Abstract
The bisphosphonates are a novel class of drug that have been registered for various clinical applications worldwide. Bisphosphonates, and in particular the aminobisphosphonates (nBPs), are known to have a number of side-effects including a rise in body temperature and accompanying flu-like symptoms that resemble a typical acute phase response. The mechanism for this response has been partially elucidated and appears to be associated with the release of tumour necrosis factor (TNF)α and interleukin (IL)6, although the effector cells that release these cytokines and the mechanism of action remain enigmatic. Here, we show that the nBP-induced acute phase response differs from the typical acute phase response in that CD14+ cells such as monocytes and macrophages are not the primary cytokine producing cells. We show that by inhibiting the mevalonate pathway, nBPs induce rapid and copious production of TNFα and IL6 by peripheral blood γδ T cells. Prior treatment with statins, which inhibit 3-hydroxy-3-methylglutaryl coenzyme A (HMG CoA) reductase, blocks nBP-induced production of these proinflammatory cytokines by γδ T cells and may offer a means of avoiding the associated acute phase response. In addition, our findings provide a further mechanism for the anti-inflammatory effects attributed to inhibitors of HMG CoA reductase.
Keywords: bisphosphonates, acute phase response, proinflammatory cytokines, γδ T cells, aminobisphosphonates, statins
Introduction
Bisphosphonates (BPs) are currently in use for the treatment of Paget's disease [1,2], osteoporosis [3], hypercalcaemia [4], multiple myeloma and metastatic bone disease [5–7]. The clinical success of the BPs etidronate and clodronate in the 1970s and 1980s led to trials of BPs with different alkyl chains. In particular, bisphosphonates containing an amino group in their alkyl chain, such as pamidronate and alendronate, were found to be 10- to 100-fold more potent at inhibiting bone resorption than the early nonamino bisphosphonates [8]. The latest generation of bisphosphonates, such as risedronate and zoledronate, contain a nitrogen atom within a heterocyclic ring [9] and have been shown to be up to 10,000-fold more potent than etidronate in experimental systems [10]. Recent mechanistic studies indicate that the bisphosphonates can, broadly speaking, be classified into two groups based on their mode of action: (i) those resembling pyrophosphate (e.g. etidronate, clodronate, and tiludronate) that can be incorporated into cytotoxic ATP analogs; and (ii) the more potent nitrogen-containing bisphosphonates that interfere with other metabolic pathways (e.g. the mevalonate pathway) (reviewed in [11]). Bisphosphonates, and in particular the aminobisphosphonates (nBPs), are known to have a number of side-effects (reviewed in [11]) including a rise in body temperature and accompanying flu-like symptoms that resemble a typical acute phase response; these clinical features occur in over a third of patients receiving treatment for the first time [12,13]. The mechanism for this response has been partially elucidated and appears to be associated with the release of TNFα and IL6 [14–16], although the effector cells that release these cytokines and the mechanism of action remain obscure. nBPs are known to inhibit farnesyl pyrophosphate (FPP) synthase [17, 18, 19, 20, 21, 22]. It has recently been shown that this inhibition of the mevalonate pathway leads to an accumulation of metabolic intermediates including isopentenyl pyrophosphate (IPP) [23]. IPP is a potent activator of human peripheral blood γδ T cells [24,25] and nBPs have also been shown to activate these cells [23, 25, 26, 27, 28, 29]. As the acute phase response has not been observed with the nonaminobisphosphonates etidronate, clodronate or tiludronate, and is thus a specific feature of the nBPs, it seems feasible that this phenomenon is mediated through γδΤ cell activation. Here, we show that nBPs induce rapid and copious production of TNFα and IL6 by peripheral blood γδ T cells. Blockade of HMG CoA reductase by statin pretreatment abrogates this effect.
Materials and methods
Drugs
The following bisphosphonates and statins were used: disodium etidronate (Procter & Gamble Pharmaceuticals Ltd, Staines, UK), disodium clodronate (Roche Products Ltd, Welwyn Garden City, UK), disodium pamidronate (Novartis, Camberley, UK), risedronate sodium (Procter & Gamble Pharm), simvastatin (Ranbaxy Laboratories Ltd, Ealing, UK), pravastatin (Bristol-Myers Squibb Pharmaceuticals, Hounslow, UK) and fluvastatin (Sandoz Pharmaceuticals, Surrey, UK).
γδ T cell culture
γδ T cells were isolated from human peripheral blood using a magnetic bead-based separation kit (TCR γ/δ Microbead Kit and MS columns, Miltenyi Biotech, Surrey, UK). Once isolated, cells were cloned by limiting dilution in T cell medium (RPMI supplemented with penicillin, streptomycin and glutamine, 10% fetal calf serum (FCS), 10% T-STIM (BD Biosciences, Oxford, UK), 200 U/ml Proleukin (Chiron, Cranford, UK)) containing 2 × 106γ-irradiated human peripheral blood mononuclear cells (PMBC)/ml from at least 3 unrelated donors and 2 µg/ml phytohaemagglutinin (PHA). Clones were maintained with T cell medium, and restimulated with mixed irradiated PBMC and PHA every three weeks. The IMGT system of TCR nomenclature is used throughout this work [30]. Cells that grew were confirmed to be γδ T cells by flow cytometry with TCR chain-specific monoclonal antibodies (mAbs).
Antibodies
The following antibodies were used for flow cytometric analysis: fluorescein isothiocyanate (FITC)-conjugated mouse anti-human Vδ2 TCR mAb clone B6·1 (Pharmingen, Oxford, UK), FITC-conjugated mouse anti-human Vγ9 mAb clone 7A5 (Endogen, Perbio Science UK Ltd, Cramlington, UK), FITC-conjugated mouse anti-human Vδ1 mAb clone TS8·2 (Endogen), FITC-conjugated mouse anti-human CD14 mAb clone UCHMT1 (BD Biosciences), phycoerythrin (PE)-conjugated mouse anti-human Vγ9 mAb clone B3·1 (Pharmingen), PE-conjugated mouse anti-human IL6 clone AS12 (BD Biosciences), peridinin chlorophyll protein (PerCP)-conjugated mouse anti-human CD3 mAb clone SK7 (BD Biosciences), allophycocyanin (APC)-conjugated mouse anti-human IL2 clone MQ1–17H12 (Caltag-Medsystems Ltd, Towcester, UK), APC-conjugated mouse anti-human TNFα clone mAb11 (Pharmingen), and APC-conjugated mouse anti-human IFNγ mAb clone B27 (Pharmingen).
IFNγ ELISpot and TNFα ELISA
γδ T cells were washed in RPMI and incubated overnight in R10 at 37°C. 96-well nitrocellulose plates (Millipore Ltd, Watford, UK) were incubated overnight at 4°C with 15 µg/ml anti-human-IFNγ primary antibody (clone 1-D1K; Mabtech, Nacka Strand, Sweden). The plates were then washed twice with RPMI and blocked with R10 for 3 h at 37°C. R10 was decanted by inversion and assays applied to each well before incubation at 37°C as detailed below. Assays were terminated by washing once in water, followed by 5 washes in PBS. Secondary antibody (anti-human IFNγ-biotin clone 7-B6-1; Mabtech) was applied at 1 µg/ml and the plate incubated for 100 min at room temperature (RT). The plate was washed 6 times with PBS before application of streptavidin-alkaline phosphatase (AP) (1 : 1000 in PBS; Mabtech) for 40 min at RT. After 6 further washes in PBS, spots were revealed by incubation for 15 min at RT with developing buffer (Bio-Rad AP conjugate substrate kit) and counted mechanically using an ELISpot Reader System ELR02 (Autoimmun Diagnostika; Strassberg, France). An ELISA kit (Peprotech EC Ltd, London, UK) was used to quantify TNFα release into the assay supernatant according to the manufacturer's instructions.
