Table 1). We review briefly the key members of each of these groups and discuss their mode of action before considering their use in immunosuppressive regimens.
Table 1.
Currently available immunosuppressants.
| Immunosuppressant class | Immunosuppressant agent |
|---|---|
| General immunosuppressants | Corticosteroids |
| Calcineurin inhibitors (CNI) | Cyclosporin and tacrolimus |
| Anti-metabolites | 6-mercaptoprine, mycophenolate mofetil, azathioprine |
| Inhibitors of TOR | Sirolimus and everolimus |
| Antibodies | OKT3, IL-2R antibodies, campath 1H |
| Novel agents | FTY720, leflunomide, FK778, FK779 |
TOR = target of rapamycin; IL-2R = interleukin 2 receptor.
Corticosteroids
Corticosteroids are non-specific anti-inflammatory agents, the primary action of which is inhibition of cytokine gene transcription. The hydrophobic structure permits access into the cell by simple membrane diffusion, after which the steroid complexes with cytosolic receptors and translocates to the nucleus, binding to glucocorticoid response elements in the promoter regions of cytokine genes [6]. By inhibiting cytokine production, steroids prevent T cell recruitment and activation and are thus potent immunosuppressants [7]. Steroid usage is associated with a multitude of side-effects. In common with all potent immunosuppressants, sepsis and infection are frequently encountered as are hypertension, Cushingoid appearance, personality changes, development of cataracts, weight gain, dyslipidaemia, osteoporosis, hyperglycaemia and diabetes.
Calcineurin inhibitors (CNI)
Calcineurin is a key enzyme in the production of IL-2 by T cells. IL-2 is crucial to the recruitment and activation of CD4 T-cells and also, via the induction of other cytokines, orchestrates the actions of cytotoxic CD8 cells and natural killer cells, and also stimulates B cell activation. The amount of IL-2 produced by activated CD4 cells appears to be one of the major determinants of the magnitude of the immune response to a donor allograft [7]. Thus inhibiting calcineurin, and thereby impairing IL-2 transduction, has a profound effect on the immune process of rejection. The two calcineurin inhibitors, cyclosporin and tacrolimus (FK506), have become the cornerstone of immunosuppressive therapy in solid organ transplantation. Both agents are similar in that they bind to cytosolic proteins termed immunophilins, the resulting complex acting to inhibit the activity of calcineurin, but differ in that cyclosporin binds to cyclophilins while tacrolimus binds to FK506 binding proteins [8]. The two CNIs have differing side-effect profiles, which in part are related to this slight difference in mode of action. Cyclophilins are distributed ubiquitously and this may explain the diverse range of side effects associated with cyclosporin.
Dosage is monitored by measuring blood levels; despite centres having developed target levels (which may vary according to the time after transplantation), there is no close correlation between level and immunosuppressive activity. Target levels are therefore based more on experience than objective data. For cyclosporin it has been suggested, although not universally adopted, that the 2-hour post-dose level may better reflect immunosuppression than the trough level and lead to a better outcome [9,10].
Side effects are common and there is no close correlation between efficacy and toxicity. The most significant side-effect of CNIs is nephrotoxicity, occurring in 40–70% patients, and occurs primarily as a consequence of intrarenal vasoconstriction. Renal impairment is the most common indication for dose reduction or cessation of treatment. Acute nephrotoxicity is reversible, whereas chronic toxicity is not; up to 5% may eventually require renal support [11]; the onset of late renal failure is determined largely by the amount of exposure to CNI in the first year. Other frequent side effects include hypertension, venous thrombosis, tremor, headache, fits, parasthesia, hyperkalaemia, gout and gingival hyperplasia. Tacrolimus appears to be more potent than cyclosporin in inhibiting IL-2 synthesis and has a slightly different side-effect profile. It has the same degree of nephrotoxicity, but has a lower incidence of hypertension and hyperlipidaemia but increased rates of diabetes and neurotoxicty [12]. Overall, tacrolimus is broadly similar to cyclosporin in terms of both graft and patient survival, but with fewer episodes of rejection and less need for steroids [13]. Of recent interest is the observation that cyclosporin, but not tacrolimus, will inhibit hepatitis C viral (HCV) replication in vitro[14]; the relevance for patients with HCV is not yet established.
Azathioprine
Azathioprine, a prodrug form of 6-mercaptopurine, has a number of intracellular actions including inhibition of DNA synthesis, negative feedback on purine metabolism and reduction in nucleotide synthesis. These actions result in the inhibition of T cell activation, reduction in antibody production and a decrease in the level of circulating monocytes and granulocytes [15]. Azathioprine alone is relatively effective in the prevention of rejection but has very little effect upon an established immune response [7]. It is usually well tolerated in the dose range utilized in transplantation (1·5–2·0 mg/kg/day), but does have significant side effects, including bone marrow suppression (especially in those who have low levels of thiopurine methyltransferase) [16,17]. Less common adverse effects include nausea, vomiting, pancreatitis, hepatotoxicity and, as a consequence of reduced immunosurveillance, neoplasia.
