Abstract
The chronic hepatitis B virus (HBV) carrier exhibits ongoing replication of HBV and expresses abundant amounts of HBV-related antigens in the liver. However, HBV-specific immune responses are either absent or narrowly focused in these subjects. With the postulation that impaired functional abilities of liver dendritic cells (DCs) might be responsible for this, we assessed the functions of liver DCs in HBV transgenic mice (HBV-TM), an animal model of the HBV carrier state. Liver DCs were isolated from normal C57BL/6 mice and HBV-TM without the use of cytokines or growth factors. Lymphoproliferative assays were conducted to evaluate the ability of liver DCs to induce the proliferation of allogenic T lymphocytes and hepatitis B surface antigen (HBsAg)-enriched T lymphocytes. Liver DCs were stimulated with viral and bacterial products to assess their cytokine-producing capacities. In comparison to liver DCs from normal C57BL/6 mice, liver DCs from HBV-TM exhibited significantly decreased T cell proliferation-inducing capacities in allogenic mixed leucocyte reaction (P < 0·05) and HBsAg-enriched T lymphocytes proliferation assays (P < 0·05). Liver DCs from HBV-TM produced significantly lower levels of interleukin-12p70, tumour necrosis factor-alpha, interferon-gamma, and interleukin-6 (P < 0·05) compared to liver DCs from normal C57BL/6 mice. This study provides evidence that liver DCs from HBV-TM had impaired ability to induce both innate and adaptive immune responses. This might account for a weak and almost undetectable HBV-specific immune response in chronic HBV carriers. This inspires hope that up-regulation of the functions of liver DCs in situ may have therapeutic implications in chronic HBV carriers.
Keywords: antigen processing, cytokines/interleukins, dendritic cells, hepatitis B virus, liver immunology/disease
Introduction
About 350 million people in the world are chronically infected with the hepatitis B virus (HBV). Considerable numbers of chronic HBV carriers will develop progressive liver diseases such as liver cirrhosis and hepatocellular carcinoma. Also, all chronic HBV carriers are permanent sources of HBV infection and are able to transmit the HBV to uninfected healthy people [1,2]. The exact mechanism underlying the pathogenesis of the HBV carrier state is not well understood; however, the immune responses of the host are presumed to play a critical role in this regard. Patients who control HBV after an acute infection are characterized by having a clearly detectable HBV-specific CD4 and CD8 response for a wide range of different epitopes within the HBV core, polymerase and envelope proteins [3]. In contrast, circulating HBV-specific T cells are very difficult to find in chronic HBV carriers, and when they are present they are often few and specific for single epitopes [4].
As the interaction between the virus and the host determines the magnitude of the virus-specific immune response, defective processing and presentation of the HBV by antigen-presenting dendritic cells (DCs) [5,6] might be responsible for the impaired HBV-specific immune responses in chronic HBV carriers. DCs originate in the bone marrow and migrate through the blood to different non-lymphoid tissues, where the phenotypes and functions of DCs undergo modifications. This is evident from the phenotypic and functional diversities of DCs in different tissues such as the liver, lung, small intestine, large intestine, heart, kidney and skin [7,8]. DCs in these non-lymphoid tissues recognize, capture and process viruses in their endosomal compartments, and express the antigenic peptides of the virus on their surface. From this scenario, it is evident that improper handling and processing of HBV by liver DCs might be responsible for defective HBV-specific immune responses in chronic HBV carriers. Currently, there is no study describing the functions of liver DCs in human and murine HBV carriers.
Some studies have reported on the functions of peripheral blood and spleen DCs in humans and murine chronic HBV carriers, respectively [9–11]. Although these studies represent the pioneer research on HBV/DC interactions in chronic HBV infections, they have major and fundamental limitations. In fact, blood and spleen DCs are not related directly to the recognition, capturing and processing of HBV in chronic HBV carrier [12].
This study was conducted to determine if the functions of liver DCs were impaired in murine HBV carriers. It is very difficult to isolate adequate numbers of liver DCs from normal mice for functional studies because there are very few DCs in the liver. Accordingly, some investigators have expanded liver DCs by administering fms-like tyrosine kinase-3 ligand in vivo[13]. Others have cultured precursor or progenitor populations of liver DCs from normal mice with cytokines, growth factors and collagenase in vitro to obtain adequate numbers of liver DCs [14,15]. However, most of these agents induce either maturation or activation of liver DCs, therefore altering the functions of liver DCs and potentially compromising our study.