Intracellular cytokine staining
106 fresh PBMC were incubated in FACS tubes with brefeldin A (10 µg/ml in R10) for 5 h after incubation with relevant antigens for 1 h. The cells were then washed and stained with PE-conjugated anti-Vγ9, FITC-conjugated anti-Vδ2 or anti-CD14 mAbs, and for viability with 7-AAD for 20 min on ice. The cells were then washed again and permeabilized in 10% FACSLyse (BD Biosciences), washed twice in ice cold PBS/0·1% BSA and stained on ice with pretitred APC-conjugated anti-IFNγ, anti-TNFα, anti-IL-2 for 20 min. For single cytokine analysis, cells were stained with PE-conjugated anti-IL6, APC-conjugated anti-TNFα and FITC-conjugated anti-Vγ9 antibodies. In each case, cells were then washed, resuspended in PBS and analysed immediately.
γδ T cell depletion from PBMC
108 human PBMC were incubated with 10 µg/ml anti-human pan-γδ antibody for 30 min on ice [25]. Cells were washed once in 20X labelling volume with PBS/0·1%BSA and resuspended at 4 × 107 cells/ml in PBS/0·1%BSA. 2 × 107 anti-mouse IgG1 Dynabeads (Dynal Biotech ASA, Norway) washed in PBS/0·1%BSA were added and the cells incubated for 1 h at 4°C with gentle rotation. The tube was then placed in a magnetic particle concentrator (Dynal) and left to separate for 30 min. The supernatant was transferred to a fresh tube. Depletion efficiency was determined by FACS analysis with the anti-CD3 and pan-γδ antibodies detailed above, and was > 95% efficient in all cases shown.
Results
nBPs induce rapid and copious production of TNFα by peripheral blood γδ T cells
The dominant subset of human γδ T cells in peripheral blood bears T cell receptors (TCRs) comprising variable regions encoded by the Vγ9 and Vδ2 genes. These cells are known to activate in response to alkylphosphate, alkylamine and some bisphosphonate antigens. It is known that this recognition is a characteristic of cells bearing a Vγ9 chain in conjunction with a JP joining region [31]. Vγ9JP-expressing cells are highly enriched in the peripheral blood when compared to percentages in thymocytes or cord blood [31,32]. The peripheral expansion of Vγ9 cells using JγP appears to be antigen-driven. γδ T cells in cord blood mononuclear cells (CBMC) express a diverse repertoire of γδ TCRs [33]. Stimulation of CBMC with alkylphosphate antigen, but not with PHA, induces the preferential expansion of T cells bearing Vγ9Vδ2 TCRs; 70% of those TCRs expanded with alkyl-phosphate antigen use a JγP joining region compared to just 20% of those cells expanded with PHA [33].
We grew several Vγ9JPVδ2 T cell clones as described in the Materials and methods. TCR usage in these clones was characterized with a molecular analysis of gene expression [25]. These cells were shown to activate after exposure to the nBPs pamidronate and risedronate, but not the nonamino-BPs etidronate and clodronate, by IFNγ ELISpot (Fig. 1a). Vγ9JPVδ2 T cells also made large amounts of TNFα after exposure to nBPs (Fig. 1c). Production of TNFα was both copious and rapid (Fig. 1c). A Vγ9JPVδ1-expressing clone was unable to recognize these ligands [25], indicating that the δ2 chain also plays an essential role in the response of Vγ9JPVδ2 T cells to nBPs. This result is in agreement with those of a previous study that showed Vγ9Vδ1 T cell clones did not respond functionally to monoethyl phosphate [34]. Pamidronate and risedronate were also able to induce the rapid production of large amounts of TNFα from direct ex vivo human peripheral blood mononuclear cells (PBMC) (Fig. 1b). The results above suggest that Vγ9Vδ2 T cells in human PBMC might be the source of nBP-induced proinflammatory cytokines. We tested this hypothesis by depleting γδ T cells from PBMC. Magnetic depletion of γδ T cells removed the ability of PBMC to produce TNFα in response to pamidronate (Fig. 2a) and risedronate (Fig. 2b). We used flow cytometry to characterize further the cellular source of acute phase response-inducing cytokines after exposure of human PBMC to nBPs. Intracellular cytokine staining (ICS) showed that exposure of PBMC to nBPs activated only the peripheral blood lymphocyte population bearing a Vγ9 TCR and not other lymphocytes (Fig. 2c). Vγ9-expressing cells were shown to make both IL6 and TNFα in response to pamidronate and risedronate by ICS (Fig. 2d and data not shown). Several recent reports show that nBPs can activate γδ T cells in vitro or directly ex vivo[23, 25, 26, 27, 28, 29]. Kunzmann et al. [35] found that 4/10 patients given 60 or 90 mg infusions of pamidronate had an acute phase reaction. All of these patients showed a substantial increase in the number of circulating γδ T cells when measured 1 and 3 weeks post-infusion [35]. In one patient, γδ T cells expanded from 4·6% to 70% of CD3+ cells post-infusion. Thus, nBPs are likely to activate γδ T cells in vivo to produce acute phase response-inducing cytokines.
Fig. 1.
nBPs induce rapid and copious production of TNFα by peripheral blood γδ T cells. (a) nBPs, but not nonaminoBPs, activate the Vγ9JPVδ2 T cell clone Bob in IFNγ ELISpot. The TCR of this clone has been sequenced and is published elsewhere [25]. ELISpots were performed with 1000 Vγ9JPVδ2 T cells and 25 000 spinner HeLa cells as antigen presenting cells per well and incubated for 6 h prior to development. Standard deviation from the mean of two replicate assays is shown, although in all cases these errors are smaller than the plot symbol. (b) nBPs stimulate TNFα production from direct ex vivo human PBMC. 106 fresh human PBMC were incubated in 75 × 5mm FACS tubes at 37°C and 5% CO2 in 1 ml of R10 (RPMI, 10% FCS, 100 U/ml penicillin, 100 µg/ml streptomycin), l ml of R10 + 10 µm risedronate or 1 ml of R10 + 100 µm pamidronate. 60 µl aliquots were removed at the specified times and added to TNFα ELISA assays. Error bars show standard deviation from the mean. (c) Activation of Vγ9JPVδ2 T cell clones by risedronate is extremely rapid. Standard deviation from the mean of two replicate TNFα ELISA assays is shown for three separate clones expressing a Vγ9JPVδ2 TCR. Clone P expresses CD8α; clones M and Bob are CD8–. Clone P appears to make more TNFα and to produce it earlier. 106 T cells were activated by 10 µm risedronate in 1 ml R10 in 75 × 5mm FACS tubes at 37°C and 5% CO2. 60 µl aliquots were removed at the specified times and added to TNFα ELISA assays. Background TNFα production after a similar time period in the absence of risedronate was negligible (data not shown).