Mycophenolate
Mycophenolate is a selective inhibitor of de novo purine synthesis and is a potent inhibitor of both B and T cell proliferation [18]. The principle side effects limiting tolerance and use are gastrointestinal upset and bone marrow suppression, although its teratogenicity may reduce its use in some women [7]. The usual maintenance dose is 2 g/day (for the mofetyl ester); monitoring of blood levels is not usually required. Recently, an enteric coated preparation has been licenced.
Sirolimus and everolimus
Sirolimus is related structurally to tacrolimus and forms a complex with FK506 binding protein but does not inhibit calcineurin. Instead, it appears to induce cell-cycle arrest at the G1 to S phase of the cell cycle via mechanisms involving the interruption of IL-2R post-receptor signalling pathways [19]. Thus it acts at points distinct from calcineurin inhibitors and mycophenolate or azathioprine and therefore could be synergistic with either group. In the United Kingdom, sirolimus is licensed for use in combination with cyclosporin and corticosteroids or with corticosteroids alone, but clinical studies suggest it is effective as monotherapy.
The principal side effects of sirolimus include poor wound healing, hyperlipidaemia, thrombocytopaenia, anaemia, leucopoenia and peripheral oedema, although oral ulceration and pneumonitis have also been reported; the side-effect profile appears worse in liver than other solid organ transplantation; in early studies, an increase in the incidence of hepatic artery thrombosis led to concern, but later studies have largely allayed this fear [20]. When used on its own, sirolimus does not cause significant nephrotoxicity [21]. Serum levels do not equilibrate for 3–5 days and facilities for measuring drug levels are limited to specialist centres. Of potential benefit is the observation that sirolimus has antitumorogenic effects, possibly mediated by inhibiting tumour angiogenesis [22]. Studies in humans grafted with malignancy have not yet shown whether this effect is clinically important.
Everolimus is 4()-o-2-hydroxyethyl sirolimus and exhibits improved bioavailability and shorter half-life than sirolimus. In phase I trials it appeared to be well tolerated by liver transplant recipients [23].
Polyclonal antibodies
Polyclonal antibody or polyclonal antilymphocyte globulins are gamma-globulin fractions from animals inoculated with human lymphocytes, thymocytes or cultured lymphoblasts [7]. The IgG fraction contains variable amounts of specific antibodies against T cells resulting in complement and cell-mediated lymphocyte depletion. It is this variability that leads to unpredictable levels of immune suppression and side effects. The principle side effects are related to over-immunosuppression and include sepsis and lymphoproliferative disease as well as those related to immune response to foreign serum (serum sickness, thrombocytopaenia, leucopenia and anaemia).
Monoclonal antibodies
OKT3
OKT3 is a murine monoclonal antibody (MoAb) against CD3 which acts to modulate the T cell receptor (TCR) complex, inactivating both naive T cells and activated cytotoxic T cells. Although a potent immunosuppressive agent, it has several significant side effects. The principle adverse effect is a first-dose effect seen in almost all patients in which there is a large release of cytokines resulting in fever, flu-like symptoms, hypotension and rarely bronchospasm [1, 7, 24]. Rarely, this can be fatal. OKT3 is also highly immunogenic, inducing a human antimurine antibody response which acts to inhibit OKT3 function after several days and limits its efficacy.
IL-2R antibodies
IL-2R is expressed by activated lymphocytes and thus agents that inhibit specifically the function of IL-2R are likely to be more specific immunosuppressants than the previous generation of agents. Currently two chimeric IL-2R-inhibiting MoAbs, Basiliximab and Daclizumab, are commercially available [25,26]. These are directed at the Tac antigen, an element of the IL-2R complex which is expressed only following T cell activation. There is some redundancy within the IL-2R complex that permits IL-2R signalling in the presence of high IL-2 levels, despite anti-Tac antibody use. Thus, these anti-Tac IL-2 R MoAbs require the concomitant use of other agents, such as calcineurin inhibitors, that reduce IL-2 levels and overcome this redundancy [27].
Anti-CD52 (Campath-1H)
Campath-1H (Alemtuzumab) is a humanized anti-CD52 (a pan T cell, B cell natural killer cell and monocyte marker [28]. The scant data available suggest this is a promising agent and may not only have immunosuppressive properties but may, by virtue of its action against so many arms of the immune response, have a role in tolerance induction (see below).
Novel agents (FTY720, leflunomide, FK778 and FK779)
FTY720
FTY720 is novel immunosuppressant that has no effect on T cell activation, cytokine production or B cell proliferation [29]. It induces sequestration of T and B cells into peripheral lymph nodes, mesenteric lymph nodes and Peyer patches by a mechanism involving the sphyngosine-1-phosphate receptor on lymphocytes. The exact mechanism is still unclear, but is coupled to G protein Rho activation and results in altered patterns of lymphocyte homing [30]. In experimental models, FTY 720 has been shown to prolong the survival of solid organ transplants, including liver transplantation [31], and the only significant side effect in phase II trails is bradycardia, because of cross-reactivity with heart spingosine-1-phosphate [32]. Notably, it does not appear to have significant renal toxicity or to be associated with increased episodes of sepsis. In animal models of renal transplantation it demonstrates a significant synergy with cyclosporin [33].
