Abstract
Corticosteroids are highly effective anti-inflammatory or immunosuppressive drugs used commonly to treat human systemic lupus erythematosus (SLE). All-trans-retinoic acid (ATRA), which belongs to a class of retinoids that exert immunomodulatory and anti-inflammatory functions, can also suppress the development of lupus nephritis in an animal model. However, both agents can inflict serious adverse effects. Here, we have asked whether ATRA can serve as a steroid-sparing drug in the treatment of lupus nephritis. To examine the efficacy of combining predonisolone (PSL) with ATRA, we treated intraperitoneally New Zealand black/white F1 (NZB/W F1) mice with PSL, ATRA or both agents. Survival rate and proteinuria were determined once a month. Cytokine and anti-DNA antibody production were determined by enzyme-linked immunosorbent assay (ELISA) and reverse transcription-polymerase chain reaction (RT-PCR). Renal histopathology was observed by haematoxylin and periodic acid Schiff (PAS), immunoperoxidase and immunohistochemical assay. Survival rate and proteinuria were improved in all experimental groups, and were much improved in the mice receiving the combination of ATRA and PSL (P < 0·05). A single administration of ATRA reduced the Th1 [interleukin (IL)-2, interferon (IFN)-γ and IL-12], and a Th2 (IL-4) cytokine level, as effectively as administration of PSL. ATRA also suppressed the expression of inducible nitric oxide synthetase (iNOS) and monocyte chemoattractant protein-1 (MCP-1) in the kidney. The combination of PSL and ATRA significantly reduced IgG2 (especially IgG2b)-specific anti-DNA antibody levels in comparison with administration of either agent alone. These data suggest that ATRA might have the potential to act as a new therapeutic and steroid-sparing drug against lupus nephritis.
Keywords: all-trans-retinoic acid, corticosteroids, murine lupus, nephritis, Th1/Th2
Introduction
Lupus nephritis is a significant cause of morbidity and mortality among patients with systemic lupus erythematosus (SLE) and is the major cause of death in the New Zealand black/white (NZB/W) F1 mice, which are model mice of SLE. We have described previously the effects of all-trans-retinoic acid (ATRA) on lupus nephritis in NZB/W F1 mice [1]. ATRA-treated mice survived longer and exhibited a significant reduction of proteinuria and renal pathological findings, including glomerular IgG deposits and serum anti-DNA antibodies. On the other hand, corticosteroids are known to improve remarkably the prognosis of many autoimmune diseases, including SLE. However, retinoids and corticosteroids may induce many serious adverse reactions such as infectious diseases, osteoporosis, growth retardation, delayed wound healing, teratogenicity, weight loss, bone fracture, anaemia and liver damage [1].
Retinoids are a group of natural and synthetic derivatives of vitamin A that exert antineoplastic and immunomodulatory actions [2–4], and they have been used for the treatment of acute promyelocytic leukaemia [5] and inflammatory disorders such as psoriasis [6], acne [7] and rheumatoid arthritis [8]. Retinoids have various effects on cytokines and inhibit interleukin (IL)-12 production by macrophages, leading to a reduction of interferon (IFN)-γ and an induction of IL-4 by CD4+ T cells [9]. Th1 cytokines such as IL-2, IFN-γ and IL-12 and Th2 cytokines such as IL-4, -5, -10 and -13 are associated with the pathogenesis of SLE and NZB/W F1 mice, and inhibition of these cytokines is thought to be associated with the improvement of lupus nephritis in NZB/W F1 mice.
In an attempt to determine whether ATRA can help corticosteroids to treat lupus nephritis, we treated NZB/W F1 mice with prednisolone (PSL) and ATRA both singly and in combination, and observed the clinical course, cytokine production, serum anti-DNA antibody level and histopathology of the kidney. We also studied the effects of ATRA on monocyte chemoattractant protein (MCP)-1 and inducible nitric oxide synthesis (iNOS). MCP-1, which is a potent chemoattractant of monocytes, T cells and natural killer cells [10], may also be responsible for inducing tubular and/or glomerular damage in the kidney. In addition, iNOS in mesangial cells causes oxidative injury in various forms of glomerular inflammation [11]. Considering these findings, the inhibition of MCP-1 or iNOS may also be an effective anti-inflammatory strategy.
In the present study, we found that beneficial effects on survival rate and proteinuria were much improved in the mice receiving the combination of ATRA and PSL, and discuss the possibility of ATRA acting as a new therapeutic and steroid-sparing drug against lupus nephritis.