Fig. 2.
The TNFα and IL6 produced by PBMC in response to nBPs is derived from γδ T cells. Magnetic depletion of γδ T cells from human PBMC removes their ability to manufacture TNFα in response to 100 µm pamidronate (a) and 10 µm risedronate (b). 5 × 106 PBMC from a healthy donor ± magnetic depletion of γδ T cells were suspended in 1 ml of R10 ± antigen in 75 × 5mm FACS tubes at 37°C and 5% CO2 for the times shown. 60 µl of cell supernatant was removed, applied to TNFα ELISA plates in duplicate and developed according to the manufacturers’ instructions. Standard deviation from the mean of the two replicate ELISAs is shown although in most cases this error is smaller than the plot symbol. (c) Exposure to nBPs activates only lymphocytes that express a Vγ9 receptor. 106 fresh human PBMC were exposed to R10 (top panel) or R10 + 10 µm risedronate (bottom panel) for 6 h in an intracellular cytokine staining (ICS) assay. Plots show all the cells in the lymphocyte gate stained for PE-Vγ9 and APC-cytokines (TNFα, IL2, and IFNγ) as described previously [25]. Exposure to risedronate induces cytokine production only in lymphocytes that express a Vγ9 TCR. The percentage of total lymphocytes in the Vγ9+cytokine+ gate shown is indicated in the upper right of each panel. Almost 10% of the lymphocytes expressing a Vγ9 receptor are activated by exposure to nBPs. Similar results were observed with 100 µm pamidronate (data not shown). It is noticeable that exposure to risedronate lowers the expression of the Vγ9 TCR. (d) Intracellular cytokine staining (ICS) shows that nBPs induce direct ex vivo Vγ9-expressing T cells to make TNFα and IL6. Plots are gated to show only Vγ9-expressing lymphocytes. The left hand panels show IL6 production induced by 100 µm pamidronate and the right hand panels show TNFα production induced by 10 µm risedronate. The percentage of cytokine positive cells (fluorescence intensity > 20) is shown in the upper right corner of each panel.
Cells of the monocytic lineage are not the primary source of nBP-induced TNFα or IL6
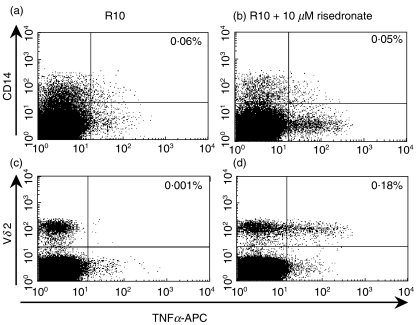
TNFα and IL6 are known to play central roles in the ‘typical’ acute phase response to infectious tissue injury [36]. While both TNFα and IL6 can be produced by many cell types, monocytes and macrophages at the site of inflammation are the major source of these cytokines during a typical acute phase response [36]. BPs are known to affect monocytic cells and have been observed to depress the accessory function of monocytes in lymphocyte proliferation assays [37]. Makkonen et al. [38] showed that pretreatment of RAW 264 macrophages with the nBP alendronate augments LPS-stimulated release of cytokines. However, alendronate itself did not induce cytokine release. These authors also showed that the nBP ibandronate enhances LPS-induced stimulation of IL6 in macrophages [39]. Again, there was no indication that the nBP per se induces activation. The fact that monocytes and macrophages are the primary source of TNFα and IL6 in a typical acute phase response, combined with knowledge that these compounds can affect such cells, has lead to the suggestion that cells of the monocytic lineage might be the primary source of nBP-induced cytokines [15]. However, there has been no formal testing of this hypothesis. The fact that γδ T cell-depleted PBMC, which still contain monocytes, do not manufacture TNFα in response to nBPs (Fig. 2a,b) suggests that this hypothesis is incorrect. To exclude monocyte lineage cells formally as the primary cellular source of the nBP-induced proinflammatory response, we positively identified these cells using an anti-CD14 monoclonal antibody to confirm that they do not produce TNFα in our assays (Fig. 3a,b). Parallel stains with a Vδ2-specific monoclonal antibody clearly showed that TNFα is produced by this subset of γδ T cells (Fig. 3c,d).
Fig. 3.
Incubation with nBPs does not activate cells of the monocytic lineage. Six hour exposure to 10 µm risedronate does not activate CD14+ cells. 106 fresh human PBMC were exposed to R10 (a,c) or R10 + 10 µm risedronate (b,d) in an ICS assay. (a,b) show all live cells as determined by dead cell exclusion with 7-amino-actinomycin D (7-AAD; BD BioSciences). CD14+ cells such as monocytes and macrophages do not produce TNFα when exposed to nBP for this duration. The cells that produce TNFα in this assay do not express CD14 (compare bottom right quadrants in (a) and (b)). The cells that do respond in this assay express a Vδ2 TCR (c,d). The number of cells in the upper right quadrant of each plot as a percentage of total live cells is indicated in the upper right corner. There were approximately 30% fewer CD14+ cells after 6 h treatment with nBP compared to treatment with R10 alone as a fraction of the CD14+ cells that became 7-AAD+.
Statins inhibit nBP-induced activation of γδ T cells
Recent studies indicate that nBPs may exert their effect on the activation of Vγ9JPVδ2 T cells via the mevalonate pathway [23,25] rather than acting as direct ligands for the γδ TCR as had been thought in previous studies [26, 27, 28, 29,40]. nBPs inhibit FPP synthase [17–21] and cause an accumulation of IPP within the cell [23] (Fig. 4). IPP is a potent activator of Vγ9Vδ2 T cells [24] and has recently been shown to activate these cells directly ex vivo[25]. Blocking the mevalonate pathway above IPP should prevent build up of IPP (Fig. 4). Indeed, inhibitors of HMG CoA reductase, the key enzyme at the beginning of this metabolic pathway, were able to inhibit nBP-induced activation of γδ T cells directly ex vivo(Fig. 5). Pravastatin, simvastatin and fluvastatin were all able to inhibit TNFα production by fresh PBMC as measured by ELISA (Fig. 5a). Addition of IPP was able to rescue TNFα production, thus ruling out the possible toxic effects of these statins at the concentrations used. However, statins and bisphosphonates have inhibitory effects on almost all cells as blocking the mevalonate pathway leads to the loss of Ras and other receptor signals due to the blockade of protein prenylation [41]. nBPs block FFP synthase and may act synergistically with inhibitors of 3-hydroxy-3-methylglutaryl coenzyme A (HMG CoA) reductase to kill cells. Any such effects may be overcome by the replenishment of cells with metabolites in the mevalonate pathway such as IPP. Thus, we felt it was important to examine the combined effects of statins and nBPs using flow cytometry. Pravastatin, simvastatin and fluvastatin were shown to inhibit the activation of Vγ9 cells by ICS (Fig. 5b). Once again, addition of IPP in addition to the statin was able to rescue activation and rule out toxic effects. Flow cytometric analyis using the viability marker 7-AAD showed that the γδ T cell population remained alive throughout the experiment when the statin and nBP were combined without addition of IPP (row 4 in Fig. 5b) (data not shown). Thus, prior administration of a statin is able to prevent the rapid and copious production of acute phase response-inducing cytokines by peripheral blood γδ T cells in response to nBPs.