Hence, we isolated fresh liver DCs from HBV transgenic mice (HBV-TM) and normal C57BL/6 mice without the use of growth factors, cytokines or collagenase. This was achieved by killing 10–15 normal mice or HBV-TM for each experiment. After confirming the phenotype and functions of liver DCs, we compared the T cell proliferation-inducing capacities of liver DCs between HBV-TM and normal C57BL/6 mice. Our study revealed that the liver DCs from HBV-TM were inefficient in inducing proliferation of both allogenic T cells and antigen-specific T cells. The mechanism underlying the impaired T cell proliferation-inducing capacity of liver DCs of HBV-TM was studied from their capacity to produce proinflammatory cytokines in response to different bacterial and viral products.
Methods
Mice
The HBV-TM (official designation, 1.2HB-BS10) were prepared by microinjecting the complete genome of HBV plus 619 base pairs of HBV DNA into the fertilized eggs of C57BL/6 mice. The HBV-TM expressed mRNAs of 3·5, 2·1, and 0·8 kilo base pairs of HBV in the liver and high levels of the hepatitis B surface antigen (HBsAg) in the sera [16].
We purchased normal C57BL/6 mice (C57BL/6 J Jel, H-2b) and normal C3H/He mice (C3H/HeN Jcl, H-2k) from CLEA Japan Inc. (Tokyo, Japan). HBV-TM and normal mice (C57BL/6 and C3H/He) were maintained separately in the animal house at Ehime University School of Medicine, Ehime, Japan, under controlled conditions (22°C, 55% humidity and 12-h day/night rhythm), and were provided with an unlimited supply of standard laboratory feed and water. All animals received humane care and the study protocols conformed to institutional guidelines.
Detection of HBsAg and HBV DNA
The levels of HBsAg and HBV DNA in the sera of HBV-TM were estimated using the chemiluminescence enzyme immunoassay method (Tokyo Institute of Immunology, Tokyo, Japan) and the real time polymerase chain reaction method (Special Reference Laboratory, Osaka, Japan), respectively.
Isolation of liver DCs
Liver DCs were isolated from mouse liver using a methodology described by Lu et al., with some modifications [14]. The mice were anaesthetized with pentobarbital sodium (Dai Nippon Pharmaceutical Co., Ltd, Osaka, Japan). After opening the abdomen, a 24-gauge intravenous canulla (VasculonTM, Becton Dickinson, Helsborge, Sweden) was inserted into the portal vein and the liver was perfused with 5 ml of phosphate-buffered saline. The inferior vena cava was cut to ensure the free outflow of blood. We removed the liver and disrupted the perfused liver using the flat portion of a plunger from a 10-ml syringe. The cell suspensions were then passed through a sterile 75-µm pore size steel mesh (Morimoto Yakuhin Co, Matsuyama, Japan), pelleted and resuspended in 35% Percoll solution (PercollTM, Amersham Bioscience Co, Tokyo, Japan) containing 100 µ/ml of heparin. After centrifugation at 450 g for 15 min at room temperature, non-parenchymal cells (NPCs) of the liver were collected.
In order to isolate liver DCs, NPCs were fractionated based on CD11c expression with immunomagnetic beads (MicroBeads Miltenyi Biotech, Bergisch Gladbach, Germany) according the manufacturer's instructions, after blocking Fc receptors with monoclonal antibody 2.4G2 (BD Biosciences Pharmingen, San Jose, CA, USA).
Preparation and isolation of HBsAg-enriched T lymphocytes
In order to prepare HBsAg-enriched lymphocytes, normal C57BL/6 mice were injected with 10 µg of recombinant HBsAg in aluminium hydroxide, twice at 2-week intervals, as described previously [17]. Allogenic T lymphocytes and HBsAg-enriched T lymphocytes were isolated from C3H/He mice and HBsAg-immunized C57BL/6 mice by magnetic cell sorting using auto-MACS (Miltenyi Biotec). Single cell suspension of the spleen was incubated with immunomagnetic beads coated with monoclonal antibodies to mouse CD90 (Thy 1·2) (Miltenyi Biotec). These cells were then washed twice through a MACS column. The positively selected cells were collected as T lymphocytes.
Flow cytometry
After blocking the Fc receptor and then staining the liver DCs with fluorescence isothiocyanate-conjugated, phycoerythrin-conjugated or peridinin chlorophyll protein-conjugated antibody, flow cytometry was performed using a FACSCalibur flow cytometer (BD Biosciences). Liver DCs were stained for DC (CD11c, HL3), lineage markers (CD3, 17A2; CD8α, 53–6·7, CD11b, M1/70, B220, RA3–6B2), major histocompatibility complex (MHC) class I (AF6-88·5), MHC class II (AF6-120·1) and co-stimulatory molecules (CD80, 16–10A1; CD86, GL1). Analysis of the flow cytometry data was conducted using CellQuest software (BD Biosciences).