Leflunomide
Leflunomide is an antiproliferative agent that is used at present as a disease-modifying agent; it acts to inhibit de novo pathways of pyrimidine synthesis [34]. Because the products of this pathway are vital to progression form G1 to S phase for activated T and B cells, leflunomide acts to suppress T cell proliferation. It also has unexplained antiviral action against CMV and herpes simplex. Leflunomide, licensed for use in patients with rheumatoid arthritis, has been employed in renal transplantation in patients with deteriorating graft function and was associated with improvement in renal function [35], and has also been employed in a few cases of liver transplantation [36]. The principle side effect in liver allograft recipients was an increase in liver function tests that resolved upon withdrawal of the drug.
FK778 and FK779
The malononitrilamides FK778 (MNA715) and FK779 (MNA279) are new derivatives of A771726, the active metabolite of leflunomide. They appear to have potential activity as immunosuppressants, while FK778 also has vasculoprotective actions independent of its immunosuppressive action, possibly by inhibiting PDGF receptor tyrosine kinases. Preliminary data from rat models indicate that both FK778 and FK 779 have synergistic activity with tacrolimus in cardiac and renal transplantations [37], permitting lower effective doses of tacrolimus [38].
Current immunosuppressive strategies and regimens
The last few years have been associated with significant changes in the management of immunosuppression in liver allograft recipients, often without good evidence that the change in immunosuppression is associated with improved outcomes or less toxicity. Thus, in North America, most recipients now receive tacrolimus in place of cyclosporin; azathioprine has been replaced by mycophenolate and while anti-T cell antibodies are used less rarely, IL-2R antibodies are being used increasingly for induction.
Standard therapy
Standard therapy in many centres involves the combination of corticosteroids (hydrocortisone at induction and then tapering oral prednisolone), in conjunction with a calcineurin inhibitor and an antiproliferative agent. Current data from North America (United Network for Organ Sharing Database) demonstrate an overall 1-year survival of 82% for patients who have received a primary liver allograft. Triple therapy of tacrolimus, azathioprine and prednisolone is associated with a 1-year graft survival rate of 81%[39].
Standard regimens are usually dynamic, with doses of calcineurin inhibitor and steroid being reduced after the initial induction phase [40]. Dual regimens of steroid and calcineurin inhibitor have also been assessed and appear as efficacious as triple therapy but are associated with lower incidences of thrombocytopenia and leukopenia [41]. It is not uncommon for patients initiated on triple therapy to be weaned onto monotherapy with a calcineurin inhibitor [39,41].
Mycophenolate and mycophenolate mofetil (MMF)-based regimens
Recent studies suggest that mycophenolate or mycophenolate with its morpholino ester, MMF, may be superior to azathioprine. In prospective randomized trails of primary immunosuppression, MMF was more effective in preventing acute rejection than azathioprine when used in combination with calcineurin inhibitors [42] and steroids [43]. MMF is also advocated as an agent to allow the dosage reduction or discontinuation of calcineurin inhibitors in patients experiencing significant renal toxicity [1]. Complete withdrawal of CNI was achieved in over 60% of patients with chronic renal failure attributed to CNI [18]. However, there are concerns that use of monotherapy is associated with an increased incidence of acute and chronic rejection that may lead to graft loss [44]. There is no significant difference between MMF and mycophenolate.
Sirolimus
Sirolimus has a proven role in renal transplantation, permitting the withdrawal of cyclosporin, with associated improvement in serum creatinine but no reduction in graft survival [45]. Alternatively, where sirolimus was compared to cyclosporin as part of MMF/steroid triple therapy, renal graft survival rates were not different but the sirolimus patients had significantly better renal function [46]. However, this is not the case for liver transplantation, in which the incidence of side effects appears to be greater [47]. In a phase II study comparing tacrolimus and steroids with sirolimus, steroids and reduced tacrolimus there was a fivefold increase in hepatic artery thrombosis in the sirolimus group, resulting in discontinuation of the study [48]; however, subsequent studies have suggested that this may not be a major problem. The renoprotective effect appears to persist in liver patients but the side-effect profile indicates that it should be used with caution [19]. It may have a limited role as a renal sparing agent, as it appeared to stabilize renal function in patients with calcineurin nephrotoxicity [49].
Acute rejection treatment regimen
High-dose steroids (such as prednisolone 200 mg or methylprednisolone 1000 mg for 3 days) have been used historically by most centres to treat acute rejection. However, this practice may become somewhat controversial with the realization that some acute episodes of acute rejection resolve spontaneously and others may resolve with an increase in the dose of CNI [1]; regimens using less corticosteroid may be more effective and safer [50]. Alternative treatments for rejection include monoclonal therapy with OKT3, Basaliximab, rabbit antithymocyte globulin or Daclizumab. There is little evidence base to advocate any one regimen.
Induction regimens
Historically, induction therapy has been used infrequently in liver transplantation (6% of US transplants) [51]. However, the use of IL-2 receptor antagonists at induction are being considered as part of calcineurin-sparing or steroid-sparing protocols. A regimen of mycophenolate, corticosteroid and a single dose of Daclizumab in the immediate postoperative phase, with the introduction of low-dose tacrolimus delayed until day 7, was associated with fewer cases of acute rejection and with a lower serum creatinine than standard therapy [52].