Methods
Mice
Three-month-old female NZB/W F1 mice obtained from the Shizuoka Laboratory Animal Center (Shizuoka, Japan) were divided into six groups (n = 8–16) and injected intraperitoneally with 0·1 mg of ATRA (Sigma, St Louis, MO, USA) alone, 0·3 or 0·6 mg of PSL (Shionogi, Osaka, Japan) alone, a combination of PSL and ATRA, or saline three times per week for 5 months. One group of mice was also treated for 9 months to determine their survival rate and measure their urinary protein.
Clinical evaluation and laboratory tests
Urine was tested monthly for proteinuria with a test strip (Hema-Combistix, Bayer/Sankyo, Tokyo, Japan) and graded semiquantitatively (0, none; 1, 30–99 mg/dl; 2, 100–299 mg/dl; 3, 300–999 mg/dL; 4, 1000 mg/dl or more of albumin). The data from each mouse were determined by the mean of the results for each mouse made on 3 sequential days. Serum samples were obtained by cardiac puncture, and the weight of the spleen and the body was measured at the time of sacrifice or spontaneous death. To evaluate the adverse and therapeutic effect of ATRA and PSL treatment, we examined weight loss, skin lesions, bone fracture, liver damage and renal function in mice at 8 months. Serum samples were analysed by blood urea nitrogen (BUN), creatine (Cr), aspartate aminotransferase (AST), alanine aminotransferase (ALT) and glucose (GLU).
Renal histopathology
Kidneys were either snap-frozen in optimal cutting temperature (OCT) compound (Miles Scientific, Naperville, IL, USA) for cryostat sectioning or fixed in 10% neutral-buffered formalin. Formalin-fixed tissue was embedded in paraffin, and 4-µm sections were stained with haematoxylin and periodic acid Schiff (PAS) and evaluated by light microscopy [12]. Glomerular pathology was assessed by counting 30 glomerular cross-sections (gcs) per kidney and scoring each of them on a semiquantitative scale: 0, normal (35–40 cells/gcs); 1, mild (few lesions with slight proliferative changes and hypercellularity) (41–50 cells/gcs); 2, moderate hypercellularity (51–60 cells/gcs) with segmental and/or diffuse proliferative changes, hyalinosis, and moderate exudates; 3, severe hypercellularity (>60 cell/gcs) with segmental or global sclerosis and/or severe necrosis, crescent formation and severe exudation. Tubular pathology was assessed based on the percentage of tubules (magnification ×200) showing damage (dilatation, atrophy or necrosis) among 200 tubules in a randomly chosen field. Perivascular cell accumulation was also evaluated by counting the number of cell layers surrounding 10 randomly chosen inter- and intralobular arteries (magnification ×400) (0, none; 1, <five layers surrounding less than half the diameter of the vessel; 2, five to 10 layers surrounding more than half of the diameter of the vessel; 3, >10 layers surrounding more than half the diameter of the vessel).
Cryostat-sectioned kidneys were stained by an immunoperoxidase method, for T cells with anti-CD4 and anti-CD8 monoclonal antibodies; for B cells with rat anti220 monoclonal antibody (PharMingen, San Diego, CA, USA); and for macrophages (Mø) with F4/80 hybridoma culture supernatant (HB198; American Type Culture Collection, Rockville, MD, USA). Numbers of T cells, B cells and macrophages in the kidney were represented as those per periglomerular or perivascular area [13] (magnification ×400).
Immunofluorescence analysis
To evaluate the glomerular and perivascular deposition of immunoglobulins, we washed cryostat sections of the kidney with phosphate-buffered saline (PBS) and pretreated the sections with 10% goat serum for 30 min at room temperature. These sections were incubated with fluorescein isothiocyanate (FITC)-conjugated goat antimouse total IgG, IgG1, IgG2a and IgG2b antibodies (Caltag, Burlingame, CA, USA) for 30 min at 37°C, washed with PBS, and then analysed using a fluorescence microscope (Labophot, Nikon, Tokyo, Japan). The intensity of the fluorescence was assessed by scoring 10 randomly chosen glomeruli on a semiquantitative scale (0, no deposition; 1, weak; 2, moderate; 3, strong) and five randomly chosen inter- or intralobar vessels on a two-part scale (negative, no deposition; positive, deposition). Specific staining was visualized using a fluorescence microscope (Zeiss, Heideberg, Germany). Tissue sections were evaluated randomly by two pathologists (magnification ×400). Scores were shown as the mean ± s.d. of nine specimens.