Proliferation-inducing capacities of liver DCs
T cells and DCs were cultured in 96-well U-bottomed plates (Corning, Tokyo, Japan) for 4 days, as described [17]. During the last 12 h, [3H]-thymidine, 1·0 µCi/ml (Amersham Biosciences UK Limited, Buckinghamshire, UK) was added to the culture. The levels of incorporation of [3H]-thymidine were determined using a liquid scintillation counter (Beckman LS 6500, Beckman Instruments, Inc., Fullerton, CA, USA) and the blastogenesis levels were expressed as counts per minute (cpm).
Cytokines production by liver DCs
To assess cytokine production, 4 × 105 immunomagnetic bead-purified CD11c+ liver DCs were cultured in 200 µl of medium in a 96-well U-bottomed Corning tissue culture plate. Lipopolysaccharides, 5 µg (Sigma, St Louis, MO, USA), Staphylococcus aureus, Cowan strain-1 (SACS-1, 0·01%, Pansorbin, no. 507861: Calbiochem, La Jolla, CA, USA) and herpes simplex virus-1 (HSV-1, 1 × 105 plaque-forming units, kindly provided by Dr Masaki Yasukawa, Japan) were added to the cultures. After 24 h, the culture supernatants were harvested and the levels of interleukin (IL)-6, IL-10, IL-12p70, tumour necrosis factor (TNF)-α and interferon (IFN)-γ (BD Pharmingen) were calculated using a commercial kit for the cytometric bead array, as described [18]. The standard negative control containing 0 pg/ml of different cytokines and the standard positive control containing 5000 pg/ml of different cytokines were provided with the kit. The mean fluorescence intensities of the standard negative control, different dilutions of standard positive controls and culture supernatants were measured using flow cytometry (FACSCalibur flow cytometer). A standard curve was prepared from the mean fluorescence intensities of the negative control and various dilutions of the positive controls using Cytometric Bead Array Software (BD Biosciences). The levels of cytokines in the culture supernatants were calibrated from the mean fluorescence intensities of the samples by Cytometric Bead Array Software (BD Biosciences) using a Macintosh computer (SAS Institute, Cary, NC, USA).
Statistical analysis
The data are shown as mean ± standard deviation. Means were compared using Student's t-test. For differences determined by F-test, the t-test was adjusted for unequal variances (Mann–Whitney U-test). P < 0·05 was considered to be statistically significant. Statistical calculations were performed using statview (version 5·0) statistical computer program.
Results
Features of isolated liver DCs
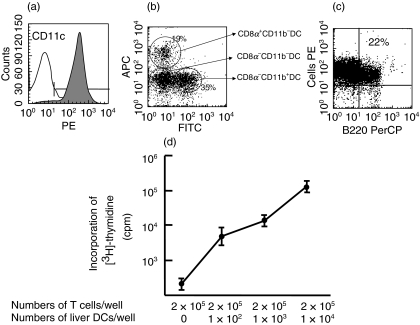
In this study, DCs were isolated from mouse liver using perfusion, density centrifugation and magnetic cell sorting without using cytokines or growth factors. Phenotypic analyses revealed that more than 95% of the liver DCs expressed CD11c (Fig. 1a). Different subtypes of liver DCs were also detected based on their expression of CD8α and CD11b (Fig. 1b). About one-fourth of the liver DCs expressed the B220 antigen, indicating that these liver DCs represented plasmacytoid DCs (Fig. 1c). Functional analysis revealed that liver DCs stimulated allogenic T cells from C3H/He mice in a dose-dependent manner (Fig. 1d). Histopathological analysis revealed no features of hepatitis in HBV-TM (data not shown).
Fig. 1.
Phenotypic and functional characterization of liver DCs. Liver DCs phenotypes were assessed in five separate experiments. The flow cytometric profile of one representative staining pattern was shown. (a) Flow cytometric analysis revealed that >95% of the liver DCs were expressing CD11c. (b) Liver DCs were subdivided into three subsets based on the expression of CD8α and CD11b. (c) Approximately one-fourth of the liver DCs expressed B220, a marker of plasmacytoid DCs. (d) The T cell proliferation-inducing capacities of liver DCs from five separate experiments are shown. Liver DCs from C57BL/6 mice stimulated allogenic T cells from C3H/He mice in a dose-dependent manner.