Fig. 4.
The mevalonate biosynthetic pathway. nBPs inhibit farnesyl pyrophosphate (FPP) synthase and lead to a build up of IPP [23]. Statins inhibit HMG CoA reductase, a proximal enzyme in this pathway.
Fig. 5.
Statins inhibit nBP-induced TNFα production by human PBMC. (a) Pretreatment of human PBMC with 1 µm pravastatin, 100 n m simvastatin or 100 n m fluvastatin for 2 h inhibits their ability to manufacture TNFα in response to 10 µm risedronate. Addition of IPP restores TNFα production and controls for any toxicity effects of these statins. 5 × 105 statin-treated or untreated cells/well in 200 µl R10 were incubated with nBP for 12 h at 37°C and 5% CO2 in 96 well U-bottomed tissue culture plates ± 1 µm IPP. 60 µl of supernatant was assayed for TNFα content by ELISA (Peprotech). Assays were performed in duplicate. Bars show standard deviation from the mean of two replicate assays. (b) ICS shows that pretreatment of PBMC with statins inhibits nBP-induced activation of Vγ9-expressing T cells. FACS plots show results with 1 µm pravastatin. Panels show results of incubation for 6 h in R10 only on the top row, R10 + 10 µm risedronate in row 2, R10 + 10 µm IPP in row 3, R10 + 10 µm risedronate + pravastatin in row 4 and R10 + 10 µm risedronate + pravastatin + 10 µm IPP on the bottom row. Pravastatin inhibits nBP-induced activation of Vγ9-expressing T cells (compare rows 2 & 4). Addition of IPP with pravastatin (bottom row) rescues activation and controls for any toxic effect of the statin. The Vγ9-expressing cell population (less than 3% of total lymphocytes) is shown in black. Vγ9 cells expressing cytokines appear in the upper right quadrant. The percentage of Vγ9 cells expressing cytokine is shown in the upper right corner of each plot. (c) Experiments performed as for (b) but with with 100 n m simvastatin or 100 n m fluvastatin instead of pravastatin. Experiments with pravastatin, simvastatin and fluvastatin were performed with different PBMC and show that between 2 and 8% of Vγ9-expressing T cells make cytokines when exposed to 10 µm risedronate. Bar charts show the percentage of Vγ9+cytokine+ cells from flow cytometry plots for PBMC exposed to R10, R10 + 10 µm risedronate, R10 + 10 µm IPP, R10 + 10 µm risedronate + statin and R10 + 10 µm risedronate + statin + 10 µm IPP. Simvastatin and fluvastatin inhibit nBP-induced activation of Vγ9-expressing T cells. Addition of IPP with the statin rescues activation and controls for any toxic effect of the statins. Flow cytometric analysis allowed confirmation that cells remained alive during the experiment and showed that non-Vγ9-expressing cells such as monocytes and macrophages did not make cytokines in response to nBP during the experiment (data not shown).
Discussion
nBPs inhibit bone resorption by inhibiting FPP synthase and preventing protein prenylation of GTP-binding proteins that are necessary for osteoclast function [41–44]. nBPs are known to have a number of adverse side-effects (reviewed in [11]). The principal side-effect, the induction of an acute phase response, was described over 15 years ago [12,13]. The mechanism for this response has only been partially elucidated and appears to be associated with the release of cytokines, in particular TNFα and IL6 [14–16]. The cellular mechanism for the action of nBPs leading to production of TNFα and IL6 has remained unclear. Current thinking has homed in on monocytes and/or macrophages as likely cellular sources of these proinflamatory agents [15]. Here, we challenge this view by showing that nBPs induce rapid and copious production of these proinflammatory cytokines by human peripheral blood γδ T cells (Figs 1, 2 and 5). Monocytes and macrophages can be activated by proinflammatory cytokines and it is possible that these cells are activated at later time points as an indirect result of γδ T cell activation. However, several lines of evidence point against cells of the monocytic lineage being the primary source of acute phase response-inducing cytokines in our experiments. First, γδ T cell-depleted PBMC, which still contain monocytes and macrophages, do not manufacture TNFα in response to nBP treatment (Fig. 2a,b). Second, we do not observe cytokine production by non-Vγ9 expressing cells in our assays (Fig. 2c). Third, only cells expressing a Vδ2 chain produce TNFα in response to risedronate treatment (Fig. 3c,d). Fourth, the cells that do make cytokines in our assays do not express CD14 (Fig. 3a,b). Thus, in contrast to the typical acute phase response, cells of the monocytic lineage are likely not the primary producers of acute phase response-inducing cytokines after exposure to nBPs.
We also demonstrate that statins, which inhibit HMG CoA reductase, are able to block nBP-induced activation of γδ T cells (Fig. 5). This result is important for two reasons. First, it helps to isolate the nBP γδ T cell-activating effects to intermediates in the mevalonate pathway. Second, it suggests that administration of a statin prior to treatment with nBPs might inhibit the acute phase response. Future trials should aim to determine whether this common class of drug can inhibit the nBP-induced acute phase response, an effect that would be useful in many clinical settings given the wide range of applications for nBPs.
Risedronate was a more potent activator of γδ T cells than pamidronate, inducing more TNFα production at a 10 fold lower concentration (Figs 1a, b and 2a,b). This potency correlates with the ability of these drugs to inhibit FPP synthase [18,22]. Risedronate inhibits FPP synthase with an IC50 of 0·01 ± 0·002 µm compared to a value of 0·2 ± 0·1 µm for pamidronate [18]. Inhibition of FPP synthase in cellular extracts requires higher concentrations (0·1 ± 0·02 µm risedronate and 0·85 ± 0·34 µm pamidronate [18]). In most cases, nBP-induced activation of γδ T cells appears to occur at clinically relevant concentrations. The serum Cmax for pamidronate is approximately 10 µm[45]. Activation of Vγ9Vδ2 T cells is known to occur at such concentrations [46]. Zoledronate is the most potent nBP inhibitor of FPP synthase [46] and activates γδ T cells at concentrations as low as 1 µm[46], a concentration exceeded in vivo[47]. Risedronate may be different than other nBPs; while it is a very potent inhibitor of FPP synthase [22] and activates γδ T cells at concentrations as low as 1 µm[25,46], maximum serum concentrations have been reported to be below this threshold (Cmax < 25 n m after oral administration of 30 mg of risedronate [48]). nBPs are currently believed to function by inhibiting FPP synthase [43]. The reported Cmax value for risedronate (<25 n m) [48] is lower than the IC50 for inhibition of FPP synthase in cellular extracts (100 n m) [18]. There may be a number of possible explanations for this discrepancy. First, the Cmax for risedronate may be greater than claimed. Second, the intracellular, and specifically intraosteoclast, concentration of risedronate may be significantly higher than that measured in serum. Third, risedronate may function to inhibit bone resorption through an FPP synthase-independent mechanism. A study by Procter and Gamble Pharmaceuticals of 66 subjects taking risedronate concluded that, in contrast to other primary alkyl bisphosphonates, risedronate does not induce an acute phase reaction [48]. Thus it is possible that risedronate concentrations in vivo do not exceed the threshold required to trigger activation of γδ T cells and the ensuing acute phase response. However, further comparative, independent studies will be needed to confirm this finding.