Isolation of CD4+ T lymphocytes
The spleen was obtained from each mouse, and the splenocytes were used for the following assay. After removing red cells by haemolysis using distilled water, we used cells that passed through a column containing nylon-wool fibre (Wako Pure Chemical, Osaka, Japan) as T cells. The purity of the CD3+ T cells in these cells was >94% as determined by flow cytometry. CD4+ T cells were purified by positive selection using biotinated anti-CD4 monoclonal antibody (RM4-5, PharMingen, San Diego, CA, USA), streptavidin-labelled magnetic beads (Dinal Beads M-280, Dynal AS, Oslo, Norway) and a magnet (Dinal M, Dynal AS).
Cytokine and IgG measurement
Concentration of cytokines (IL-2, IL-4, IL-12 and IFN-γ) in the serum was determined by using an enzyme-linked immunosorbent assay (ELISA) kit for each (PharMingen). Titres of Ig class-specific anti-DNA antibodies were measured by ELISA as described elsewhere [14]. Each well of flat-bottomed 96-well plates (Costar, Cambridge, MA, USA) was coated with calf-thymus DNA (Sigma), dried at 37°C and washed with PBS. Non-specific protein binding was blocked by coating the plate with 3% bovine serum albumin (BSA) in PBS. Each serum sample (100 µl) was diluted appropriately with PBS and incubated for 2 h at 37°C. Then, each well was washed and the contents were allowed to react with 100 µl of appropriately diluted HRP-conjugated goat antimouse IgG, IgG1, IgG2a and IgG2b antibodies for 2 h at 37°C. Pooled serum from NZB/W F1 mice (8 months of age) was used as the standard. Titres of the standard serum were defined as follows: for total IgG, 1000 U/ml; IgG1, 170 U/ml; IgG2a, 340 U/ml; IgG2b, 220 U/ml. Absorbance at 450 nm of each well was measured by a microplate reader (Benchmark, Bio-Rad, Richmond, CA, USA). Antibody titre of the samples was determined from the absorbance using a standard curve constructed for each IgG subclass.
Expression of mRNAs for IL-2, IL-4, IFN-γ and IL-12 in the CD4+ T cells, and iNOS and MCP-1 in the kidney was detected by reverse transcription-polymerase chain reaction (RT-PCR), and a housekeeping gene, 18S rebosomal RNA, served as an inner control. Total RNA was extracted by RNeasy® Mini Kit (Qiagen, Hilden, Germany) from CD4+ T cells and snap-frozen kidneys. Complementary DNA (cDNA) was synthesized from 1 µg of RNA by RT reaction using oligo(dt)12−18 (Pharmacia, Piscataway, NJ, USA) and M-MLV reverse transcriptase (Gibco-BRL, New York, NY, USA) and deoxynucleotides (dNTP). Real-time quantitative PCR analysis was performed using an ABI 7700 sequence detector system (Perkin Elmer-Applied Biosystems, Foster City, CA, USA). FAM (6-carboxyfluorescein)-labelled primers were used as the target hybridization probes for IL-2, IL-4, IFN-γ, IL-12, iNOS and MCP-1. A different fluorescent dye, VIC-labelled primer, was used as the control hybridization probe for 18S. PCR reactions were performed in triplicate.
Statistical analysis
Statistical analysis was performed using Student's t-test. The 95% confidence limit was taken for significant difference of the data between two groups. The survival rate of curve was made according to the Kaplan–Meier method.
Results
PSL + ATRA-treated mice survive longer than PSL-treated mice
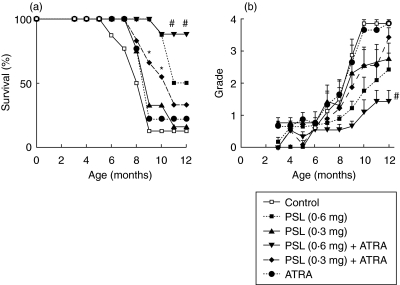
Figure 1a shows the survival rate (%) of the controls and the experimental mice for 12 months. The survival rates of the control mice at 7 and 10 months were 77·5% and 12·5%, respectively, and those of the experimental groups were improved to various extents compared to those of the control mice. At 7 months there were no significant differences among all mice. At 9 and 10 months, the survival rates were much improved in the mice receiving the combination of PSL (0·3 mg) + ATRA compared to the single administration of PSL (0·3 mg) or ATRA, P < 0·05 significant. Furthermore, at 11 and 12 months, the survival rates were much improved in the mice receiving the combination of PSL (0·6 mg) + ATRA compared to the single administration of PSL (0·6 mg), P < 0·03 significant.
