Abstract
Haemolytic uraemic syndrome (HUS) is caused by Shiga-toxin-producing Escherichia coli (STEC). Although, Shiga toxin type 2 (Stx2) is responsible for the renal pathogenesis observed in patients, the inflammatory response, including cytokines and polymorphonuclear neutrophils (PMN), plays a key role in the development of HUS. Previously, we demonstrated that Stx2 injection generates an anti-inflammatory reaction characterized by endogenous glucocorticoid (GC) secretion, which attenuates HUS severity in mice. Here, we analysed the effects of Stx2 on the pathogenic function of PMN and the potential role of endogenous GC to limit PMN activation during HUS development in a murine model. For this purpose we assessed the functional activity of isolated PMN after in vivo treatment with Stx2 alone or in simultaneous treatment with Ru486 (GC receptor antagonist). We found that Stx2 increased the generation of reactive oxygen intermediates (ROI) under phobol-myristate-acetate (PMA) stimulation and that the simultaneous treatment with Ru486 strengthened this effect. Conversely, both treatments significantly inhibited in vitro phagocytosis. Furthermore, Stx2 augmented in vitro PMN adhesion to fibrinogen (FGN) and bovine serum albumin (BSA) but not to collagen type I (CTI). Stx2 + Ru486 caused enhanced adhesion to BSA and CTI compared to Stx2. Whereas Stx2 significantly increased migration towards N-formyl-methionyl-leucyl-phenylalanine (fMLP), Stx2 + Ru486 treatment enhanced and accelerated this process. The percentage of apoptotic PMN from Stx2-treated mice was higher compared with controls, but equal to Stx2 + Ru486 treated mice. We conclude that Stx2 activates PMN and that the absence of endogenous GC enhances this activation suggesting that endogenous GC can, at least partially, counteract PMN inflammatory functions.
Keywords: Stx2, glucocorticoids, HUS, neutrophils, inflammatory response
Introduction
The typical form of haemolytic uraemic syndrome (HUS) is caused by particular serotypes of enterohaemorrhagic Escherichia coli (EHEC) of which O157:H7 is the most frequent. During the first period of the disease, the pathogen colonizes the gastrointestinal tract without invading the blood stream, yet causing diarrhoea, haemorrhagic colitis and intestinal inflammation [1]. Full development of HUS is characterized by thrombocytopenia, haemolytic anaemia and acute renal failure, which appear a few days after the infection and as a result of the production of Shiga toxin (Stx) by the bacteria. It is also well known that the endothelial damage induced by Stx2 is a central pathogenic process for the development of the disease [2], although the toxicity of Stx alone is not enough to cause the extent of damage observed in HUS patients.
Several authors have reported the importance of the immune response in the development of HUS. In fact, a number of studies suggest the participation of polymorphonuclear neutrophils (PMN) in the disease, and that a poor prognosis is accounted by a high peripheral PMN count at presentation [1,3]. Moreover, activation and degranulation of PMN have also been demonstrated by the presence of high levels of elastase and IL-8 in the serum of patients, as well as increased PMN adhesive capacity and subsequent endothelial damage [4,5].
An inflammatory stimulus is usually followed by an anti-inflammatory reaction to control the aggressive potential of the immune response generated after infection [6]. In this regard, in a previous manuscript we demonstrated that Stx2 per se is able to stimulate the Hypothalamus-Pituitary-Adrenal axis in mice inducing the secretion of glucocorticoids (GC) [7]. We also showed that the depletion of endogenous GC by adrenalectomy or treatment with a GC antagonist (Ru486) increased renal damage and mortality induced by Stx2. These results revealed the capacity of endogenous GC to partially attenuate Stx2-toxicity [7]. Although, it is extensively accepted that GC released under physiological conditions contribute to the control of inflammation [8] down-regulating macrophage function [9] or inhibiting PMN response [10], the mechanisms involved in the protection provided by endogenous GC against Stx2-toxicity has not been previously investigated. Since PMN are central effector cells in the pathogenesis of HUS, endogenous GC could be counteracting pro-inflammatory functions of PMN involved in the endothelial damage triggered by Stx2. Therefore, the objective of the present report was to study the effects of Stx2 on PMN functionality in vivo, in the presence or absence of endogenous GC effects, by blocking the GC receptor (GR) with Ru486 treatment.
We found that Stx2 in vivo was able to induce the activation of PMN by increasing the respiratory burst, adhesion and migration capacity and that the absence of GC action exacerbated these effects. These results suggest that endogenous GC protect, at least partially, against Stx2-toxicity reducing PMN pro-inflammatory activity.
Materials and methods
Antibodies and reagents
Ru486 [17-hydroxy-11-(4-dimethylaminophenyl) 17-(1-propynyl) estra-4,9-diene-3-one], dihydrorhodamine-123 (DHR-123), Phorbol Myristate Acetate (PMA), Propidium Iodide (PI), Trypsin, Zymozan A (Zy), neutrophil chemoattractant N-formyl-Met-Leu-Phe (fMLP), fluorescein isiothiocyanate (FITC), acid soluble type-I rat tail collagen (CTI), bovine serum albumin (BSA) and mouse fibrinogen (FGN) were obtained from Sigma (St. Louis, MO, USA).
Mice
BALB/c mice were bred in the animal facility of the Department of Experimental Medicine, Academia Nacional de Medicina, Buenos Aires. Male mice aged 9–16 weeks and weighing 20–25 g were used throughout the experiments. They were maintained under a 12-h light-dark cycle at 22 ± 2°C and fed with standard diet and water ad libitum. The experiments performed herein were conducted according to principles set forth in the Guide for the Care and Use of Laboratory Animals (National Institute of Health, 1985).
Stx2 preparation
Dr Sugiyama Junichi kindly provided Stx2 from Denka Seiken CO Ltd. (Nigata, Japan). Purity was analysed by the supplier, which showed only one peak in HPLC. Stx2 preparation was checked for endotoxin contamination by the Limulus amoebocyte lysate assay given that 1 IU/ml is equal to 0·1 ng/ml of United States Pharmacopea standard E. coli endotoxin [11]. Stx2 preparation contained less that 40 pg LPS/µg of Shiga toxin protein. Stx2 was tested for cytotoxic activity on Vero cells in the Instituto Nacional de Enfermedades Infecciosas, ANLIS, DrC.G. Malbran (Buenos Aires, Argentina) as previously described [12].