Steroid sparing regimens
Steroid withdrawal is relatively safe in liver transplant recipients and has been practised by some centres for over 10 years [53]. When achieved within the first few months of transplantation, it is associated with minimal increases in acute rejection episodes and no increase in graft loss [54]. More recently steroid avoidance has been explored using regimens of tacrolimus and MMF, with only a single intraoperative dose of steroid [55] or a regimen of a very short course of steroid (days) combined with maintenance by sirolimus and a CNI [56]. Both regimens demonstrated rejection rates similar to historical controls. Steroid avoidance was associated with lower serum cholesterol and less hypertension in the tacrolimus and MMF trial; this was not seen when cyclosporin was used.
Novel approaches and regimens: tolerance induction
Aims to induce tolerance (a lack of immune response to a donor antigen while maintaining full reactivity to other antigens [57]) contrasts with the classical approach to immunosuppression which suppresses response to all antigens. Strategies include the generation of a bone marrow chimera of host and donor cells which is transplanted to the host after initial marrow ablation (irradiation or T cell depleting chemotherapy or immunotherapy) followed by grafting of the solid organ. Tolerance can also be induced by immunotherapy directed at professional antigen-presenting cells (anti CD28-B7 and CD40-CD40L) given immediately post-transplant. If cyclosporin is added at this stage, signalling through TCR is inhibited and the tolerance induction is lost. Thus tolerance induction requires engagement of the immune system. It is currently believed that tolerance induction involves the activation of CD4 cells which are then (due to the immunotherapy employed) unable to stimulate effectors of the immune response. In essence this process permits an immune response to the allograft, but the immune response has no deleterious effect. This can therefore be considered as a form of apparent or ‘prope tolerance’, rather than true tolerance [58]. The concept of prope tolerance (also termed MIST—minimal immunosuppression tolerance) may be developed more in the future as new therapeutic agents are introduced into clinical practice.
Although tolerance in rodents is relatively easy to achieve with pharmacological manipulation, extrapolation to higher animals and to humans is not so easy. At present the evidence for tolerance induction is based almost entirely on animal experiments, with minimal data obtained from clinical experience. Even so, it is clear that a proportion of humans will not need long-term immunosuppression. Use of donor blood transfusions, for example, appears to be ineffective in inducing tolerance in humans.
The use of anti-CD52 (Campath-1H), in conjunction with low-dose tacrolimus, has also been advocated as a method of permitting slow engagement with the host immune system due to its widespread immunological suppression. Initial results in liver transplantation are encouraging, with significantly less episodes of acute rejection, lower tacrolimus doses and increased median time to rejection being found with this regimen than with standard regimens [28].
Thus, possible routes to clinically relevant immune tolerance may involve either chimeric bone marrow transplantation or the application of tolerance-inducing immunotherapy (with or without low-dose conventional immunosuppressants). If tolerance-inducing immunotherapy were to be employed the concurrent use of immunosuppressants in the induction phase would need to be avoided or reduced.
Disease-specific regimens
It is important to consider the pre-existing liver pathology because this may influence outcome and choices for therapy post-transplantation. Following transplantation for HCV the allograft is universally reinfected and undergoes accelerated progression to cirrhosis, which is potentiated by excessive or changing levels of immunosuppression [59]. The recurrence of PBC is significantly greater with tacrolimus compared with cyclosporin-based regimens [60]. Recurrence of autoimmune hepatitis (AIH) is reduced by continued steroid usage.
Cardiovascular protection
Cardiovascular morbidity and mortality are major causes of premature death and loss of allograft function and are related to an accelerated vasculopathy [61]. This is due in part to the side effects of immunosuppressive agents, in particular CNIs, sirolimus and steroids, which are associated with impaired glucose tolerance, diabetes, hypertension and hyperlipidaemia. Statins (of which pravastatin is favoured as it has less interaction with CNI metabolism) improve lipid profiles and reduces transplant vasculopathy, but there is an increased risk of myositis [62,63]. Statins also have an immunosuppressive action in their own right, possibly by altering MHC or leucocyte adhesion molecule expression [64]. Thus, in those patients considered at risk for atherosclerosis (patients with diabetes, hyperlipidaemia and hypertension), CNIs may be avoided and statins should be considered. Hypertension, as an independent risk factor, should be controlled in all patients.
A logical algorithm for immunosuppression following liver transplantation
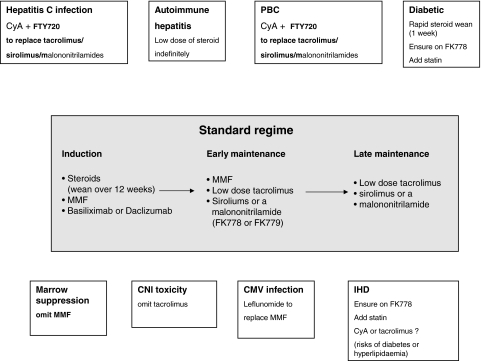
A logical approach to immunosuppression requires a clear understanding of the mechanism of rejection and tolerance induction; furthermore, there needs to be a balance of efficacy and toxicity and consideration of cost-effectiveness. The algorithm shown in Fig. 1 indicates a possible standard strategy (central panel) that could be employed for the majority of patients while the peripheral panels indicate potential adjustments or deviations from the standard regimen that may be required in specific situations. Underpinning much of this suggested model is the concept of synergy, the usage of combined low dose therapies that maximize the immunosuppressive action but limit side effects. We accept that this is speculative as there is little, if any, evidence from prospective randomized control trials to support many of these proposals.