Fig. 1.
(a) Survival rate of the experimental and control mice. (b) Proteinuria was graded semiquantitatively monthly (0, none; 1, 30–99 mg/dl; 2, >100–299 mg/dl; 3, >300–999 mg/dl; 4, >1000 mg/dl or more of albumin). PSL (0·3 mg) + ATRA, n = 11 versus PSL (0·3 mg), n = 9; ATRA, n = 9; control, n = 16 (*P < 0·05) and PSL (0·6 mg) + ATRA, n = 12 versus PSL (0·6 mg), n = 8; PSL (0·3 mg) + ATRA; PSL (0·3 mg); ATRA; control (#P < 0·03).
PSL + ATRA improves proteinuria compared to PSL or ATRA
Figure 1b shows the changes of the mean scores of proteinuria. They stayed at less than grade 1 until 6 months. From 7 to 10 months, the scores increased promptly in the control and ATRA-treated mice, and reached grade 4 at 12 months. In contrast, the scores in the other groups increased slowly, and the increase of scores in the mice given PSL (0·6 mg) and PSL (0·6 mg) + ATRA were small, especially in the latter group [P < 0·05, PSL (0·6 mg) versus PSL (0·6 mg) + ATRA at 12 months]. The changes of the positive rate of proteinuria were similar to those of the scores. The rate (%) of each group at 12 months was: PSL (0·6 mg) + ATRA, 33; PSL (0·6 mg) alone, 54; PSL (0·3 mg) + ATRA, 63; PSL (0·3 mg) alone, 72; ATRA alone, 100; control, 100.
Effects on spleen and body weight
The spleen weights at 5 months after beginning treatment were lighter in all the experimental groups than those in the control mice (Table 1). The body weights in all the experimental groups tended to be lower than those in the control group, although the difference did not reach significance. There were no differences in body weight among the experimental groups (Table 1).
Table 1.
Body and spleen weight of the experimental groups and control mice after 5 months of treatment.
| Treatment | Body weight (g) | Spleen weight (g) |
|---|---|---|
| PSL (0·6 mg) | 36·1 ± 1·5 | 0·12 ± 0·02** |
| PSL (0·6 mg) + ATRA | 36·3 ± 1·8 | 0·14 ± 0·03** |
| PSL (0·3 mg) | 37·4 ± 1·2 | 0·14 ± 0·03** |
| PSL (0·3 mg) + ATRA | 35·7 ± 1·9 | 0·12 ± 0·01** |
| ATRA | 37·6 ± 1·8 | 0·18 ± 0·03* |
| Control | 39·6 ± 1·5 | 0·28 ± 0·03 |
Mean ± s.d. is shown.
P < 0·05
P < 0·005 versus control mice.
Expression of mRNAs for cytokines, MCP-1 and iNOS
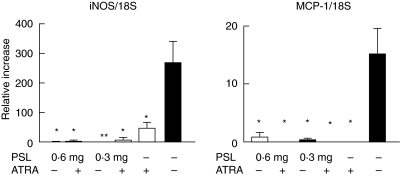
Expression of the mRNAs for IL-2, IL-4, IFN-γ and IL-12 by CD4+ T cells in most of the experimental groups was remarkably lower than that in the control mice. IL-2 mRNA expression in the mice given PSL (0·6 mg, 0·3 mg) + ATRA was significantly lower than in PSL (0·6 mg, 0·3 mg) alone, respectively (P < 0·05). IFN-γ mRNA expression in the mice given PSL (0·3 mg) + ATRA was lower than in those given PSL (0·3 mg) alone. IL-12 mRNA expression in all experimental mice was lower than that in the control mice (Fig. 2). Expression of the mRNAs for iNOS and MCP-1 in the kidney was reduced in all experimental mice compared to that in the control mice (Fig. 3).
Fig. 2.
Expression of mRNAs for cytokines in the CD4+ T cells after 5 months (n = 8, *P < 0·05, **P < 0·005, ***P < 0·001 versus control mice, †P < 0·05; PSL (0·6 mg or 0·3 mg) alone versus combination of each dose of PSL + ATRA).