Considerable publicity has been given to gastrointestinal disturbances associated with oral administration of the nBP alendronate [49]. Similar problems had earlier led to the discontinuation of oral pamidronate for osteoporosis [50] and the administration of these compounds by intravenous or intramuscular injection is increasing in popularity worldwide. The gastrointestinal tract is the main reservoir of γδ T cells in the body, probably as it is the main portal of entry for bacterial pathogens. The predominant populations of γδ T cells in the gut may also activate in response to nBPs. Thus, it is possible that some of the various complications that have been described after oral administration of nBPs may result from effects mediated through γδ T cells in the gastrointestinal epithelia.
Both simvastatin and fluvastatin inhibited nBP-induced activation of Vγ9Vδ2 T cells at a concentration of 100 n m (Fig. 5). This is close to the concentration of simvastatin found in vivo (Cmax = ∼80 n m) and well below the clinically relevant concentration of fluvastatin (Cmax > 1 µm) [51]. It is further noteworthy that statins have anti-inflammatory properties [52–64] that appear to be mediated through nonsterol mevalonate products [65] and to be paralleled by a dose dependent reduction in IL6 production [57, 60, 65–67]. Statins also appear to promote a Th2 cytokine bias in vivo[68]. The therapeutic relevance of these pharmacologic effects is becoming increasingly apparent. The mechanism(s) by which statins decrease inflammation and autoimmunity are still under debate. Several potential explanations have been put forward (reviewed in [69,70]). First, it has been established that some statins can inhibit interactions between cellular adhesion molecules by binding to leucocyte function antigen-1 [71]. However, it is not clear whether this function is applicable to the majority of statins. Second, it is known that statins block the isoprenylation of molecules such as Ras and Rho that are important for lymphocyte function [72,73]. Third, statins have been shown to decrease IFNγ-induced MHC class II expression [74]. Our work provides a further mechanism by which statins could have anti-inflammatory effects by showing that these drugs inhibit the activation of human peripheral blood γδ T cells in response to endogenous nonsterol mevalonate products.
Several studies have established that γδ T cells play a role in allergic airway inflammation [75–78]. Recent results in an OVA-induced airway inflammation model show that the promotion of airway hyper-reactivity and inflammation is dependent on the Vγ chain these cells express [79]. Curiously, simvastatin has just been shown to modulate airway inflammation in this system [62]. Thus, this model may prove ideal for direct testing of whether the anti-inflammatory effects of statins are mediated primarily by the inhibition of γδ T cell activation in vivo. However, it is not yet clear whether some murine γδ T cells activate in response to nonsterol mevalonate derivatives. Thus, future studies in humans, where it is established that statins can inhibit γδ T cell activation in vitro in response to nBP-induced accumulation of mevalonate intermediates or endogenous mevalonate metabolites in tumour cells (Fig. 5) [23], should aim to determine whether the anti-inflammatory effects attributed to statins are mediated through γδ T cells.
During the preparation of this manuscript, another group has demonstrated that statins prevent nBP-induced γδ T cell proliferation in vitro[80]. While this study did not specifically identify the cellular source of nBP-induced proinflammatory cytokines within ex vivo PBMC, it does have a number of complementary findings. First, inhibition of protein isoprenylation with specific protein prenyl transfer inhibitors did not stimulate γδ T cell proliferation [80]. Second, products of the mevalonate pathway downstream of FFP synthase did not prevent nBP-induced γδ T cell proliferation [80]. These findings lend further support to the notion that nBP-induced activation of γδ T cells occurs via mevalonate pathway intermediates upstream of FFP synthase such as IPP. Curiously, Thompson and Rogers did not observe IL6 release after nBP treatment of PBMCs in vitro, although it is not clear whether PBMC from several different individuals were examined [80]. In our study, nBP-induced IL6 production by γδ T cells was observed in only one of three donors, while all donors had γδ T cells that made TNFα. As only a minority of patients suffers an nBP-induced acute phase response, it is tempting to speculate that this reflects a particular subset of IL6-producing γδ T cells present only in some individuals. Further work is required to test this possibility.
Acknowledgments
This work was funded by the Wellcome Trust. AKS is a Wellcome Trust Senior Fellow. AEG is a Wellcome Trust Prize Student. DAP is a Medical Research Council Clinician Scientist Fellow. REH was funded by the Multiple Sclerosis Society of Great Britain and Northern Ireland.
References
- 1.Roux C, Dougados M. Treatment of patients with Paget's disease of bone. Drugs. 1999;58:823–30. doi: 10.2165/00003495-199958050-00005. [DOI] [PubMed] [Google Scholar]
- 2.Delmas PD, Meunier PJ. The management of Paget's disease of bone. N Engl J Med. 1997;336:558–66. doi: 10.1056/NEJM199702203360807. [DOI] [PubMed] [Google Scholar]
- 3.Delmas PD. Treatment of postmenopausal osteoporosis. Lancet. 2002;359:2018–26. doi: 10.1016/S0140-6736(02)08827-X. [DOI] [PubMed] [Google Scholar]
- 4.Berenson JR. Treatment of hypercalcemia of malignancy with bisphosphonates. Semin Oncol. 2002;29:12–8. doi: 10.1053/sonc.2002.37417. [DOI] [PubMed] [Google Scholar]
- 5.Neville-Webbe HL, Holen I, Coleman RE. The anti-tumour activity of bisphosphonates. Cancer Treat Rev. 2002;28:305–19. doi: 10.1016/s0305-7372(02)00095-6. [DOI] [PubMed] [Google Scholar]
- 6.Coleman RE. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev. 2001;27:165–76. doi: 10.1053/ctrv.2000.0210. [DOI] [PubMed] [Google Scholar]