In vivo treatments: Stx2 and Ru486
Stx2 lethality was evaluated by treating mice with serial dilutions of Stx2 in pyrogen-free saline 0·2% FCS, via intravenous (i.v.) injection in the retro-orbital plexus, as described in a previous manuscript [12]. The dose selected was approximately 250 pg/mouse, which induced ≈ 50% mortality between 72 and 96 h after injection. The same batch and dose of Stx2 was used for all experiments.
To evaluate the role of endogenous GC on the inflammatory function of PMN triggered by Stx2, we used the GC and progesterone antagonist Ru486 (mifepristone). Since we used males, we could ascribe Ru486 effects only to its blocking activity on the GC receptor (GR), therefore depleting the tissues from the action of endogenous GC. The metabolic half life of Ru486 is of about 20–24 h [13], and repeated intraperitoneal administration of Ru486 daily in mice has been shown to completely deplete mice from endogenous GC effects [14]. Moreover, Ru486 is used to determine the specificity of GC-receptor mediated functions in diverse models of inflammation [15]. Mice were treated with Stx2 and with 600 µg/mouse of Ru486 intreaperitoneally (i.p.) every day until the end of the experiment [13] and the first dose was always given in simultaneous with Stx2.
PMN purification and blood leucocyte count
PMN were isolated from a pool of heparinized blood obtained from 2 to 3 mice. The cells were harvested by Ficoll-Hypaque separation, followed by dextran sedimentation as previously described [16]. Total leucocyte count was determined in a Neubauer chamber, by means of an optical microscope, after dilution of the blood samples in a 2% acetic acid solution. Blood samples were obtained by puncture of the retro-orbital plexus at 2, 6, 24, 48 and 72 h after treatment. The total count and percentage of circulating PMN were determined after differential count on May – Grunwald Giemsa-stained blood smears.
Flow cytometry studies
Reactive Oxygen Intermediates generation (ROI)
To determine the production of reactive oxygen intermediates (ROI) by flow cytometry DHR-123, a derivative of rhodamine 123, was used following the protocol described by Leech et al. [14]. Briefly, isolated PMN (2 × 105) were incubated 15 min at 37°C with 1 µm DHR-123. Subsequently, the cells were incubated with or without PMA 10 ng/ml for 15 min at 37°C 5% CO2 in a humidified atmosphere. Immediately after, the cells were washed with medium (RPMI) and green fluorescence was measured as previously described.
Phagocytosis
Zymozan particles (2 mg/ml) were conjugated with FITC (2 mg/ml) in carbonate-bicarbonate pH 9·5 1 m (Zy-FITC) for 4 h at room temperature. Isolated PMN (2 × 105 cells) were incubated with 100 µl Zy-FITC (0·1 mg/ml) for 30 min at 37°C 5% CO2. Immediately after, the cells were treated with Trypsin 1% in PBS 5 min at 37°C to remove particles deposited on the surface of the cells and finally, the cells were fixed with 0·5% paraformaldehyde and measured by flow cytometry.
Quantification of neutrophil apoptosis by propidium iodide staining
The percentage of neutrophils that displayed a hypodiploid DNA peak, i.e. apoptotic cells, was determined using a modification of Nicoletti's protocol [17]. Purified PMN, extracted from mice treated in vivo as explained above, were incubated for 18 h with RPMI 2% FCS at 37°C 5% CO2 in a humidified atmosphere. Then, the cells were resuspended 0·5 ml of cold medium 50% FCS and then in 1·5 ml of 70% ice-cold ethanol solution, drop by drop. Finally, fixed neutrophils were washed and suspended in a propidium iodide (PI) (50 µg/ml) solution for 30 min at room temperature. The red fluorescence of PI of the cells was measured using a FACScan flow cytometer (Becton Dickinson). The forward scatter and side scatter of particles were simultaneously measured. Cell debris was excluded from analysis by appropriately raising the forward-scattered threshold.
Adhesion assay
PMN adhesion assays were performed in triplicates in 96-well tissue culture plates (Costar, Cambridge, MA) precoated overnight at 37°C with the extracellular matrix proteins (ECM), bovine serum albumin (BSA) (30 µg/ml), fibrinogen (FGN) (200 mg/ml) or collagen type-I (CTI) (30 µg/ml) as previously described [18]. A total of 100 µl of the PMN suspension (2 × 105 cells/well) in RPMI with 0,5% BSA was loaded onto the wells and allowed to settle for 60 min, and then incubated at 37°C without (basal) or with PMA (20 ng/ml) (stimulated). Non-adherent cells were carefully removed from the plates and adherent cells were washed and fixed with 1·1% (v/v) glutaraldehyde for 15 min. After extensive washing with distilled water, cells were coloured 0·1 ml crystal violet (0·1% in 20% methanol). The adhesion of PMN was evaluated as the absorbance at 550 nm in a microtiter plate reader.
Chemotaxis assay
Neutrophil chemotaxis was quantified using a modification of the Boyden chamber technique [19]. A cell suspension (50 µl) containing 2 × 106 cells/ml in RPMI with 0·5% BSA, was placed in the top wells of a 48-well microchemotaxis chamber. A PVP-free polycarbonate membrane (3 µm pore size; Neuro Probe Inc. Gaithersburg MD, USA) separated the cells from lower wells containing either RPMI or fMLP (10−8 M). The chamber was incubated for 90 min at 37°C in a 5% CO2 humidified atmosphere. After incubation, the filter was stained with TINCION-15 (Biopur SRL, Rosario, Argentina), and the number of PMN on the undersurface of the filter was counted in a five random high-power fields (HPF) × 400 for each of triplicate filters.
Statistical analysis
All data correspond to the mean ± SEM of individual mice. Statistical differences were determined using the one-way analysis of variance (anova) followed by Bonferroni multiple comparison test and P < 0·05 was considered significant.