Fig. 3.
Expression of mRNAs for MCP-1 and iNOS in the kidney after 5 months (n = 8; *P < 0·05 versus control mice; **P < 0·005).
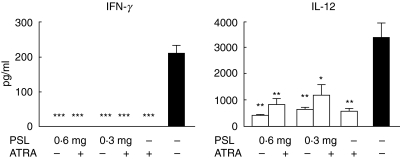
Serum cytokine levels
Serum levels of IFN-γ in all the experimental groups were reduced to an undetectable range. Serum levels of IL-12 were also reduced to various extents regardless of the treatment, although there were no differences among these groups (Fig. 4). IL-2 and IL-4 levels were undetectable in all the groups.
Fig. 4.
Effects of ATRA on the serum levels of IFN-γ and IL-12 at 8 months (n = 8, *P < 0·05, **P < 0·005, ***P < 0·001 versus control mice).
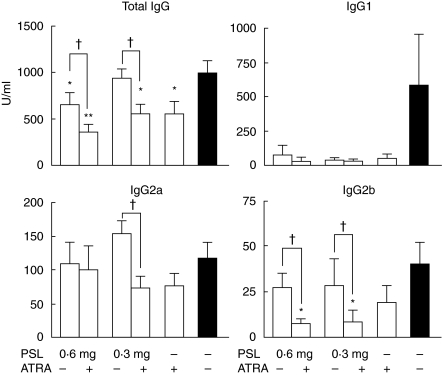
Serum titres of anti-DNA antibody
Figure 5 shows the serum titres of the anti-DNA antibody of the total IgG and its subclasses. Those of the total IgG class were reduced significantly in the mice given PSL (0·6 mg, 0·3 mg) + ATRA, PSL (0·6 mg) and ATRA alone, but not in the mice given PSL (0·3 mg), as compared with the control mice. Serum titres of the total IgG-specific anti DNA antibody in the mice given a combination of PSL (0·6 mg, 0·3 mg) + ATRA were reduced significantly compared to those in mice given PSL (0·6 mg, 0·3 mg, respectively) alone (P < 0·05, each). The titres of IgG1-specific anti-DNA antibody tended to be reduced in all the experimental groups. There were no differences in the serum titres of IgG2a-specific anti-DNA antibody among all the treatment groups. Titres of IgG2b-specific anti-DNA antibody were reduced in all experimental groups compared to those in the control mice, the reductions being significantly greater in the mice given a combination of PSL (0·6 mg, 0·3 mg) + ATRA in comparison with the mice given PSL (0·6 mg, 0·3 mg, respectively) alone. IgG3-specific anti-DNA antibody was undetectable in the serum from all the groups.
Fig. 5.
Effects of ATRA on the serum levels of anti-DNA antibody of various isotypes at 8 months (n = 8, *P < 0·05, **P < 0·005 versus control mice, †P < 0·05; PSL (0·6 mg or 0·3 mg) alone versus combination of each dose of PSL + ATRA).
Renal histopathology and immunohistochemical analysis
Figure 6a shows representative photographs in the mice given PSL (0·6 mg) + ATRA (Fig. 6b,e) and control (Fig. 6a,c,d). In the control mice, glomeruli contained segmental or global mesangial hypercellularity, increased mesangial matrix, capillary wall thickening, fibrin deposits, focal or global necrosis and crescents. The interstitium contained atrophy and dilation of renal tubules (Fig. 6a). In the glomeruli, mononuclear cell infiltration was reduced significantly in all the experimental groups including ATRA-treated mice compared to that in the control mice, as seen in Fig. 6a,b. In the interstitium, mononuclear cell infiltration was also reduced in all the experimental groups compared to that in the control mice. In particular, mononuclear cell infiltration was reduced by a combination of PSL (0·3 mg) + ATRA than by PSL (0·3 mg) alone. In the perivascular area, mononuclear cell infiltration was significantly reduced in the mice given PSL (0·6 mg), PSL (0·6 mg) + ATRA and PSL (0·3 mg) + ATRA, but was similar in the mice given PSL (0·3 mg) or ATRA alone compared to that in the control mice (Fig. 6b). The number of CD4+ T cells infiltrating the periglomerular areas tended to decrease in all experimental groups compared to that in the control mice, although there were no significant differences among these groups. The number of CD4+ T cells infiltrating the perivascular areas tended to reduce in the mice given PSL (0·6 mg) and the combination of PSL (0·6 mg, 0·3 mg) + ATRA compared to that in the mice given PSL (0·3 mg) or ATRA alone or in the control mice (Fig. 6a,c,e). The number of macrophages infiltrating the periglomerular areas tended to be reduced in the mice given PSL (0·6 mg) + ATRA or PSL (0·6 mg) compared to that in the control mice and to that in the other groups. The number of macrophages infiltrating the perivascular areas was reduced significantly in the mice given PSL (0·6 mg) and tended to be reduced in the other experimental groups of mice compared to that in the control mice (Fig. 6a,d,f). There were no differences in the number of CD8+ T cells or B220+ B cells infiltrating the periglomerular areas and the interstitium, including the perivascular areas among the groups (data not shown).