Phenotypes of liver DCs from normal C57BL/6 mice and HBV-TM
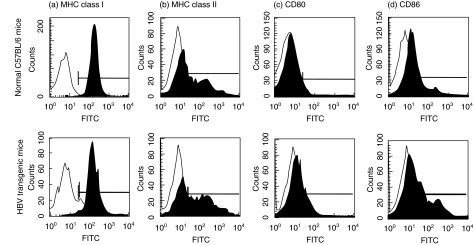
The expression levels of MHC class I, MHC class II, CD80 and CD86 on liver DCs from normal C57BL/6 mice and HBV-TM did not exhibit any significant difference. The data shown in Fig. 2 are the representative flow cytometric profiles of five separate experiments of liver DCs from age- and sex-matched normal C57BL/6 mice and HBV-TM.
Fig. 2.
There was no significant difference in the expression of MHC class I, MHC class II, CD80, and CD86 on liver DCs from normal C57BL/6 mice and HBV-TM. Liver DCs were isolated from the mouse liver without use of cytokines or growth factors. Five separate experiments were conducted to evaluate the expression of MHC class I, MHC class II, CD80 and CD86 on liver DCs using direct flow cytometry. The data of one representative experiment are shown.
Low T cell proliferation-inducing capacity of liver DCs from HBV-TM
The T cell proliferation-inducing capacities of liver DCs were assessed in an allogenic mixed lymphocyte reaction (MLR), where allogenic T lymphocytes from C3H/He mice were cultured with liver DCs from normal C57BL/6 mice or HBV-TM. To evaluate the ability of liver DCs to induce proliferation of HBsAg-enriched lymphocytes, HBsAg-enriched T lymphocytes were cultured with liver DCs either without or with HBsAg. The blastogenesis levels in lymphocyte proliferative assays were measured in cpm. The blastogenesis levels in allogenic MLR containing liver DCs from normal C57BL/6 mice and liver DCs from HBV-TM were 101 299 ± 13 293 (n = 5), and 14 689 ± 6308 (n = 5) cpm, respectively (P < 0·01) (Table 1).
Table 1.
Low T cell proliferation-inducing ability of liver DCs from HBV transgenic mice.
| Mouse | T cell proliferation-inducing capacity (cpm) | Mean ± standard deviation (cpm) |
|---|---|---|
| Normal C57BL/6 mice | ||
| Experiment 1 | 78 873 | |
| Experiment 2 | 101 001 | |
| Experiment 3 | 105 306 | 101 299 ± 13 293 |
| Experiment 4 | 113 169 | |
| Experiment 5 | 108 144 | |
| HBV-transgenic mice | ||
| Experiment 1 | 12 828 | |
| Experiment 2 | 7 097 | |
| Experiment 3 | 20 419 | 14 689 ± 6308* |
| Experiment 4 | 21 950 | |
| Experiment 5 | 11 151 | |
Liver DCs from normal C57BL/6 mice and HBV transgenic mice were cultured with allogenic T cells from C3H/He mice. The blastogenesis levels were assessed by incorporating [3H]-thymidine in T lymphocytes from 12 microtitre wells and measured as counts per minutes (cpm). Mean and standard deviation of cpm of five separate experiments are shown. The mean cpm in cultures containing only 2 × 105 T lymphocytes or only 1 × 104 liver DCs were less than 500 and 100 cpm, respectively.
P = 0·009, compared to the mean cpm of cultures containing liver DCs from normal C57BL/6 mice.
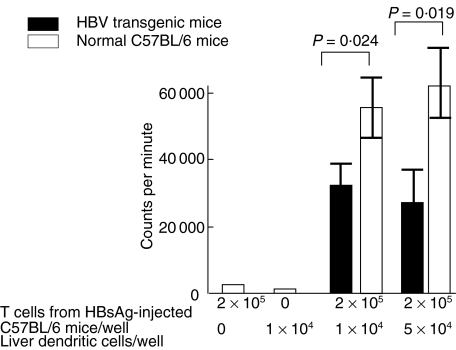
The ability of liver DCs from HBV-TM and normal C57BL/6 mice to induce proliferation of HBsAg-enriched lymphocytes was evaluated in lymphoproliferative assays. In comparison to liver DCs from normal C57BL/6 mice, liver DCs from HBV-TM had significantly decreased capacity to stimulate HBsAg-enriched lymphocytes (Fig. 3). Although the blastogenesis levels increased along with increased amounts of HBsAg in culture, liver DCs from HBV-TM induced significantly lower levels of proliferation of HBsAg-enriched lymphocytes at all doses of HBsAg (data not shown).
Fig. 3.