Fig. 1.
Proposed algorithm for immunosuppression following liver transplantation. The central panel indicates the standard regimen that would be employed in routine transplantation. The peripheral panels indicate modifications that would be considered in specific circumstances. These may be either disease-specific modifications or side-effect specific modifications, as outlined in the text. We have included the addition of statins in certain circumstances because they have potential both as immunomodulators and as agents that may address the increased burden of cardivascular disease seen in transplant recipients.
Tailored immunosuppression is a much-discussed yet rarely implemented concept, yet it is clear that we should be moving towards regimens tailored to the individual that take into account both disease-specific and side effect-specific modifications. We do not consider that tolerance induction is sufficiently advanced to be incorporated into the algorithm at this time.
Assessing and developing new treatments in liver allograft recipients is becoming increasingly problematic. There are many reasons for this. The current graft survival rate exceeds 90% at 1 year, so large numbers of patients will be required to demonstrate a statistically significant benefit in survival. There are many factors that will affect patient and graft survival, such as the quality of the donor liver and technical factors that will not be affected by immunosuppression. Use of surrogate end-points, such as the rates of acute rejection, are not validated and may give rise to irrelevant or misleading conclusions. Many of the complications of immunosuppression are not manifest for several years after transplantation (such as risk of cancers associated with immunosuppression, renal failure and diabetes associated with CNI), so long-term follow-up is needed; this is often impractical and expensive. Subgroup analyses are required for different diseases: the choice of immunosuppression may affect disease recurrence. Because trials take years between setting-up and publication, developments in immunosuppression may invalidate the conclusions. Finally, the increasing burden of regulation and the associated costs will deter both individuals and pharmaceutical companies from sponsoring or participating in clinical studies. However, despite these hurdles it is imperative that we consider assessing more logical forms of immunosuppression if we are to further improve allograft and patient survival in the face of increasingly marginal donor organs.
References
- 1.Vilatoba M, Contreras JL, Eckhoff DE. New immunosuppressive strategies in liver transplantation: balancing efficacy and toxicity. Current Opin Organ Transplant. 2003;8:139–45. [Google Scholar]
- 2.Sherlock S, Dooley J. Diseases of the liver and biliary system. 11. Oxford: Blackwell Science Ltd; 2002. [Google Scholar]
- 3.Wiesner RH, Demetris AJ, Belle SH, et al. Acute hepatic allograft rejection: incidence, risk factors, and impact on outcome. Hepatology. 1998;28:45. doi: 10.1002/hep.510280306. [DOI] [PubMed] [Google Scholar]
- 4.Neuberger J, Adams DH. What is the significance of acute liver allograft rejection? J Hepatol. 1998;29:143–50. doi: 10.1016/s0168-8278(98)80190-6. [DOI] [PubMed] [Google Scholar]
- 5.Lowes JR, Hubscher SG, Neuberger JM. Chronic rejection of the liver allograft. Gastroenterol Clin North Am. 1993;22:401–20. [PubMed] [Google Scholar]
- 6.Pfeilschifter J, Muhl H. Immunopharmacology: anti-inflammatory therapy targeting transcription factors. Eur J Pharmacol. 1999;375:237–45. doi: 10.1016/s0014-2999(99)00361-1. [DOI] [PubMed] [Google Scholar]
- 7.Mueller XM. Drug immunosuppression therapy for adult heart transplantation. Part 1. Immune response to allograft and mechanism of action of immunosuppressants. Ann Thorac Surg. 2004;77:354–62. doi: 10.1016/j.athoracsur.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 8.Jiang H, Kobayashi M. Differences between cyclosporin A and tacrolimus in organ transplantation. Transplant Proc. 1999;31:1978–80. doi: 10.1016/s0041-1345(99)00235-3. [DOI] [PubMed] [Google Scholar]
- 9.Sterneck M, Zadeh KM, Groteluschen R, Broring D, Rogiers X, Fischer L. Clinical use of C2 monitoring in long-term liver transplant recipients. Transplant Proc. 2002;34:3304–6. doi: 10.1016/s0041-1345(02)03567-4. [DOI] [PubMed] [Google Scholar]