- 7.Coleman RE. Bisphosphonates for the prevention of bone metastases. Semin Oncol. 2002;29:43–9. doi: 10.1053/sonc.2002.37415. [DOI] [PubMed] [Google Scholar]
- 8.Shinoda H, Adamek G, Felix R, Fleisch H, Schenk R, Hagan P. Structure-activity relationships of various bisphosphonates. Calcif Tissue Int. 1983;35:87–99. doi: 10.1007/BF02405012. [DOI] [PubMed] [Google Scholar]
- 9.Rogers MJ, Watts DJ, Russell RG. Overview of bisphosphonates. Cancer. 1997;80:1652–60. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1652::aid-cncr15>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 10.Fleisch H. Bisphosphonates: mechanisms of action. Endocr Rev. 1998;19:80–100. doi: 10.1210/edrv.19.1.0325. [DOI] [PubMed] [Google Scholar]
- 11.Russell RG, Croucher PI, Rogers MJ. Bisphosphonates: pharmacology, mechanisms of action and clinical uses. Osteoporos Int. 1999;9(Suppl. 2):S66–80. doi: 10.1007/pl00004164. [DOI] [PubMed] [Google Scholar]
- 12.Adami S, Bhalla AK, Dorizzi R, et al. Salvagno, and V. Lo Cascio. The acute-phase response after bisphosphonate administration. Calcif Tissue Int. 1987;41:326–31. doi: 10.1007/BF02556671. [DOI] [PubMed] [Google Scholar]
- 13.Gallacher SJ, Ralston SH, Patel U, Boyle IT. Side-effects of pamidronate. Lancet. 1989;2:42–3. doi: 10.1016/s0140-6736(89)90277-8. [DOI] [PubMed] [Google Scholar]
- 14.Schweitzer DH, Oostendorp-van de Ruit M, Van der Pluijm G, Lowik CW, Papapoulos SE. Interleukin-6 and the acute phase response during treatment of patients with Paget's disease with the nitrogen-containing bisphosphonate dimethylaminohydroxypropylidene bisphosphonate. J Bone Miner Res. 1995;10:956–62. doi: 10.1002/jbmr.5650100617. [DOI] [PubMed] [Google Scholar]
- 15.Sauty A, Pecherstorfer M, Zimmer-Roth I, et al. Interleukin-6 and tumor necrosis factor alpha levels after bisphosphonates treatment in vitro and in patients with malignancy. Bone. 1996;18:133–9. doi: 10.1016/8756-3282(95)00448-3. [DOI] [PubMed] [Google Scholar]
- 16.Thiebaud D, Sauty A, Burckhardt P, et al. An in vitro and in vivo study of cytokines in the acute-phase response associated with bisphosphonates. Calcif Tissue Int. 1997;61:386–92. doi: 10.1007/s002239900353. [DOI] [PubMed] [Google Scholar]
- 17.Thompson K, Dunford JE, Ebetino FH, Rogers MJ. Identification of a bisphosphonate that inhibits isopentenyl diphosphate isomerase and farnesyl diphosphate synthase. Biochem Biophys Res Commun. 2002;290:869–73. doi: 10.1006/bbrc.2001.6289. [DOI] [PubMed] [Google Scholar]
- 18.Dunford JE, Thompson K, Coxon FP, Luckman SP, Hahn FM, Poulter CD, Ebetino FH, Rogers MJ. Structure-activity relationships for inhibition of farnesyl diphosphate synthase in vitro and inhibition of bone resorption in vivo by nitrogen-containing bisphosphonates. J Pharmacol Exp Ther. 2001;296:235–42. [PubMed] [Google Scholar]
- 19.Bergstrom JD, Bostedor RG, Masarachia PJ, Reszka AA, Rodan G. Alendronate is a specific, nanomolar inhibitor of farnesyl diphosphate synthase. Arch Biochem Biophys. 2000;373:231–41. doi: 10.1006/abbi.1999.1502. [DOI] [PubMed] [Google Scholar]
- 20.van Beek E, Pieterman E, Cohen L, Lowik C, Papapoulos S. Nitrogen-containing bisphosphonates inhibit isopentenyl pyrophosphate isomerase/farnesyl pyrophosphate synthase activity with relative potencies corresponding to their antiresorptive potencies in vitro and in vivo. Biochem Biophys Res Commun. 1999;255:491–4. doi: 10.1006/bbrc.1999.0224. [DOI] [PubMed] [Google Scholar]
- 21.van Beek E, Pieterman E, Cohen L, Lowik C, Papapoulos S. Farnesyl pyrophosphate synthase is the molecular target of nitrogen-containing bisphosphonates. Biochem Biophys Res Commun. 1999;264:108–11. doi: 10.1006/bbrc.1999.1499. [DOI] [PubMed] [Google Scholar]
- 22.Sanders JM, Gomez AO, Mao J, et al. 3-D QSAR investigations of the inhibition of Leishmania major farnesyl pyrophosphate synthase by bisphosphonates. J Med Chem. 2003;46:5171–83. doi: 10.1021/jm0302344. [DOI] [PubMed] [Google Scholar]
- 23.Gober HJ, Kistowska M, Angman L, Jeno P, Mori L, De Libero G. Human T cell receptor gammadelta cells recognize endogenous mevalonate metabolites in tumor cells. J Exp Med. 2003;197:163–8. doi: 10.1084/jem.20021500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanaka Y, Morita CT, Nieves E, Brenner MB, Bloom BR. Natural and synthetic non-peptide antigens recognized by human gamma delta T cells. Nature. 1995;375:155–8. doi: 10.1038/375155a0. [DOI] [PubMed] [Google Scholar]
- 25.Green AE, Lissina A, Hutchinson SL, et al. Recognition of nonpeptide antigens by human V gamma 9V delta 2 T cells requires contact with cells of human origin. Clin Exp Immunol. 2004;136:472–82. doi: 10.1111/j.1365-2249.2004.02472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kunzmann V, Bauer E, Feurle J, Weissinger F, Tony HP, Wilhelm M. Stimulation of gammadelta T cells by aminobisphosphonates and induction of antiplasma cell activity in multiple myeloma. Blood. 2000;96:384–92. [PubMed] [Google Scholar]
- 27.Das H, Wang L, Kamath A, Bukowski JF. Vgamma2Vdelta2 T-cell receptor-mediated recognition of aminobisphosphonates. Blood. 2001;98:1616–8. doi: 10.1182/blood.v98.5.1616. [DOI] [PubMed] [Google Scholar]
- 28.Kato Y, Tanaka Y, Miyagawa F, Yamashita S, Minato N. Targeting of tumor cells for human gammadelta T cells by nonpeptide antigens. J Immunol. 2001;167:5092–8. doi: 10.4049/jimmunol.167.9.5092. [DOI] [PubMed] [Google Scholar]
- 29.Kato Y, Tanaka Y, Tanaka H, Yamashita S, Minato N. Requirement of species–specific interactions for the activation of human gamma delta T cells by pamidronate. J Immunol. 2003;170:3608–13. doi: 10.4049/jimmunol.170.7.3608. [DOI] [PubMed] [Google Scholar]
- 30.Lefranc M-P, Lefranc G. The T Cell Receptor Factsbook. London: Academic Press; 2001. [Google Scholar]
- 31.Miyagawa F, Tanaka Y, Yamashita S, Mikami B, Danno K, Uehara M, Minato N. Essential contribution of germline-encoded lysine residues in Jgamma1.2 segment to the recognition of nonpeptide antigens by human gammadelta T cells. J Immunol. 2001;167:6773–9. doi: 10.4049/jimmunol.167.12.6773. [DOI] [PubMed] [Google Scholar]