Decreased ability of liver DCs from HBV-TM to induce proliferation of HBsAg-enriched lymphocytes. HBsAg-enriched lymphocytes (2 × 105) were produced by injecting HBsAg in adjuvant to normal C57BL/6 mice, as described in the Methods section. HBsAg-enriched lymphocytes were cultured with liver DCs from normal C57BL/6 mice and HBV-TM (1 × 104 and 5 × 104) for 4 days in the presence of HBsAg (50 ng/ml), as described in the Methods section. The incorporation of [3H]-thymidine during the last 12 h of the culture was assayed and expressed as counts per minute (cpm). The mean levels of cpm of five separate experiments are shown. The mean cpm in cultures containing only 2 × 105 T lymphocytes or only 1 × 104 liver DCs were less than 500 and 100 cpm, respectively. HBsAg-enriched lymphocytes did not proliferate if HBsAg was not added to the cultures.
Decreased levels of cytokines in the supernatants of allogenic MLR containing liver DCs from HBV-TM
The T cell proliferation-inducing capacities of liver DCs from HBV-TM were significantly lower than those of liver DCs from normal C57BL/6 mice. However, the expression of surface antigens was almost the same on liver DCs from HBV-TM when compared to normal C57BL/6 mice. To determine the underlying causes, we estimated the levels of various cytokines in the supernatants of allogenic MLR. As shown in Table 2, the levels of IL-12p70, TNF-α, IFN-γ and IL-10 were significantly lower in the supernatants of allogenic MLR cultures containing liver DCs from HBV-TM compared to cultures containing liver DCs from normal C57BL/6 mice. However, the levels of IL-6 in allogenic MLR supernatants containing liver DCs from normal C57BL/6 mice and HBV-TM are almost comparable (Table 2).
Table 2.
Decreased levels of cytokines in the supernatants of allogenic MLR cultures containing liver DCs from HBV transgenic mice.
| IL-12p70 | TNF-α | IFN-γ | IL-6 | IL-10 | |
|---|---|---|---|---|---|
| Normal C57BL/6 mice | 101 ± 43 | 422 ± 98 | 2825 ± 608 | 63 ± 32 | 1494 ± 820 |
| HBV transgenic mice | 13 ± 7* | 167 ± 45* | 1018 ± 293* | 71 ± 41 | 227 ± 56* |
Liver DCs were isolated from normal C57BL/6 and HBV transgenic mice. Liver DCs (1 × 104) and allogenic T cells (2 × 105) were cultured for 4 days. The levels of cytokines in the culture supernatants were measured using the cytometric bead array method and are expressed as pg/ml. Data are shown as mean ± standard deviation of duplicate cultures of five separate experiments.
P < 0·05 compared to normal C57BL/6 mice.
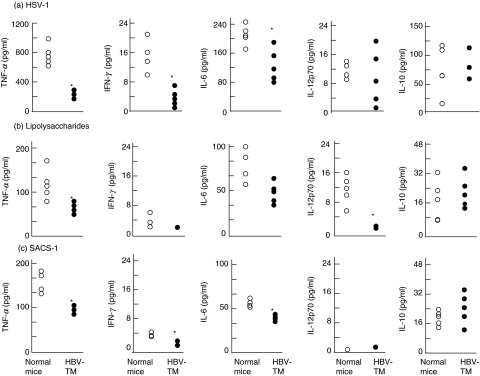
Decreased cytokine-producing ability of liver DCs from HBV-TM in response to bacterial and viral products
The levels of various cytokines in allogenic MLR culture supernatants containing liver DCs from HBV-TM were low. However, this does not reflect the cytokine-producing ability of only liver DCs because both T cells and DCs produce cytokines in allogenic MLR. To study the cytokine-producing ability of the pure population of liver DCs, we cultured liver DCs from normal C57BL/6 mice and HBV-TM with different viral and bacterial products, such as lipopolysaccharides, SACS and HSV-1. The levels of various cytokines produced by liver DCs varied considerably based on the nature of the stimulants. However, liver DCs from HBV-TM produced lower levels of various cytokines, except IL-10, when compared to those produced by liver DCs from normal C57BL/6 mice (Fig. 4).
Fig. 4.
Decreased production of proinflammatory cytokines from liver DCs of HBV-TM due to stimulation with viral and bacterial products. Liver DCs from normal (open circles) and HBV-TM (filled circles) were stimulated with HSV-1 (1 × 105 plaque forming unit) (a), lipopolysaccharides (5 µg) (b) and SACS (0·01%) (c) for 24 h. The levels of cytokines in the culture supernatants were measured using the cytometric bead array method and expressed as pg/ml. *P < 0·05 compared to the levels of cytokines produced by liver DCs from normal C57BL/6 mice.