Results
PMN reactive oxygen intermediates (ROI) generation
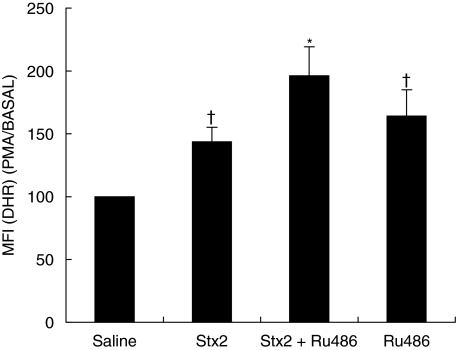
The functional activation of PMN was studied by measuring the oxidative burst at 24 and 72 h after in vivo treatment. Basal production of ROI in PMN isolated from Stx2 treated mice was not affected neither at 24 h after injection (MIF: saline = 31 ± 1; Stx2 = 40 ± 3, n = 6) nor at 72 h (saline 27 ± 4; Stx2 = 33 ± 2), but in vitro stimulation with PMA (10 ng/ml) caused a significant increase in the respiratory burst compared with control mice (saline) at 24 h (Fig. 1) but not at 72 h (data not shown). The participation of endogenous GC in the modulation of ROI generation was evaluated by simultaneous treatment of mice with Stx2 and Ru486. This treatment rendered a higher respiratory burst than Stx2 alone at 24 h (Fig. 1) and 72 h (MFI: Stx2 = 144 ± 13; Stx2 + Ru486 = 162 ± 16, n = 6; P < 0·01). Ru486 per se also showed an increase in ROI production at both time points (24 h in Fig. 1 and 72 h MFI Ru486 : 158 ± 17%, Saline: 100%n = 6, P < 0·01), reflecting the physiologic control of PMN activity by endogenous GC.
Fig. 1.
Effect of Stx2 and GC on neutrophil ROI, 24 h post injection. Neutrophils (2 × 105 cells) were incubated first with DHR-123 (1 µm) and then with PMA (10 ng/ml) for 15 min each. The mean fluorescence intensity (MFI) was determined by flow cytometry. Data are shown as percentage of increase of MFI in ROI generation of PMA/BASAL saline; each value represents the mean ± SEM from six separate experiments performed with a pool of 3 mice/group. †P < 0·05 compared with saline; *P < 0·001 versus saline and P < 0·05 versus Stx2.
PMN phagocytosis
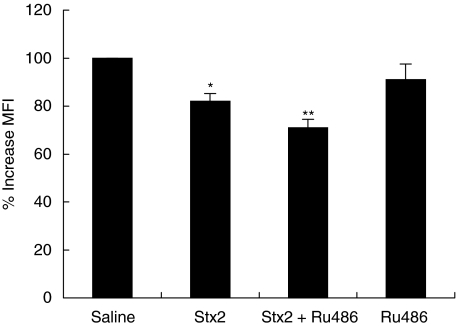
The phagocytic function was studied in isolated PMN 24 and 72 h after treatment evaluating Zy-FITC particle ingestion by flow cytometry. Stx2-injection induced a significant decrease in the phagocytic capacity 24 h after treatment (Fig. 2) which remained low at 72 h post treatment (data not shown). To evaluate the participation of endogenous GC on PMN phagocytosis under the effects of Stx2, we treated mice with Stx2 and Ru486. This treatment resulted in a further inhibition of PMN phagocytosis, compared with Stx2. Ru486 injection did not cause differences in the phagocytic function of PMN in comparison with saline treated mice.
Fig. 2.
Effect of Stx2 and GC on neutrophil phagocytosis of Zy-FITC particles 24 h post treatment. Neutrophils (2 × 105 cells) were incubated with zymozan-FITC particles for 30 min. Later, MFI of phagocytic cells was determined by flow cytometry. Data are shown as percentage of increase compared with saline; each value represents the mean ± SEM from six separate experiments, performed with neutrophils isolated from a pool of 3 mice/group. *P < 0·05 compared with saline; **P < 0·05 compared with Stx2.
Specific PMN adhesion to ECM molecules
During the process of PMN–endothelial cell interaction, PMN do not only recognize the adhesion molecules ELAM-1 and ICAM-1 on the endothelium [20] but they can also interact with the basement membrane of the blood vessels and the interstitial matrix of the stroma [21]. Furthermore, expression and deposition of coagulation proteins are both up-regulated in the interstitium of inflamed and injured tissues [22]. Therefore, we analysed the influence of Stx2 treatment on the adhesion capacity of isolated PMN to FBG, BSA and CTI, which involves different adhesion molecules [23] 24 and 72 h after in vivo treatments.
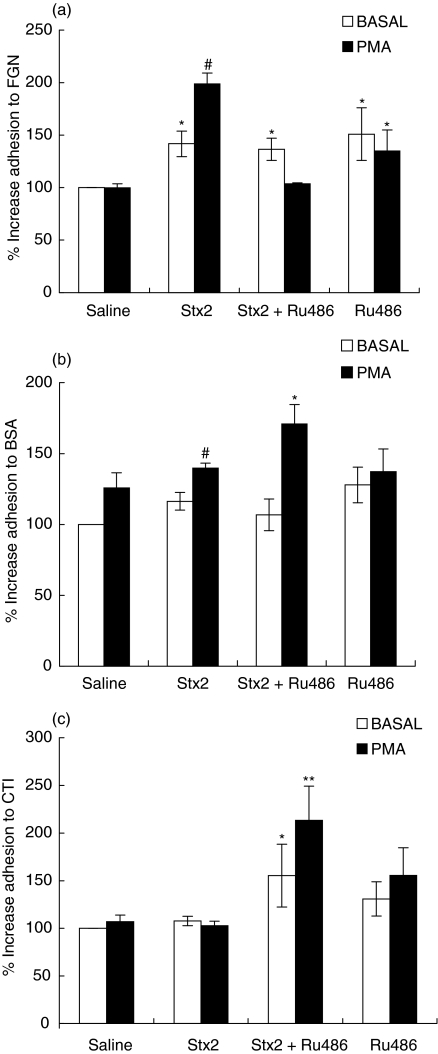
Early isolation of PMN (24 h) rendered no differences in the adhesive capacity of PMN under all treatments (data not shown). As can be seen in Fig. 3, in vivo injection with Stx2 induced a ligand-dependent increase in neutrophil adhesion at 72 h-post injection. Adhesion to FBG-coated plates (Fig. 3a) was increased under basal or PMA-stimulated conditions. Adhesion to BSA (Fig. 3b) was slightly but significantly enhanced only upon PMA stimulation. On the contrary, adhesion to CTI was not modified neither under basal, nor under PMA-stimulated conditions (Fig. 3c).