Fig. 6.
Pathological and immunohistochemical findings of the kidney. (A) PAS and immunohistochemical staining. Representative photographs (a–e) of a mouse given PSL (0·6 mg) + ATRA (b,e) and control (a,c,d) are shown. (b) PAS (magnification × 400), (c) CD4+, (d) F4/80+, (e) CD4+ and (f) F4/80+ cells. T cells and macrophages (brown) are present in the periglomerular, interstitial and perivascular areas (magnification ×200). Control mice (a) had glomerulosclerosis (GS), leukocytic infiltrates (arrow), tubular atrophy (TA) and casts (magnification ×400). In contrast, the renal histology of all the experimental groups of mice remained nearly normal. (B) Renal pathology was evaluated by scoring in the PAS-stained sections. Renal pathology was evaluated by scoring in the PAS-stained sections (n = 8, *P < 0·05, **P < 0·005, ***P < 0·001 versus control mice).
IgG deposition in the kidney
Table 2 shows the extent of deposition of total IgG and its subclasses in the intraglomerular and perivascular areas. Intraglomerular deposition of IgG, and its subclasses, was reduced in all the experimental groups compared to that in the control mice, although there were no significant differences in the extent of deposition among the experimental groups. On the other hand, treatment with PSL + ATRA reduced IgG deposition to a greater degree than PSL alone at the same dose.
Table 2.
Immunoglobulin deposition in the kidney.
| Intraglomerular | Perivascular | ||||
|---|---|---|---|---|---|
| Treatment | IgG | IgG1 | IgG2a | IgG2b | IgG |
| PSL (0·6 mg) | 0·9 ± 0·2** | 0·3 ± 0·1*** | 0·9 ± 0·3 | 0·1 ± 0·1*** | 38% |
| PSL (0·6 mg) + ATRA | 0·6 ± 0·2*** | 0·8 ± 0·3** | 0·2 ± 0·1** | 0·9 ± 0·4* | 25% |
| PSL (0·3 mg) | 0·8 ± 0·3** | 0·4 ± 0·3** | 0·3 ± 0·2* | 0·4 ± 0·4* | 75% |
| PSL (0·3 mg) + ATRA | 0·6 ± 0·2*** | 1·0 ± 0·3* | 0·5 ± 0·2 | 0·4 ± 0·2** | 38% |
| ATRA | 1·2 ± 0·3* | 0·8 ± 0·2** | 0·5 ± 0·2 | 0·7 ± 0·5 | 75% |
| Control | 2·1 ± 0·3 | 2·0 ± 0·3 | 1·0 ± 0·2 | 1·9 ± 0·3 | 75% |
Deposits of IgG and its subclasses were analysed by immunohistochemistry. The intensity of the fluorescence in the glomeruli was assessed by a semiquantitative scale (0, no deposition; 1, weak; 2, moderate; 3, strong) (n = 9
P < 0·05
P < 0·005
P < 0·001 versus control mice. Values are mean ± s.d.). Perivascular IgG deposition is represented as the positive rate of five randomly chosen inter- or intralobular vessels.
Laboratory tests
Table 3 shows laboratory data on the age of 8 months. BUN levels were significantly lower in the mice given PSL (0·6 mg), PSL (0·6 mg) + ATRA, and PSL (0·3 mg) + ATRA than those in the control mice (P < 0·05). BUN levels were reduced more in the mice given the combination of PSL (0·6 mg, 0·3 mg) + ATRA than in the mice given PSL (0·6 mg, 0·3 mg) alone, respectively. Cr levels were also significantly lower in all groups than in the control mice (P < 0·05). There were no significant differences in the serum levels of AST, ALT and GLU among the all groups. There were no signs of progression of renal failure, liver damage, glucose intolerance, bone fracture and skin lesion by administration of ATRA or PSL.