Discussion
The purpose of this study was to assess the functional ability of liver DCs in HBV-TM and to determine the role, if any, of liver DCs in impaired HBV-specific immune responses in HBV-TM. There were some distinctive features to this study. First, we used HBV-TM, an animal model of the HBV carrier state. HBV-TM have been used widely for studying immunopathogenesis of the chronic HBV carrier state when it could not be studied directly in human subjects [19,20]. These HBV-TM express HBV-related mRNAs (3·5, 2·1 and 0·08 kb) and antigens (HBsAg and hepatitis B core antigen) in the liver [16]. Furthermore, liver DCs expressed HBV DNA. Therefore, liver DCs of HBV-TM reside in a tissue microenvironment, where HBV is replicating continuously. We isolated fresh liver DCs, which were not expanded in vivo or cultured in vitro by cytokines or other immune modulators because these mediators induce maturation and activation of liver DCs [12–14]. In addition, all functional assays were conducted just after isolation of the liver DCs. This study was conducted using liver DCs which mostly retained their in situ phenotypes and functions.
There was no significant difference in the frequencies of liver DCs between normal C57BL/6 mice and HBV-TM. Also, the viability of liver DCs did not differ between these mice (data not shown). However, in comparison to liver DCs from normal C57BL/6 mice, the T cell proliferation-inducing capacities of liver DCs from HBV-TM were significantly lower. This was evident in allogenic MLR and HBsAg-enriched lymphoproliferative assays. Furthermore, liver DCs from HBV-TM produced significantly lower amounts of IL-12p70, IFN-γ and TNF-α in response to viral and bacterial products.
Human liver DCs secrete significant amounts of IL-10 and very little IL-12p70 [21]. Moreover, Hyodo et al. have reported that hepatitis B core antigen stimulates IL-10 secretion from peripheral blood T cells and monocytes from patients with chronic hepatitis B [22]. We also found high levels of IL-10 and low levels of IL-12 in cultures containing liver DCs from normal C57BL/6 mice and HBV-TM. However, IL-10 production by liver DCs due to stimulation with HSV-1, lipopolysaccharides and SACS was not significantly different between normal C57BL/6 mice and HBV-TM. It would be interesting to evaluate IL-10 production from hepatitis B core antigen-stimulated DCs.
To the best of our knowledge, this is the first study about liver DCs in HBV carriers, although there are some studies about spleen and blood DCs in HBV carriers [9–11]. Although those studies provided some insights into DC/HBV interactions, blood and spleen DCs are not involved in the recognition, capture and processing of viruses. In fact, these DCs represent the overall status of DCs under pathological conditions and their functions show extreme heterogeneity among infected subjects. This has been supported by studies regarding DCs in chronic hepatitis C virus infection. Bain et al. have shown a decrease in the allostimulatory ability and lower IL-12 production by blood DCs in patients with chronic hepatitis C [23], but Longman et al. did not find any functional defects of blood DCs in these patients [24].
However, the most important question is why the functions of liver DCs were impaired in HBV-TM. This is an unresolved but important issue regarding the DC/virus interaction. Possibly, the HBV was not properly recognized by liver DCs in the HBV-TM in spite of having abundant amounts of HBV and their antigens around liver DCs. Liver DCs from HBV-TM produced lower levels of proinflammatory cytokines. Ridge et al. have shown that proinflammatory cytokines provide the inflammatory mucosal milieu, which are required for recognition of viruses as ‘danger’ and ‘harmful entities’ by DCs [25]. In addition, the HBV may interfere with the antigen capturing and processing abilities of liver DCs. Some viruses also block the maturation of DCs. Further studies are required to clarify the underlying mechanism which impairs the functional capabilities of liver DCs in HBV-TM.
To our knowledge, there is no report about the phenotypes and functions of liver DCs in other transgenic mouse models. We checked the functions of liver DCs in HBV-TM; however, it is important to characterize liver DCs in patients with chronic hepatitis B in order to transfer what is learned in the laboratory to the patient's bedside. We have reported previously about the localization of CD83-positive liver DCs in patients with chronic hepatitis B [26]. At this time, there is no established methodology of isolating liver DCs from human livers for a functional study, although it might be possible to isolate liver DCs from a transplanted liver. Furthermore, it might be possible to check the cytokine-producing ability of liver DCs by isolating the liver-infiltrating cells and then checking their expression of intracellular cytokines.