Fig. 3.
Effect of Stx2 and Ru486 on PMN adhesion 72 h post treatment. PMN (1·5 × 105) were incubated with saline solution (BASAL) (□) or PMA (10 ng/ml) 60 min at 37°C (▪) and seeded on plates coated with (a) FGN (b) BSA and (c) CTI. Adhesion was revealed by crystal violet staining. The results are expressed as percentage of increase with respect to BASAL saline and each value represents the mean ± SEM from 4 to 6 separate experiments performed with a pool of 3 mice/group. (a) *P < 0·01 compared with saline (BASAL); #P < 0·001 versus all groups; (b) #P < 0·05 compared with saline (PMA) and Stx2 (BASAL), *P < 0·05 compared with Stx2 (PMA) and Stx2 + Ru486 (BASAL) (c) *P < 0·05 versus Stx2 (BASAL) and **P < 0·001 versus all groups.
Then, we analysed whether endogenous GC were able to modulate PMN adhesion properties under the effects of Stx2. Simultaneous treatment of mice with Stx2 + Ru486 caused the same level of PMN basal adhesion than Stx2 alone to FBG and BSA (Figs 3a, b) but augmented PMN stickiness to CTI (Fig. 3c). Upon PMA stimulation, Stx2 + Ru486 treatment counteracted the effects observed in the adhesion of PMN to FBG (Fig. 3a) but increased the adhesion to BSA and CTI (Figs 3b,c). Treatment with Ru486 alone caused a significant increase in PMN adhesion to FBG-coated plates under basal or stimulated conditions (Fig. 3a) but did not modify PMN adhesive capacity to BSA or CTI (Figs 3b,c).
Migration assays
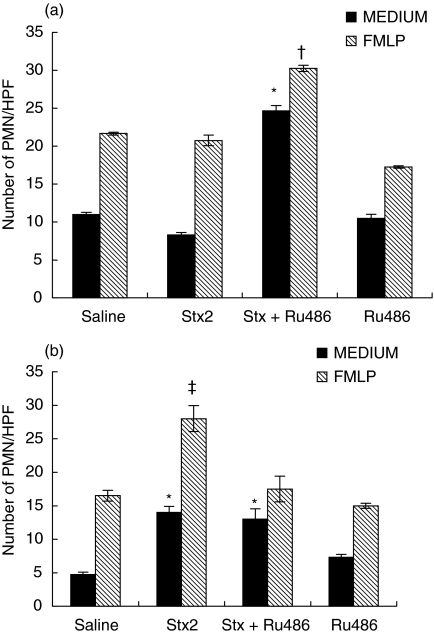
We investigated the effect of in vivo treatment with Stx2 on PMN migration properties, in response to a chemotactic gradient of FMLP. PMN migration towards MEDIUM or FMLP was not affected by Stx2 treatment at 24 h (Fig. 4a), but was significantly enhanced at 72 h (Fig. 4b). PMN from Stx2 + Ru486 treated mice had a higher migratory capacity as soon as 24 h (Fig. 4a) returning to Stx2 levels with MEDIUM or to saline-levels with FMLP at 72 h (Fig. 4b).
Fig. 4.
Chemotactic migration of neutrophils against FMLP. Neutrophils (1 × 105) were allowed to migrate through polycarbonate filters (3 µm) for 90 min at 37°C with CO2 in response to MEDIUM (control) (▪) or FMLP (10−8m) ( ). Then, the filter was fixed and stained and the number of chemotactic cells was counted in five random high-power fields (HPF) × 400 for each of triplicate filters. The data are shown as the Number of PMN/HPF × 400. (a) 24 h (b) 72 h post treatments. *P < 0·001 compared with all groups with MEDIUM, †P < 0·001 compared with all groups; ‡P < 0·001 compared with all groups.
). Then, the filter was fixed and stained and the number of chemotactic cells was counted in five random high-power fields (HPF) × 400 for each of triplicate filters. The data are shown as the Number of PMN/HPF × 400. (a) 24 h (b) 72 h post treatments. *P < 0·001 compared with all groups with MEDIUM, †P < 0·001 compared with all groups; ‡P < 0·001 compared with all groups.
White blood cells (WBCs) and PMN in circulation
Increased leucocyte number appears as a consequence of an inflammatory process and it is known that both exogenous GC [24] and Stx2 [12] induce an increase in WBC count as well as a marked neutrophilia after 24 h of injection. Therefore, we studied the influence of endogenous GC on the evolution of the leucocyte number under the effects of Stx2. All groups were bled at 2, 6, 24, 48 and 72 h post treatment, and although Stx2 and Stx2 + Ru486 groups showed a gradual increase in total leucocyte and PMN count at 2 h post injection (data not shown), the differences between them were significant at 48 h. At this time point, PMN number was significantly higher in Stx2 + Ru486 treated mice than in the control groups. Ru486 alone also increased the total leucocyte and PMN count, but these values returned to control levels at 72 h, unlike Stx2 and Stx2 + Ru486 groups, whose PMN counts remained high over time (Table 1).
Table 1.
Effect of Stx2 and Ru486 on total leucocyte and PMN count.
| 48 h post treatment | 72 h post treatment | |||
|---|---|---|---|---|
| Treatments | Leucocytes (cells/l) (%) | PMN (cells/l) (%) | Leucocytes (cells/l) (%) | PMN (cells/l) (%) |
| Saline | 6·1 ± 2 (100%) | 1·6 ± 0·9 (26%) | 5·4 ± 0·5 (100%) | 1·1 ± 0·15 (14%) |
| Stx2 | 10·9 ± 0·2 (100%)* | 4·6 ± 0·4 * | 7·3 ± 0·3 (100%)* | 3·3 ± 0·2 (48%)* |
| Stx2+Ru486 | 11·3 ± 0·8 (100%)* | 5·9 ± 0·35 (52%)*‡ | 6·5 ± 0·8 (100%)**† | 3·2 ± 0·5 (48%)*† |
| Ru486 | 9·7 ± 0·4 (100%)* | 4·9 ± 0·21 (50%)* | 4·0 ± 0·6 (100%)** | 0·9 ± 0·3 (12%) |
Values are shown as media ± SEM. (%) is % of the total.