- 32.Davodeau F, Peyrat MA, Hallet MM, Gaschet J, Houde I, Vivien R, Vie H, Bonneville M. Close correlation between Daudi and mycobacterial antigen recognition by human gamma delta T cells and expression of V9JPC1 gamma/V2DJC delta-encoded T cell receptors. J Immunol. 1993;151:1214–23. [PubMed] [Google Scholar]
- 33.Yamashita S, Tanaka Y, Harazaki M, Mikami B, Minato N. Recognition mechanism of non-peptide antigens by human gammadelta T cells. Int Immunol. 2003;15:1301–7. doi: 10.1093/intimm/dxg129. [DOI] [PubMed] [Google Scholar]
- 34.Tanaka Y, Sano S, Nieves E, et al. Nonpeptide ligands for human gamma delta T cells. Proc Natl Acad Sci USA. 1994;91:8175–9. doi: 10.1073/pnas.91.17.8175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kunzmann V, Bauer E, Wilhelm M. Gamma/delta T-cell stimulation by pamidronate. N Engl J Med. 1999;340:737–8. doi: 10.1056/NEJM199903043400914. [DOI] [PubMed] [Google Scholar]
- 36.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–54. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 37.de Vries E, van der Weij JP, van der Veen CJ, van Paassen HC, Jager MJ, Sleeboom HP, Bijvoet OL, Cats A. In vitro effect of (3-amino-1-hydroxypropylidene)-1,1-bisphosphonic acid (APD) on the function of mononuclear phagocytes in lymphocyte proliferation. Immunology. 1982;47:157–63. [PMC free article] [PubMed] [Google Scholar]
- 38.Makkonen N, Salminen A, Rogers MJ, Frith JC, Urtti A, Azhayeva E, Monkkonen J. Contrasting effects of alendronate and clodronate on RAW 264 macrophages: the role of a bisphosphonate metabolite. Eur J Pharm Sci. 1999;8:109–18. doi: 10.1016/s0928-0987(98)00065-7. [DOI] [PubMed] [Google Scholar]
- 39.Monkkonen J, Simila J, Rogers MJ. Effects of tiludronate and ibandronate on the secretion of proinflammatory cytokines and nitric oxide from macrophages in vitro. Life Sci. 1998. pp. PL95–102. [DOI] [PubMed]
- 40.Gossman W, Oldfield E. Quantitative structure – activity relations for gammadelta T cell activation by phosphoantigens. J Med Chem. 2002;45:4868–74. doi: 10.1021/jm020224n. [DOI] [PubMed] [Google Scholar]
- 41.Luckman SP, Hughes DE, Coxon FP, Graham R, Russell G, Rogers MJ. Nitrogen-containing bisphosphonates inhibit the mevalonate pathway and prevent post-translational prenylation of GTP-binding proteins, including Ras. J Bone Miner Res. 1998;13:581–9. doi: 10.1359/jbmr.1998.13.4.581. [DOI] [PubMed] [Google Scholar]
- 42.Benford HL, Frith JC, Auriola S, Monkkonen J, Rogers MJ. Farnesol and geranylgeraniol prevent activation of caspases by aminobisphosphonates: biochemical evidence for two distinct pharmacological classes of bisphosphonate drugs. Mol Pharmacol. 1999;56:131–40. doi: 10.1124/mol.56.1.131. [DOI] [PubMed] [Google Scholar]
- 43.Fisher JE, Rogers MJ, Halasy JM, et al. Alendronate mechanism of action: geranylgeraniol, an intermediate in the mevalonate pathway, prevents inhibition of osteoclast formation, bone resorption, and kinase activation in vitro. Proc Natl Acad Sci USA. 1999;96:133–8. doi: 10.1073/pnas.96.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coxon FP, Helfrich MH, Van’t Hof R, Sebti S, Ralston SH, Hamilton A, Rogers MJ. Protein geranylgeranylation is required for osteoclast formation, function, and survival: inhibition by bisphosphonates and GGTI-298. J Bone Miner Res. 2000;15:1467–76. doi: 10.1359/jbmr.2000.15.8.1467. [DOI] [PubMed] [Google Scholar]
- 45.Fitton A, McTavish D. Pamidronate. A review of its pharmacological properties and therapeutic efficacy in resorptive bone disease. Drugs. 1991;41:289–318. doi: 10.2165/00003495-199141020-00009. [DOI] [PubMed] [Google Scholar]
- 46.Sanders JM, Ghosh S, Chan JM, et al. Quantitative structure-activity relationships for gammadelta T cell activation by bisphosphonates. J Med Chem. 2004;47:375–84. doi: 10.1021/jm0303709. [DOI] [PubMed] [Google Scholar]
- 47.Chen T, Berenson J, Vescio R, et al. Pharmacokinetics and pharmacodynamics of zoledronic acid in cancer patients with bone metastases. J Clin Pharmacol. 2002;42:1228–36. doi: 10.1177/009127002762491316. [DOI] [PubMed] [Google Scholar]
- 48.Mitchell DY, Eusebio RA, Sacco-Gibson NA, et al. Dose-proportional pharmacokinetics of risedronate on single-dose oral administration to healthy volunteers. J Clin Pharmacol. 2000;40:258–65. doi: 10.1177/00912700022008928. [DOI] [PubMed] [Google Scholar]
- 49.Graham DY, Malaty HM, Goodgame R. Primary amino-bisphosphonates: a new class of gastrotoxic drugs – comparison of alendronate and aspirin. Am J Gastroenterol. 1997;92:1322–5. [PubMed] [Google Scholar]
- 50.Lufkin EG, Argueta R, Whitaker MD, Cameron AL, Wong VH, Egan KS, O'Fallon WM, Riggs BL. Pamidronate: an unrecognized problem in gastrointestinal tolerability. Osteoporos Int. 1994;4:320–2. doi: 10.1007/BF01622190. [DOI] [PubMed] [Google Scholar]
- 51.Corsini A, Bellosta S, Baetta R, Fumagalli R, Paoletti R, Bernini F. New insights into the pharmacodynamic and pharmacokinetic properties of statins. Pharmacol Ther. 1999;84:413–28. doi: 10.1016/s0163-7258(99)00045-5. [DOI] [PubMed] [Google Scholar]
- 52.Kobashigawa JA, Katznelson S, Laks H, et al. Effect of pravastatin on outcomes after cardiac transplantation. N Engl J Med. 1995;333:621–7. doi: 10.1056/NEJM199509073331003. [DOI] [PubMed] [Google Scholar]
- 53.Stuve O, Prod’homme T, Slavin A, Youssef S, Dunn S, Steinman L, Zamvil SS. Statins and their potential targets in multiple sclerosis therapy. Expert Opin Ther Targets. 2003;7:613–22. doi: 10.1517/14728222.7.5.613. [DOI] [PubMed] [Google Scholar]
- 54.Stuve O, Youssef S, Steinman L, Zamvil SS. Statins as potential therapeutic agents in neuroinflammatory disorders. Curr Opin Neurol. 2003;16:393–401. doi: 10.1097/01.wco.0000073942.19076.d1. [DOI] [PubMed] [Google Scholar]
- 55.Marz W, Koenig W. HMG-CoA reductase inhibition: anti-inflammatory effects beyond lipid lowering? J Cardiovasc Risk. 2003;10:169–79. doi: 10.1097/01.hjr.0000073686.78271.6d. [DOI] [PubMed] [Google Scholar]