- 10.Levy G, Villamil F, Samuel D, et al. LIST Study Group. Results of list, a multicenter, randomized study comparing cyclosporine microemulsion with C2 monitoring and tacrolimus with C0 monitoring in de novo liver transplantation. Transplantation. 2004;77:1632–8. doi: 10.1097/01.tp.0000129095.51031.42. [DOI] [PubMed] [Google Scholar]
- 11.Ojo AO, Held PJ, Port FK, et al. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med. 2003;349:931–40. doi: 10.1056/NEJMoa021744. [DOI] [PubMed] [Google Scholar]
- 12.Kahan BD. The limitations of calcineurin and mTOR inhibitors: new directions for immunosuppressive strategies. Transplant Proc. 2002;34:130–3. doi: 10.1016/s0041-1345(01)02702-6. [DOI] [PubMed] [Google Scholar]
- 13.The US Multicenter FK506 Liver Study Group. A comparison of tacrolimus (FK 506) and cyclosporine for immunosuppression in liver transplantation. N Engl J Med. 1994;331:1110–5. doi: 10.1056/NEJM199410273311702. [DOI] [PubMed] [Google Scholar]
- 14.Nakagawa M, Sakamoto N, Enomoto N, et al. Specific inhibition of hepatitis C virus replication by cyclosporin A. Biochem Biophys Res Commun. 2004;313:42–7. doi: 10.1016/j.bbrc.2003.11.080. [DOI] [PubMed] [Google Scholar]
- 15.Nielsen OH, Vainer B, Rask-Madsen J. Review article: the treatment of inflammatory bowel disease with 6-mercaptopurine or azathioprine. Aliment Pharmacol Ther. 2001;15:1699–708. doi: 10.1046/j.1365-2036.2001.01102.x. [DOI] [PubMed] [Google Scholar]
- 16.Connell WR, Kamm MA, Ritchie JK, Lennard-Jones JE. Bone marrow toxicity caused by azathioprine in inflammatory bowel disease: 27 years of experience. Gut. 1993;34:1081–5. doi: 10.1136/gut.34.8.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Armstrong VW, Shipkova M, von Ahsen N, Oellerich M. Analytic aspects of monitoring therapy with thiopurine medications. Ther Drug Monit. 2004;26:220–6. doi: 10.1097/00007691-200404000-00024. [DOI] [PubMed] [Google Scholar]
- 18.Moreno JM, Rubio E, Pons F, et al. Usefulness of mycophenolate mofetil in patients with chronic renal insufficiency after liver transplantation. Transplant Proc. 2003;35:715–7. doi: 10.1016/s0041-1345(03)00061-7. [DOI] [PubMed] [Google Scholar]
- 19.Neff GW, Montalbano M, Tzakis AG. Ten years of sirolimus therapy in orthotopic liver transplant recipients. Transplant Proc. 2003;35:209S–216S. doi: 10.1016/s0041-1345(03)00217-3. [DOI] [PubMed] [Google Scholar]
- 20.Trotter JF. Sirolimus in liver transplantation. Transplant Proc. 2003;35:S193–200. doi: 10.1016/s0041-1345(03)00234-3. [DOI] [PubMed] [Google Scholar]
- 21.Groth CG, Backman L, Morales JM, et al. Sirolimus (rapamycin)-based therapy in human renal transplantation: similar efficacy and different toxicity compared with cyclosporine. Transplantation. 1999;67:1036–42. doi: 10.1097/00007890-199904150-00017. . Sirolimus European Renal Transplant Study Group. [DOI] [PubMed] [Google Scholar]
- 22.Koehl GE, Andrassy J, Guba M, et al. Rapamycin protects allografts from rejection while simultaneously attacking tumors in immunosuppressed mice. Transplantation. 2004;77:1319–26. doi: 10.1097/00007890-200405150-00002. [DOI] [PubMed] [Google Scholar]
- 23.Tan HP, Basu A, Shapiro R. Everolimus: an update. Current Opin Organ Transplant. 2003;8:323–6. [Google Scholar]
- 24.Go MR, Bumgardner GL. OKT3 (muromonab-CD3) associated hepatitis in a kidney transplant recipient. Transplantation. 2002;73:1957–9. doi: 10.1097/00007890-200206270-00020. [DOI] [PubMed] [Google Scholar]
- 25.Vincenti F. The role of newer monoclonal antibodies in renal transplantation. Transplant Proc. 2001;33:1000–1. doi: 10.1016/s0041-1345(00)02303-4. [DOI] [PubMed] [Google Scholar]
- 26.Calmus Y, Scheele JR, Gonzalez-Pinto I, et al. Immunoprophylaxis with basiliximab, a chimeric anti-leukin-2 receptor monoclonal antibody, in combination with azathioprine-containing triple therapy in liver transplant recipients. Liver Transplant. 2002;8:123–31. doi: 10.1053/jlts.2002.30882. [DOI] [PubMed] [Google Scholar]
- 27.Hirose R, Roberts JP, Quan D, et al. Experience with daclizumab in liver transplantation: renal transplant dosing without calcineurin inhibitors is insufficient to prevent acute rejection in liver transplantation. Transplantation. 2000;69:307–11. doi: 10.1097/00007890-200001270-00019. [DOI] [PubMed] [Google Scholar]