P < 0·001 and
P < 0·05 compared with saline;
P < 0·001 compared with Ru486;
P < 0·05 compared with Stx2 and Ru486 treatment.
PMN apoptosis
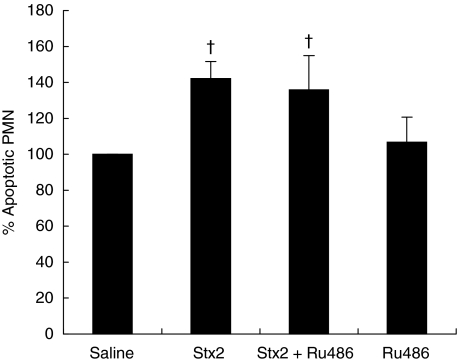
During acute inflammation, PMN infiltrate the injured tissue where they initiate and amplify the inflammatory response. The subsequent clearance of apoptotic PMN by macrophages is imperative for the control and resolution of the inflammatory process [25]. Here, we assessed the in vivo effect of Stx2 on PMN apoptosis after 18 h culture. The percentage of apoptotic PMN was not changed 24 h post treatment (Saline: 100%, Stx2: 89 ± 7%), but significantly increased at 72 h post Stx2 (Fig. 5).
Fig. 5.
Effect of Stx2 and GC on neutrophil apoptosis 72 h post treatment. Neutrophils (2 × 105 cells) were incubated overnight and the percentage of apoptotic cells was evaluated by flow cytometry. Data are shown as percentage of increase of apoptotic cells compared with saline group; each value represents the mean ± SEM from six separate experiments performed with a pool of PMN of 3 mice/group. †P < 0·001 compared with saline.
The effects of endogenous GC on PMN apoptosis in Stx2 treated mice was examined at 24 and 72 h also, but no differences were observed at 24 h post treatment (Stx2: 89 ± 7%; Stx2 + Ru486: 102 ± 2%). At 72 h, Stx2 + Ru486 treatment had no further effect on PMN apoptosis when compared with Stx2 (Fig. 5). Treatment with Ru486 alone had no direct effect on PMN survival at either time point.
Discussion
The current study addresses the role of Stx2 and endogenous GC on the inflammatory response of PMN, with special emphasis in those processes which could be involved in the endothelial damage observed in HUS: chemotactic response, general adhesive capacity, oxygen radical generation and survival.
Our results show that in vivo injection with Stx2 activates PMN increasing migration, ligand-dependent adhesion and the respiratory burst. The results presented herein confirm and extend previous data showing that PMN isolated from Stx2-treated mice are early and profoundly activated [12]. Moreover, we found that the absence of endogenous GC on Stx2 treated mice strengthened PMN activation, suggesting an inhibitory effect of endogenous GC on their pathogenic capacity.
The generation of reactive oxygen species by PMN is instrumental to endothelial or tissue damage during diverse conditions like infection, ischemic injury, arthritis and other chronic inflammatory and autoimmune disorders [26]. The increase in ROI production induced by Stx2, supports the idea that during the course of the disease PMN can effectively play a pathogenic role enhancing Stx2-direct effects on the endothelium. Increased oxidative damage and decreased antioxidant enzyme activities were also observed in red blood cells of patients with HUS, suggesting the relevance of the oxidative burst on endothelial damage and haemolytic anaemia [27].
GC are known to inhibit PMN ROI production during inflammation in a number of models [28]. In the present study we demonstrated that the absence of GC action exacerbated the respiratory burst caused by in vivo treatment with Stx2.
The opposite response observed in ROI-generation and phagocytosis has been previously reported in human PMN after in vitro incubation with Stx [29], as well as in other experimental conditions [30,31], and different hypotheses have been proposed. Wagner & Roth [30] have suggested that the phagocytic function is probably suppressed as a consequence of the toxic effects of oxygen radicals on the phagocytic machinery. Others propose that the triggering of the oxidative response promotes neutrophil apoptosis [31], and neutrophils undergoing apoptosis lose their phagocytic function [32]. In this regard, we observed a higher PMN-apoptotic rate only at 72 h after Stx2 treatment. Whether this increase is a consequence of the early oxidative response deserves further investigation.
Leucocyte activation and endothelial cell injury are both main components in the development of microangiopathic lesions seen in HUS patients [2]. Leucocyte adhesion to the endothelium and transmigration through the basement membrane depend strongly on signals triggered by soluble mediators like chemokines, cell/cell interactions with the endothelium and with extracellular matrix (ECM) proteins [23], and on the profile of expression of the adhesion molecules expressed [33]. Here, we analysed the in vitro adhesive capacity of PMN from Stx2 treated mice to FGN, BSA and CTI coated plates. We observed a significant increase in PMN adhesiveness to FGN 72 h post Stx2 treatment, either under nonstimulated or stimulated conditions, or to BSA after PMA stimulation only. These adhesive alterations are probably mediated by the enhanced expression of β2 integrins, since they have been implicated in the process of PMN adhesion to BSA and FGN [34] and their expression on PMN is increased by Stx2 in a similar time dependent manner [12]. FGN is a relevant protein involved in the coagulation cascade, a process that in HUS is augmented due to Stx2 direct and indirect pro-coagulatory properties [35]. Moreover, Stx-induced endothelial cell injury and subsequent exposure of the subendothelium, may release von Willebrand factor and fibrinogen, which contribute to the microthrombi formation observed in this pathology [36]. In this regard, an enhanced PMN adhesion to FGN after Stx2 in vivo treatment could be important in HUS development, since PMN may strengthen Stx2-mediated endothelial damage.
The simultaneous treatment of Stx2 and Ru486 caused no modifications in basal FGN adhesion when compared with Stx2, but a marked inhibition after PMA stimulus (Fig. 3a). Again, this observation is coincident with the inhibition in CD11b expression (data not shown), but further analysis is required to understand this mechanism. Instead, adhesion to BSA was strengthened in the absence of endogenous GC.