Characterization of liver DCs in chronic HBV infection is also important in the context of DC-based therapy for chronic HBV carriers. We have shown that the administration of antigen-pulsed spleen DCs induced antigen-specific immune responses in HBV-TM, but could not reduce HBV replication [27–29]. There is little information regarding the utility of antigen-pulsed tissue DCs for treatment purposes. The therapeutic utility of HBsAg-pulsed liver DCs in HBV-TM should be assessed. Furthermore, it would be interesting to see if the use of antigen-pulsed spleen DCs alters the functions of liver DCs in HBV-TM.
In conclusion, we isolated liver DCs from murine liver without using cytokines or growth factors. To the best of our knowledge, this is the first study showing that the functions of liver DCs were impaired in HBV-TM. This study also provides the first direct evidence that liver DCs might be responsible for impaired HBV-specific immune responses of HBV-TM. At the same time, this study inspires hope that DC-based therapy may be developed for chronic HBV carriers by up-regulating the functions of liver DCs.
Acknowledgments
We would like to thank San Francisco Edit (http://www.sfedit.net) for their assistance in editing this manuscript.
References
- 1.Hilleman MR. Critical overview and outlook: pathogenesis, prevention, and treatment of hepatitis and hepatocarcinoma caused by hepatitis B virus. Vaccine. 2003;21:4626–49. doi: 10.1016/s0264-410x(03)00529-2. [DOI] [PubMed] [Google Scholar]
- 2.Lok AS. Hepatitis B infection: pathogenesis and management. J Hepatol. 2000;32(Suppl.):89–97. doi: 10.1016/s0168-8278(00)80418-3. [DOI] [PubMed] [Google Scholar]
- 3.Rehermann B, Fowler P, Sidney J, et al. The cytotoxic T lymphocyte response to multiple hepatitis B virus polymerase epitopes during and after acute viral hepatitis. J Exp Med. 1995;181:1047–58. doi: 10.1084/jem.181.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chisari FV, Ferrari C. Hepatitis B virus immunopathogenesis. Annu Rev Immunol. 1995;13:29–60. doi: 10.1146/annurev.iy.13.040195.000333. [DOI] [PubMed] [Google Scholar]
- 5.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–96. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 6.Morel PA, Feili-Hariri M, Coates PT, Thomson AW. Dendritic cells, T cell tolerance and therapy of adverse immune reaction. Clin Exp Immunol. 2003;133:1–10. doi: 10.1046/j.1365-2249.2003.02161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Onji M. Dendritic cells in clinics. Tokyo: Springer; 2004. [Google Scholar]
- 8.Steinman RM. Some interfaces of dendritic cell biology. APMIS. 2003;111:675–97. doi: 10.1034/j.1600-0463.2003.11107802.x. [DOI] [PubMed] [Google Scholar]
- 9.Arima S, Akbar SMF, Michitaka K, et al. Impaired function of antigen-presenting dendritic cells in patients with chronic hepatitis B. Localization of HBV DNA and HBV RNA in blood DC by in situ hybridization. Int J Mol Med. 2003;11:169–74. [PubMed] [Google Scholar]
- 10.Beckebaum S, Cicinnati VR, Dworacki G, et al. Reduction in the circulating pDC1/pDC2 ratio and impaired function of ex vivo-generated DC1 in chronic hepatitis B infection. Clin Immunol. 2002;104:138–50. doi: 10.1006/clim.2002.5245. [DOI] [PubMed] [Google Scholar]
- 11.Akbar SMF, Onji M, Inaba K, Yamamura K, Ohta Y. Low responsiveness of hepatitis B virus-transgenic mice in antibody response to T-cell-dependent antigen: defect in antigen-presenting activity of dendritic cell. Immunology. 1993;78:468–75. [PMC free article] [PubMed] [Google Scholar]
- 12.Figdor CG, de Vries IJ, Lesterhuis WJ, Melief CJ. Dendritic cell immunotherapy: mapping the way. Nat Med. 2004;10:80. doi: 10.1038/nm1039. [DOI] [PubMed] [Google Scholar]
- 13.Steptoe RJ, Fu F, Li W, et al. Augmentation of dendritic cells in murine organ donors by Flt3 ligand alters the balance between transplant tolerance and immunity. J Immunol. 1997;159:5483–91. [PubMed] [Google Scholar]