- 28.Tzakis AG, Tryphonopoulos P, Kato T, et al. Preliminary experience with alemtuzumab (Campath-1H) and low-dose tacrolimus immunosuppression in adult liver transplantation. Transplantation. 2004;77:1209–14. doi: 10.1097/01.tp.0000116562.15920.43. [DOI] [PubMed] [Google Scholar]
- 29.Suzuki S. FTY720: mechanisms of action and its effect on organ transplantation. Transplant Proc. 1999;317:2779–82. doi: 10.1016/s0041-1345(99)00564-3. [DOI] [PubMed] [Google Scholar]
- 30.Cheuch S-CJ, Kahan BD. Update on FTY720: review of mechanisms and clinical results. Curr Opin Organ Transplant. 2003;8:288–98. [Google Scholar]
- 31.Tamura A, Li XK, Funeshima N, et al. Immunosuppressive therapy using FTY720 combined with tacrolimus in rat liver transplantation. Surgery. 2000;127:47–54. doi: 10.1067/msy.2000.100884. [DOI] [PubMed] [Google Scholar]
- 32.Sanna MG, Liao J, Jo E, et al. Sphingosine 1-phosphate (S1P) receptor subtypes S1P1 and S1P3, respectively, regulate lymphocyte recirculation and heart rate. J Biol Chem. 2004;279:13839–48. doi: 10.1074/jbc.M311743200. [DOI] [PubMed] [Google Scholar]
- 33.Schuurman HJ, Menninger K, Audet M, et al. Oral efficacy of the new immunomodulator FTY720 in cynomolgus monkey kidney allotransplantation, given alone or in combination with cyclosporine or RAD. Transplantation. 2002;74:951–60. doi: 10.1097/00007890-200210150-00009. [DOI] [PubMed] [Google Scholar]
- 34.Koch MJ, Brennan DC. Leflunomide: is there a place for its use in transplantation? Current Opin Organ Transplant. 2003;8:317–22. [Google Scholar]
- 35.Hardinger KL, Wang CD, Schnitzler MA. Prospective, pilot, open-label, short term study of conversion to leflunomide reverses chronic renal allograft dysfunction. Am J Transplant. 2002;2:867–71. doi: 10.1034/j.1600-6143.2002.20909.x. [DOI] [PubMed] [Google Scholar]
- 36.Williams JW, Mital D, Chong A, et al. Experiences with leflunomide in solid organ transplantation. Transplantation. 2002;73:358–66. doi: 10.1097/00007890-200202150-00008. [DOI] [PubMed] [Google Scholar]
- 37.Bilolo KK, Ouyang J, Wang X, et al. Synergistic effects of malononitrilamides (FK778, FK779) with tacrolimus (FK506) in prevention of acute heart and kidney allograft rejection and reversal of ongoing heart allograft rejection in the rat. Transplantation. 2003;75:1881–7. doi: 10.1097/01.TP.0000064710.78335.D3. [DOI] [PubMed] [Google Scholar]
- 38.Deuse T, Schrepfer S, Reichenspurner H. The interaction between FK778 and tacrolimus in the prevention of rat cardiac allograft rejection is dose dependent. Transplantation. 2004;77:509–13. doi: 10.1097/01.tp.0000113443.70993.8c. [DOI] [PubMed] [Google Scholar]
- 39.Neuhaus P, Langrehr JM, Williams R, Calne RY, Pichlmayr R, McMaster P. Tacrolimus-based immunosuppression after liver transplantation: a randomised study comparing dual versus triple low-dose oral regimens. Transplant Int. 1997;10:253–61. doi: 10.1007/s001470050054. [DOI] [PubMed] [Google Scholar]
- 40.Ekberg H. Tailoring minimal immunosuppression long term. Transplant Proc. 2003;35:755–7. doi: 10.1016/s0041-1345(03)00028-9. [DOI] [PubMed] [Google Scholar]
- 41.Serrano J, Gonzale M, Gomez M, et al. Tacrolimus is effective in both dual and triple regimens after transplantation. Transplant Proc. 2002;34:1529–30. doi: 10.1016/s0041-1345(02)03006-3. [DOI] [PubMed] [Google Scholar]
- 42.Fisher RA, Ham JM, Marcos A, et al. A prospective randomised trial of mycophenalate mofetil with Neoral or tacrolimus after orthoptic liver transplantation. Transplantation. 1998;66:1616–21. doi: 10.1097/00007890-199812270-00008. [DOI] [PubMed] [Google Scholar]
- 43.Wiesner R, Rabkin J, Klintmalm G, et al. A randomized double-blind comparative study of mycophenolate mofetil and azathioprine in combination with cyclosporine and corticosteroids in primary liver transplant recipients. Liver Transplant. 2001;7:442–50. doi: 10.1053/jlts.2001.23356. [DOI] [PubMed] [Google Scholar]
- 44.Stewart SF, Hudson M, Talbot D, Manas D, Day CP. Mycophenolate mofetil monotherapy in liver transplantation. Lancet. 2001;357:609–10. doi: 10.1016/s0140-6736(00)04065-4. [DOI] [PubMed] [Google Scholar]
- 45.Oberbauer R, Kreis H, Johnson RWG, et al. Long term improvement in renal function with sirolimus after early cyclosporine withdrwal in renal transplant recipients: 2 year results of the Rapamune maintenance regimen study. Transplantation. 2003;76:364–70. doi: 10.1097/01.TP.0000074360.62032.39. [DOI] [PubMed] [Google Scholar]