Collagen type I is a major component of the ECM, that promotes the adhesion of a variety of cells influencing many processes such as proliferation, differentiation, migration and activation [37]. A number of studies have implicated β1-integrins in the binding of PMA-activated human PMN to CTI [38], although integrins of the β2 class, specifically αLβ2 (CD11a/CD18, LFA-1), have also been found necessary for CTI PMN binding and signal transduction [18]. Here, we have seen that PMN from Stx2 treated mice, did not differ from the control in their adhesive capacity to CTI. Our results suggest that the promiscuous nature of the β2 integrins may not be enough to regulate PMN adhesion to ECM proteins necessary to migrate across the basement membrane [34] and a higher activation state could be required, as observed in the absence of endogenous GC.
Chemoattractants are known to induce a kinetic response of leucocytes (chemokinesis) that in the presence of a concentration gradient is manifested as a directional movement (chemotaxis). In our experimental conditions Stx2, in vivo, induced an enhanced chemokinetic (MEDIUM) and chemotactic (FMLP) in vitro response in isolated PMN, 72 h post treatment. Although Ru486 did not show any effect per se on the locomotion properties of PMN, supplied together with Stx2, enhanced and accelerated (24 h after treatment) Stx2 effects.
The study on granulocytosis reveals that Stx2 and Ru486 are independently and transiently able to increase PMN number in the periphery. When combined, neutrophil number augments significantly, but not in an additive way. It is known that GC inhibit PMN accumulation in inflamed tissue but they cause a marked increase in circulating PMN primarily by shifting PMN from the marginal pools into the circulation, with minor contribution from bone marrow release [24]. Experiments designed to demonstrate PMN mobilization from the bone marrow by Stx2 were not conclusive.
Apoptosis is the mechanism that contributes to the resolution of acute inflammation, and PMN are rapidly recruited to inflammatory sites, where the expression of their apoptotic program can be readily modified by diverse agents released by the host or the pathogen [39]. In our model, Stx2 in vivo increased the apoptotic rate after 72 h. Then, this enhancement is probably a consequence of the activation of the oxidative machinery or the action of different inflammatory mediators, instead of a direct effect of Stx2 on PMN as has previously been reported [34]. In fact, Liu et al. [40] saw the inhibition of PMN apoptosis after 24 h of in vitro incubation with Stx, although King et al. [29] reported no modification on apoptosis and necrosis rates after similar conditions of in vitro exposure to Stx. Although glucocorticoids are known to inhibit PMN apoptosis [15], in vivo treatment with Ru486 showed no alterations in PMN survival either in saline or Stx2 treated mice.
In this report, we show that PMN are affected in vivo by Stx2 and by the absence of endogenous GC differently in time and magnitude according to the function studied. Liu et al. [41] has demonstrated that Stx2 in vitro is able to cause direct activation of normal human PMN as seen in increased adhesion to human umbilical vein endothelial cells (HUVEC), expression of CD11b/CD18 and superoxide generation and decreased CD62L. We focused our studies to examine the in vivo effects of Stx2 on PMN. Experiments with Stx2 applied in vitro analyse the direct effects of the toxin on PMN function or activation, which depend on the presence of specific receptors as well as on their identity and lipid-composition, and also on the subsequent intracellular signals triggered by such interaction. Experiments with in vivo injection of Stx2 reflect the result of pleiotropic effects on different cell types and tissues, thus reproducing the complex scenario of the pathogenic condition. The involvement of PMN activation in the pathogenesis of HUS does not necessarily depend on the direct effects of Stx2 on PMN, but also on the activation of endothelium after Stx2–Gb3 interaction. Moreover the endothelial damage caused by Stx2 and pro-inflammatory cytokines (TNF-α, IL-1β) could be enough to activate PMN. In a reciprocal way, the oxidative potential of PMN could induce a positive feedback in the primary damage of the endothelium caused by Stx2.
The higher sensitivity to Stx2 in animals without the effects of endogenous GC, does not correlate with changes in systemic cytokine production [7], but rather with an exacerbated reactivity of PMN, as shown in this report. We conclude that endogenous GC could be partially protecting mice from Stx2 injury by counteracting PMN pathogenic potential on the endothelium and directly influencing the development of HUS.
However, we cannot discard additional protective effects of endogenous GC on endothelial cells. In this regard, Cronstein et al. [42] reported that GC prevent recruitment of leucocytes at endotoxin-inflamed HUVEC by inhibiting the display of adhesive molecules. Moreover, Keusch et al. [43] found that the inducing effects of the IL-1β and TNF-α on Stx1 activity were partially inhibited by DEX on HUVEC culture.
The results presented herein may have important clinical implications: if host factors, such as PMN and their products contribute to HUS pathogenesis, it is reasonable to consider the inhibition of these factors as a novel therapeutic strategy for EHEC treatment.
Acknowledgments
The authors thank Héctor Costa, Analía Trevani, Juan Portaluppi, and Antonio Morales for their excellent technical assistance. This work was supported by grants from Ministerio de Salud de la Nación ‘R. Carrillo – A. Oñativia’ 2003, Alberto J. Roemmers, and Agencia Nacional de Promoción Científica y Tecnológica, Argentina.