- 56.Crisby M. Modulation of the inflammatory process by statins. Drugs Today (Barc) 2003;39:137–43. doi: 10.1358/dot.2003.39.2.740209. [DOI] [PubMed] [Google Scholar]
- 57.Leung BP, Sattar N, Crilly A, et al. A novel anti-inflammatory role for simvastatin in inflammatory arthritis. J Immunol. 2003;170:1524–30. doi: 10.4049/jimmunol.170.3.1524. [DOI] [PubMed] [Google Scholar]
- 58.Blanco-Colio LM, Tunon J, Martin-Ventura JL, Egido J. Anti-inflammatory and immunomodulatory effects of statins. Kidney Int. 2003;63:12–23. doi: 10.1046/j.1523-1755.2003.00744.x. [DOI] [PubMed] [Google Scholar]
- 59.Weitz-Schmidt G. Statins as anti-inflammatory agents. Trends Pharmacol Sci. 2002;23:482–6. doi: 10.1016/s0165-6147(02)02077-1. [DOI] [PubMed] [Google Scholar]
- 60.Kinlay S, Schwartz GG, Olsson AG, et al. High-dose atorvastatin enhances the decline in inflammatory markers in patients with acute coronary syndromes in the MIRACL study. Circulation. 2003;108:1560–6. doi: 10.1161/01.CIR.0000091404.09558.AF. [DOI] [PubMed] [Google Scholar]
- 61.Olsson AG, Schwartz GG, Jonasson L, Linderfalk C. Are early clinical effects of cholesterol lowering mediated through effects on inflammation? Acta Physiol Scand. 2002;176:147–50. doi: 10.1046/j.1365-201X.2002.01017.x. [DOI] [PubMed] [Google Scholar]
- 62.McKay A, Leung BP, McInnes IB, Thomson NC, Liew FY. A novel anti-inflammatory role of simvastatin in a murine model of allergic asthma. J Immunol. 2004;172:2903–8. doi: 10.4049/jimmunol.172.5.2903. [DOI] [PubMed] [Google Scholar]
- 63.Pate GE, Tahir MN, Murphy RT, Foley JB. Anti-inflammatory effects of statins in patients with aortic stenosis. J Cardiovasc Pharmacol Ther. 2003;8:201–6. doi: 10.1177/107424840300800305. [DOI] [PubMed] [Google Scholar]
- 64.Sparrow CP, Burton CA, Hernandez M, et al. Simvastatin has anti-inflammatory and antiatherosclerotic activities independent of plasma cholesterol lowering. Arterioscler Thromb Vasc Biol. 2001;21:115–21. doi: 10.1161/01.atv.21.1.115. [DOI] [PubMed] [Google Scholar]
- 65.Diomede L, Albani D, Sottocorno M, Donati MB, Bianchi M, Fruscella P, Salmona M. In vivo anti-inflammatory effect of statins is mediated by nonsterol mevalonate products. Arterioscler Thromb Vasc Biol. 2001;21:1327–32. doi: 10.1161/hq0801.094222. [DOI] [PubMed] [Google Scholar]
- 66.Nawawi H, Osman NS, Yusoff K, Khalid BA. Reduction in serum levels of adhesion molecules, interleukin-6 and C-reactive protein following short-term low-dose atorvastatin treatment in patients with non-familial hypercholesterolemia. Horm Metab Res. 2003;35:479–85. doi: 10.1055/s-2003-41805. [DOI] [PubMed] [Google Scholar]
- 67.Nawawi H, Osman NS, Annuar R, Khalid BA, Yusoff K. Soluble intercellular adhesion molecule-1 and interleukin-6 levels reflect endothelial dysfunction in patients with primary hypercholesterolaemia treated with atorvastatin. Atherosclerosis. 2003;169:283–91. doi: 10.1016/s0021-9150(03)00193-x. [DOI] [PubMed] [Google Scholar]
- 68.Youssef S, Stuve O, Patarroyo JC, et al. The HMG-CoA reductase inhibitor, atorvastatin, promotes a Th2 bias and reverses paralysis in central nervous system autoimmune disease. Nature. 2002;420:78–84. doi: 10.1038/nature01158. [DOI] [PubMed] [Google Scholar]
- 69.Takemoto M, Liao JK. Pleiotropic effects of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors. Arterioscler Thromb Vasc Biol. 2001;21:1712–9. doi: 10.1161/hq1101.098486. [DOI] [PubMed] [Google Scholar]
- 70.Liao JK. Isoprenoids as mediators of the biological effects of statins. J Clin Invest. 2002;110:285–8. doi: 10.1172/JCI16421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Weitz-Schmidt G, Welzenbach K, Brinkmann V, et al. Statins selectively inhibit leukocyte function antigen-1 by binding to a novel regulatory integrin site. Nat Med. 2001;7:687–92. doi: 10.1038/89058. [DOI] [PubMed] [Google Scholar]
- 72.Czyzyk J, Brogdon JL, Badou A, Henegariu O, Preston Hurlburt P, Flavell R, Bottomly K. Activation of CD4 T cells by Raf-independent effectors of Ras. Proc Natl Acad Sci USA. 2003;100:6003–8. doi: 10.1073/pnas.1031494100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Greenwood J, Walters CE, Pryce G, Kanuga N, Beraud E, Baker D, Adamson P. Lovastatin inhibits brain endothelial cell Rho-mediated lymphocyte migration and attenuates experimental autoimmune encephalomyelitis. Faseb J. 2003;17:905–7. doi: 10.1096/fj.02-1014fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kwak B, Mulhaupt F, Myit S, Mach F. Statins as a newly recognized type of immunomodulator. Nat Med. 2000;6:1399–402. doi: 10.1038/82219. [DOI] [PubMed] [Google Scholar]
- 75.McMenamin C, Pimm C, McKersey M, Holt PG. Regulation of IgE responses to inhaled antigen in mice by antigen-specific gamma delta T cells. Science. 1994;265:1869–71. doi: 10.1126/science.7916481. [DOI] [PubMed] [Google Scholar]
- 76.Zuany-Amorim C, Ruffie C, Haile S, Vargaftig BB, Pereira P, Pretolani M. Requirement for gammadelta T cells in allergic airway inflammation. Science. 1998;280:1265–7. doi: 10.1126/science.280.5367.1265. [DOI] [PubMed] [Google Scholar]
- 77.Lahn M, Kanehiro A, Takeda K, et al. Negative regulation of airway responsiveness that is dependent on gammadelta T cells and independent of alphabeta T cells. Nat Med. 1999;5:1150–6. doi: 10.1038/13476. [DOI] [PubMed] [Google Scholar]
- 78.Hahn YS, Taube C, Jin N, et al. V gamma 4+ gamma delta T cells regulate airway hyperreactivity to methacholine in ovalbumin-sensitized and challenged mice. J Immunol. 2003;171:3170–8. doi: 10.4049/jimmunol.171.6.3170. [DOI] [PubMed] [Google Scholar]
- 79.Hahn YS, Taube C, Jin N, et al. Different potentials of gammadelta T cell subsets in regulating airway responsiveness: Vgamma1+ cells, but not Vgamma4+ cells, promote airway hyperreactivity, Th2 cytokines, and airway inflammation. J Immunol. 2004;172:2894–902. doi: 10.4049/jimmunol.172.5.2894. [DOI] [PubMed] [Google Scholar]
- 80.Thompson K, Rogers MJ. Statins prevent bisphosphonate-induced gamma,delta-T-cell proliferation and activation in vitro. J Bone Miner Res. 2004;19:278–88. doi: 10.1359/JBMR.0301230. [DOI] [PubMed] [Google Scholar]