- 14.Lu L, Maureen L, Drakes ML, Thomson AW. Isolation and propagation of mouse liver-derived dendritic cells. In: Robinson SP, Stagg AJ, editors. Dendritic cell protocols. Totowa, NJ: Humana Press; 2001. pp. 85–95. [DOI] [PubMed] [Google Scholar]
- 15.Lian Zx Okada T, He XS, et al. Heterogeneity of dendritic cells in the mouse liver: identification and characterization of four distinct populations. J Immunol. 2003;170:2323–30. doi: 10.4049/jimmunol.170.5.2323. [DOI] [PubMed] [Google Scholar]
- 16.Araki K, Miyazaki J, Hino O, Tomita N, Chisaka O, Matsubara K, Yamamura K. Expression and replication of hepatitis B virus genome in transgenic mice. Proc Natl Acad Sci USA. 1989;86:207–11. doi: 10.1073/pnas.86.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akbar SMF, Abe M, Masumoto T, Horiike N, Onji M. Mechanism of action of vaccine therapy in murine hepatitis B virus carriers: vaccine-induced activation of antigen presenting dendritic cells. J Hepatol. 1999;30:755–64. doi: 10.1016/s0168-8278(99)80125-1. [DOI] [PubMed] [Google Scholar]
- 18.Kobayashi Y, Murakami H, Akbar SM, Matsui H, Onji M. A novel and effective approach of developing aggressive experimental autoimmune gastritis in neonatal thymectomized BALB/c mouse by polyinosinic : polycytidylic acid. Clin Exp Immunol. 2004;136:423–31. doi: 10.1111/j.1365-2249.2004.02467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chisari FV. Hepatitis B virus transgenic mice. models of viral immunobiology and pathogenesis. Curr Top Microbiol Immunol. 1996;206:149–73. doi: 10.1007/978-3-642-85208-4_9. [DOI] [PubMed] [Google Scholar]
- 20.Akbar SMF, Onji M. Hepatitis B virus (HBV)-transgenic mice as an investigative tool to study immunopathology during HBV infection. Int J Exp Pathol. 1998;33:909–13. doi: 10.1046/j.1365-2613.1998.740406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goddard S, Youster J, Morgan E, Adams DH. Interleukin-10 secretion differentiates dendritic cells from human liver and skin. Am J Pathol. 2004;164:511–9. doi: 10.1016/S0002-9440(10)63141-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hyodo N, Nakamura I, Imawari M. Hepatitis B core antigen stimulates interleukin-10 secretion by both T cells and monocytes from peripheral blood of patients with chronic hepatitis B virus infection. Clin Exp Immunol. 2004;135:462–6. doi: 10.1111/j.1365-2249.2003.02376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bain C, Fatmi A, Zoulim F, Zarski JP, Trepo C, Inchauspe G. Impaired allostimulatory function of dendritic cells in chronic hepatitis C infection. Gastroenterology. 2001;120:512–24. doi: 10.1053/gast.2001.21212. [DOI] [PubMed] [Google Scholar]
- 24.Longman RS, Talal AH, Jacobson IM, Albert ML, Rice CM. Presence of functional dendritic cells in patients chronically infected with hepatitis C virus. Blood. 2004;103:1026–9. doi: 10.1182/blood-2003-04-1339. [DOI] [PubMed] [Google Scholar]
- 25.Ridge JP, Fuchs EJ, Matzinger P. Neonatal tolerance revisited: turning on newborn T cells with dendritic cells. Science. 1996;271:1723–6. doi: 10.1126/science.271.5256.1723. [DOI] [PubMed] [Google Scholar]
- 26.Tanimoto K, Akbar SMF, Michitaka K, Horiike N, Onji M. Antigen-presenting cells at the liver tissue in patients with chronic viral liver diseases: CD83-positive mature dendritic cells at the vicinity of focal and confluent necrosis. Hepatol Res. 2001;21:117–25. doi: 10.1016/s1386-6346(01)00084-5. [DOI] [PubMed] [Google Scholar]
- 27.Akbar SMF, Horiike N, Onji M. Prognostic importance of antigen presenting dendritic cells during vaccine therapy in murine hepatitis B virus carriers. Immunology. 1999;96:98–108. doi: 10.1046/j.1365-2567.1999.00668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akbar SMF, Furukawa S, Horiike N, Onji M. Vaccine therapy for hepatitis B virus carrier. Curr Drug Targets Infect Disord. 2004;4:93–101. doi: 10.2174/1568005043340885. [DOI] [PubMed] [Google Scholar]
- 29.Akbar SMF, Furukawa S, Hasebe A, Horiike N, Michitaka K, Onji M. Production and efficacy of a dendritic cell-based therapeutic vaccine for murine chronic hepatitis B virus carrier. Int J Mol Med. 2004;14:295–9. [PubMed] [Google Scholar]