References
- 1.Paton JC, Paton AW. Pathogenesis and diagnosis of shiga toxin-producing Escherichia coli. Clin Microbiol Rev. 1998;11:450–79. doi: 10.1128/cmr.11.3.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Proulx F, Seidman EG, Karpman D. Pathogenesis of Shiga toxin–associated hemolytic uremic syndrome. Pediatr Res. 2001;50:163–71. doi: 10.1203/00006450-200108000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Walters MD, Matthei IU, Kay R, Dillon MJ, Barratt TM. The polymorphonuclear leucocyte count in childhood haemolytic uraemic syndrome. Pediatr Nephrol. 1989;3:130–4. doi: 10.1007/BF00852893. [DOI] [PubMed] [Google Scholar]
- 4.Fitzpatrick MM, Shah V, Filler G, Dillon MJ, Barratt TM. Neutrophil activation in the haemolytic uraemic syndrome: free and complexed elastase in plasma. Pediatr Nephrol. 1992;6:50–3. doi: 10.1007/BF00856833. [DOI] [PubMed] [Google Scholar]
- 5.Forsyth KD, Simpson AC, Fitzpatrick MM, Barrat TM, Levinsky RJ. Neutrophil mediated endothelial injury in haemolytic uraemic syndrome. Lancet. 1989;2:411–4. doi: 10.1016/s0140-6736(89)90591-6. [DOI] [PubMed] [Google Scholar]
- 6.Salkowski CA, Vogel SN. Lipopolysaccharide increases glucocorticoid receptor expression in murine macrophages. A possible mechanism for glucocorticoid-mediated suppression of endotoxicity. J Immunol. 1992;149:4041–7. [PubMed] [Google Scholar]
- 7.Gómez S, Fernández GC, Vanzulli S, Dran G, Rubel CJ, Berki T, Isturiz MA, Palermo MS. Endogenous glucocorticoids attenuate Shiga toxin−2 induced toxicity in a mouse model of Hemolytic Uremic Syndrome. Clin Exp Immunol. 2003;133:200–7. doi: 10.1046/j.1365-2249.2003.02057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blalock EJ. The syntax of immune-neuroendocrine communication. Immunol Today. 1985;15:504–11. doi: 10.1016/0167-5699(94)90205-4. [DOI] [PubMed] [Google Scholar]
- 9.Baybutt HN, Holsboer NJ. Inhibition of macrophage differentiation and function by cortisol. Endocrinology. 1990;127:476–80. doi: 10.1210/endo-127-1-476. [DOI] [PubMed] [Google Scholar]
- 10.Goulding NJ, Euzger HS, Butt SK, Perretti M. Novel pathways for glucocorticoid effects on neutrophils in chronic inflammation. Inflamm Res. 1998;47:S158–S165. doi: 10.1007/s000110050310. [DOI] [PubMed] [Google Scholar]
- 11.Duner KI. A new kinetic single stage Limulus amoebocyte lysate method for the detection of endotoxin in water and plasma. J Biochem Biophys Meth. 1993;26:131–42. doi: 10.1016/0165-022x(93)90043-n. [DOI] [PubMed] [Google Scholar]
- 12.Fernández GC, Rubel C, Dran G, Gomez S, Isturiz MA, Palermo MS. Shiga toxin-2 induces neutrophilia and neutrophil activation in a murine model of hemolytic uremic syndrome. Clin Immunol. 2000;95:227–34. doi: 10.1006/clim.2000.4862. [DOI] [PubMed] [Google Scholar]
- 13.Sheridan PL, Evans RM, Horwitz KB. Phosphotryptic peptide analysis of human progesterone receptor. New phosphorylated sites formed in nuclei after hormone treatment. J Biol Chem. 1989;264:6520–8. [PubMed] [Google Scholar]
- 14.Leech M, Hutchinson P, Holdsworth SR, Morand EF. Endogenous glucocorticoids modulate neutrophil migration and synovial P-selectin but not neutrophil phagocytic or oxidative function in experimental arthritis. Clin Exp Immunol. 1998;112:383–8. doi: 10.1046/j.1365-2249.1998.00601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yazawa H, Kato T, Nakada T, Sendo F. Glucocorticoid hormone suppression of human neutrophil-mediated tumor cell cytostasis. Int J Cancer. 1999;81:74–80. doi: 10.1002/(sici)1097-0215(19990331)81:1<74::aid-ijc14>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 16.Coligan J, Knisblek A, Margulies D, Shevach E, Warren S. In vitro assays for mouse lymphocyte function. In: Coligan J, Knisblek A, Margulies D, et al., editors. Current Protocols in Immunology, National Institute of Health, USA. New York: Wiley & Sons, Inc.; 1994. pp. 3.20.3–4. [Google Scholar]
- 17.Nicoletti I, Migliorati G, Pagliacci MC, Grignani F, Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Meth. 1991;139:271–9. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- 18.Garnotel R, Monboisse JC, Randoux A, Haye B, Borel JP. The binding of type I collagen to lymphocyte function-associated antigen (LFA) 1 integrin triggers the respiratory burst of human polymorphonuclear neutrophils. Role of calcium signaling and tyrosine phosphorylation of LFA 1. J Biol Chem. 1995;270:27495–503. doi: 10.1074/jbc.270.46.27495. [DOI] [PubMed] [Google Scholar]
- 19.Betsuyaku T, Liu F, Senior RM, Haug JS, Brown EJ, Jones SL, Matsushima K, Link DC. A functional granulocyte colony-stimulating factor receptor is required for normal chemoattractant-induced neutrophil activation. J Clin Invest. 1999;103:825–32. doi: 10.1172/JCI5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lundgren-Akerlund E, Olofsson AM, Berger E, Arfors KE. CD11b/Cd18–dependent polymorphonuclear leucocyte interaction with matrix proteins in adhesion and migration. Scan J Immunol. 1993;37:569–74. doi: 10.1111/j.1365-3083.1993.tb02573.x. [DOI] [PubMed] [Google Scholar]
- 21.Vaday GG, Lider O. Extracellular matrix moieties, cytokines, and enzymes: dynamic effects on immune cell behaviour and inflammation. J Leukoc Biol. 2000;67:149–59. doi: 10.1002/jlb.67.2.149. [DOI] [PubMed] [Google Scholar]
- 22.Kreis C, La Fleur M, Menard C, Paquin R, Beaulieu AD. Thrombospondin and fibronectin are synthesized by neutrophils in human inflammatory joint disease and in a rabbit model of in vivo neutrophil activation. J Immunol. 1989;143:1961–8. [PubMed] [Google Scholar]