- 46.Flechner S, Goldfarb D, Modlin C, et al. Kindey transplantation without calcinueurin inhibitor drugs: a prospective, randomized trial of sirolimus versus cyclosporine. Transplantation. 2002;74:1070–6. doi: 10.1097/00007890-200210270-00002. [DOI] [PubMed] [Google Scholar]
- 47.Basu A, Tan HP, Shapiro R. Sirolimus. Curr Opin Organ Transplant. 2003;8:299–304. [Google Scholar]
- 48.Weisner RH for the Rapamune Liver Transplant Study Group. The safety and efficacy of sirolimus and low-dose tacrolimus versus tacrolimus in de-novo orthoptic liver transplant recipients. Results from a pilot study. Hepatology. 2002;36:208A. [Abstract]. [Google Scholar]
- 49.Heffron TG, Smallwood GA, Davis L. Sirolimus-based immunosuppressive protocol for calcineurine sparing in liver transplantation. Transplant Proc. 2002;34:1522–3. doi: 10.1016/s0041-1345(02)02956-1. [DOI] [PubMed] [Google Scholar]
- 50.Volpin R, Angeli P, Galioto A, et al. Comparison between two high dose methylprednisolone schedules in the treatment of acute hepatic cellular rejection in liver transplant patients: a controlled clinical trial. Liver Transplant. 2002;8:527–34. doi: 10.1053/jlts.2002.33456. [DOI] [PubMed] [Google Scholar]
- 51.Helderman JH, Bennett WM, Cibrik DM, Kaufman DB, Klein A, Takemoto SK. Immunosuppression: practice and trends. Am J Transplant. 2003;3(Suppl. 4):41–52. doi: 10.1034/j.1600-6143.3.s4.5.x. [DOI] [PubMed] [Google Scholar]
- 52.Heffron TG, Smallwood GA, Pillen T, et al. Liver transplant induction trial of daclizumab to spare calcineurin inhibition. Transplant Proc. 2002;34:1514–5. doi: 10.1016/s0041-1345(02)02952-4. [DOI] [PubMed] [Google Scholar]
- 53.Padbury RT, Gunson BK, Dousset B, et al. Long-term immunosuppression after liver transplantation: are steroids necessary? Transplant Int. 1992;5(Suppl. 1):S470–2. doi: 10.1007/978-3-642-77423-2_137. [DOI] [PubMed] [Google Scholar]
- 54.Kim DY, Stegall MD. Steroid-sparing regimens in organ transplantation. Current Opin Organ Transplant. 2001;6:313–19. [Google Scholar]
- 55.Ringe B, Braun F, Schutz E, et al. A novel management strategy of steroid-free immunosuppression after liver transplantation: efficacy and safety of tacrolimus and mycophenolate mofetil. Transplantation. 2001;71:508–15. doi: 10.1097/00007890-200102270-00005. [DOI] [PubMed] [Google Scholar]
- 56.Trotter JF, Wachs M, Bak T, et al. Liver transplantation using sirolimus and minimal corticosteroids (3-day taper) Liver Transplant. 2001;7:343–51. doi: 10.1053/jlts.2001.23012. [DOI] [PubMed] [Google Scholar]
- 57.Gudmundsdottir H. The road toward transplantation tolerance. Transplant Proc. 2003;35:758–9. doi: 10.1016/s0041-1345(03)00029-0. [DOI] [PubMed] [Google Scholar]
- 58.Calne R. Prope tolerance: a step in the search for tolerance in the clinic. Transplant Proc. 2000;32:2058–9. doi: 10.1016/s0041-1345(00)01557-8. [DOI] [PubMed] [Google Scholar]
- 59.Neuberger J. Treatment of hepatitis C virus infection in the allograft. Liver Transplant. 2003;9:S101–8. doi: 10.1053/jlts.2003.50250. [DOI] [PubMed] [Google Scholar]
- 60.Neuberger J, Gunson B, Hubscher S, Nightingale P. Immunosuppression affects the rate of recurrent primary biliary cirrhosis after liver transplantation. Liver Transplant. 2004;10:488–91. doi: 10.1002/lt.20123. [DOI] [PubMed] [Google Scholar]
- 61.Fellstrom B. Impact and management of hyperlipidemia posttransplantation. Transplantation. 2000;70:SS51–7. [PubMed] [Google Scholar]
- 62.Zachoval R, Gerbes AL, Schwandt P, Parhofer KG. Short-term effects of statin therapy in patients with hyperlipoproteinemia after liver transplantation: results of a randomized cross-over trial. J Hepatol. 2001 July;35:86–91. doi: 10.1016/s0168-8278(01)00044-7. [DOI] [PubMed] [Google Scholar]
- 63.Hedman M, Neuvonen PJ, Neuvonen M, Holmberg C, Antikainen M. Pharmacokinetics and pharmacodynamics of pravastatin in pediatric and adolescent cardiac transplant recipients on a regimen of triple immunosuppression. Clin Pharmacol Ther. 2004;75:101–9. doi: 10.1016/j.clpt.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 64.Raggatt LJ, Partridge NC. HMG-CoA reductase inhibitors as immunomodulators: potential use in transplant rejection. Drugs. 2002;62:2185–91. doi: 10.2165/00003495-200262150-00002. [DOI] [PubMed] [Google Scholar]