- 23.Frieser M, Hallmann R, Johansson S, Vestweber D, Goodman SL, Sorokin L. Mouse polymorphonuclear granulocyte binding to extracellular matrix molecules involves beta 1 integrins. Eur J Immunol. 1996;26:3127–36. doi: 10.1002/eji.1830261245. [DOI] [PubMed] [Google Scholar]
- 24.Nakagawa M, Terashima T, Dýachkova Bondy GP, Hogg JC, van Eeden SF. Glucocorticoid-induced granulocytosis. Contribution of marrow release and demargination of intravascular granulocytes. Circulation. 1998;98:2307–13. doi: 10.1161/01.cir.98.21.2307. [DOI] [PubMed] [Google Scholar]
- 25.Lagasse E, Weissman IL. bcl-2 inhibits apoptosis of neutrophils but not their engulfment by macrophages. J Exp Med. 1994;179:1047–52. doi: 10.1084/jem.179.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Condino-Neto A, Whitney C, Newburger PE. Dexamethasone but not indomethacin inhibits human phagocyte nicotinamide adenine dinucleotide phosphate oxidase activity by down-regulating expression of genes encoding oxidase components. J Immunol. 1998;161:4960–7. [PubMed] [Google Scholar]
- 27.Turi S, Nemeth I, Vargha I, Matkovics B. Oxidative damage of red blood cells in haemolytic uraemic syndrome. Pediatr Nephrol. 1994;8:26–9. doi: 10.1007/BF00868253. [DOI] [PubMed] [Google Scholar]
- 28.Dandona P, Mohanty P, Hamouda W, Aljada A, Kumbkarni Y, Garg R. Effect of dexamethasone on reactive oxygen species generation by leukocytes and plasma interleukin-10 concentrations: a pharmacodynamic study. Clin Pharmacol Ther. 1999;66:58–65. doi: 10.1016/S0009-9236(99)70054-8. [DOI] [PubMed] [Google Scholar]
- 29.King AJ, Sundaram S, Cendoroglo M, Acheson DWK, Keusch GT. Shiga toxin induces superoxide production in polymorphonuclear cells with subsequent impairment of phagocytosis and responsiveness to phorbol esters. J Infect Dis. 1999;179:503–7. doi: 10.1086/314579. [DOI] [PubMed] [Google Scholar]
- 30.Wagner JG, Roth RA. Neutrophil migration during endotoxemia. J Leukoc Biol. 1999;66:10–24. doi: 10.1002/jlb.66.1.10. [DOI] [PubMed] [Google Scholar]
- 31.Oishi K, Machida K. Inhibition of neutrophil apoptosis by antioxidants in culture medium. Scand J Immunol. 1997;45:21–7. doi: 10.1046/j.1365-3083.1997.d01-365.x. [DOI] [PubMed] [Google Scholar]
- 32.Hart SP, Ross JA, Ross K, Haslett C, Dransfield I. Molecular characterisation of the surface of apoptotic neutrophils: implications for functional downregulation and recognition by phagocytes. Cell Death Differ. 2000;7:493–503. doi: 10.1038/sj.cdd.4400680. [DOI] [PubMed] [Google Scholar]
- 33.Sixt M, Hallmann R, Wendler O, Scharffetter-Kochanek K, Sorokin LM. Cell adhesion and migration properties of beta 2-integrin negative polymorphonuclear granulocytes on defined extracellular matrix molecules. Relevance for leukocyte extravasation. J Biol Chem. 2001;276:18878–87. doi: 10.1074/jbc.M010898200. [DOI] [PubMed] [Google Scholar]
- 34.Werr J, Xie X, Hedqvist P, Ruoslahti E, Lindbom L. Integrins are critically involved in neutrophil locomotion in extravascular tissue in vivo. J Exp Med. 1998;187:2091–6. doi: 10.1084/jem.187.12.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karpman D, Papadopoulou D, Nilsson K, Sjogren AC, Mikaelsson C, Lethagen S. Platelet activation by Shiga toxin and circulatory factors as a pathogenetic mechanism in the hemolytic uremic syndrome. Blood. 2001;97:3100–8. doi: 10.1182/blood.v97.10.3100. [DOI] [PubMed] [Google Scholar]
- 36.van de Kar NC, van Hinsbergh VW, Brommer EJ, Monnens LA. The fibrinolytic system in the hemolytic uremic syndrome: in vivo and in vitro studies. Pediatr Res. 1994;36:257–64. doi: 10.1203/00006450-199408000-00019. [DOI] [PubMed] [Google Scholar]
- 37.Mori S, Heldin CH, Claesson-Welsh L. Ligand-induced ubiquitination of the platelet-derived growth factor beta-receptor plays a negative regulatory role in its mitogenic signaling. J Biol Chem. 1993;268:577–83. [PubMed] [Google Scholar]
- 38.Werr J, Johansson J, Erksson EE, Hedqvist P, Ruoslahti E, Lindbom L. Integrin α2β1 (VLA-2) is a principal receptor used by neutrophils for locomotion in extravascular tissue. Blood. 2000;95:1804–9. [PubMed] [Google Scholar]
- 39.Salamone G, Giordano M, Trevani AS, Gamberale R, Vermeulen M, Schettinni J, Geffner JR. Promotion of neutrophil apoptosis by TNF-alpha. J Immunol. 2001;166:3476–83. doi: 10.4049/jimmunol.166.5.3476. [DOI] [PubMed] [Google Scholar]
- 40.Liu J, Akahoshi T, Sasahana T, Kitasato H, Namai R, Sasaki T, Inoue M, Kondo H. Inhibition of neutrophils apoptosis by verotoxin 2 derived from Escherichia coli O157:H7. Infect Immun. 1999;67:6203–5. doi: 10.1128/iai.67.11.6203-6205.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu J, He T, He Y, Zhang Z, Akahoshi T, Kondo H, Zhong S. Prolongation of functional life-span of neutrophils by recombinant verotoxin 2. Chin Med J (Engl) 2002;115:900–3. [PubMed] [Google Scholar]
- 42.Cronstein BN, Kimmel SC, Levin RI, Martiniuk F, Weissmann G. A mechanism for the antiinflammatory effects of corticosteroids: The glucocorticoid receptor regulates leukocyte adhesion to endothelial cells and expression of endothelial-leukocyte adhesion molecule 1 and intercellular adhesion molecule 1. Proc Natl Acad Sci USA. 1992;89:9991–5. doi: 10.1073/pnas.89.21.9991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Keusch GT, Acheson WK, Aaldering L, Erban J, Jacewicz MS. Comparison of the effects of Shiga-like toxin 1 on cytokine- and butyrate-treated Human Umbilical and Saphenous Vein Endothelial Cells. J Infect Dis. 1996;173:1164–70. doi: 10.1093/infdis/173.5.1164. [DOI] [PubMed] [Google Scholar]