Table 3.
Laboratory tests in NZB/W F1 mice.
| Treatment | BUN | Cr | AST | ALT | GLU | Bone fracture | Skin lesion |
|---|---|---|---|---|---|---|---|
| PSL (0·6 mg) | 18·8 ± 1·8* | 0·06 ± 0·02* | 115 ± 8·2 | 33 ± 4·7 | 141·2 ± 11·2 | – | – |
| PSL (0·6 mg) + ATRA | 14·8 ± 1·3* | 0·08 ± 0·02* | 108 ± 10·2 | 28 ± 3·2 | 133·3 ± 14·1 | – | – |
| PSL (0·3 mg) | 40·3 ± 15·2 | 0·09 ± 0·03* | 151 ± 41·2 | 73 ± 5·1 | 161·2 ± 12·3 | – | – |
| PSL (0·3 mg) + ATRA | 31·2 ± 8·3* | 0·14 ± 0·02* | 78·5 ± 9·7 | 15·5 ± 1·9 | 111·8 ± 23·5 | – | – |
| ATRA | 87·2 ± 34·2 | 0·15 ± 0·05* | 103·7 ± 13·6 | 18·7 ± 3·1 | 113·7 ± 12·7 | – | – |
| Control | 147·7 ± 36·9 | 0·37 ± 0·02 | 108·3 ± 15·3 | 26 ± 5·7 | 135·7 ± 11·7 | – | – |
Tests were carried out in all mice at 8 months of age. Values are the mean ± s.d. (n = 5
P < 0·05 versus control mice). BUN: blood urea nitrogen; Cr: creatine; AST: asparate aminotransferase; ALT: alanine aminotransferase; GLU: glucose.
Discussion
Corticosteroid is the most effective of various anti-inflammatory or immunosuppressive drugs used commonly to treat human SLE. However, its pharmacological adverse reactions include infection, osteoporosis, diabetes mellitus and retardation of wound healing. On the other hand, we have demonstrated previously that ATRA improved the symptoms of lupus nephritis and prolonged the survival period in NZB/W F1 mice [1]. However, it is known that ATRA induces weight loss, bone fracture, anaemia, liver damage and teratosis. Here, we combined suboptimal doses of these two agents in order to decrease adverse effects and to gain a possible synergistic effect. In this study, the combination of PSL and ATRA was more effective than the single administration of PSL or ATRA in prolonging the survival period and reducing the symptoms of lupus nephritis. Furthermore, there were no remarkable symptoms and signs of adverse effects induced by a single use or combination of ATRA and PSL.
Recently, there have been several reports that abnormalities of both the Th1 and Th2 cells are associated with the pathogenesis of autoimmune disorders [15,16]. Among the Th1 cytokines IFN-γ, the production of which is promoted by IL-12, plays a key regulatory role in the development of autoimmune kidney diseases, and deletion of IFN-γ receptor or IFN-γ genes or administration of anti-IFN-γ monoclonal antibody improved the survival rate of lupus-prone mice [17–20]. IL-12 has been reported to promote T cell proliferation [21,22] and differentiation of CD4+ T cells into Th1 cells [23], and its production by tubular epithelial cells (TEC) and macrophages is up-regulated along with the development of lupus nephritis in MRL-Fas (lpr) mice [24]. Among the Th2 cytokines, IL-4 enhances the differentiation of both B cells and T cells (especially Th2 cells), and increased production of IL-4 is also associated with the pathogenesis of SLE [25,26] and lupus nephritis [27]. In this study, we found a synergistic effect of PSL and ATRA in mRNA expression of IFN-γ and IL-12, although there were no differences in their serum levels between the combination of PSL + ATRA and the single administration of PSL or ATRA alone. On the other hand, serum titres of IgG1-specific anti-DNA antibodies, which include IL-4-dependent and IL-12 or IFN-γ-dependent subtypes [28], were reduced by ATRA alone as much as they were reduced in the presence of PSL. The finding that serum titres of total IgG-specific anti-DNA antibodies and IgG2 (especially IgG2b)-specific anti-DNA antibodies, whose production is driven by IFN-γ, were reduced more effectively by the combination of PSL and ATRA than by PSL or ATRA alone also supports this synergistic effect.
Deposition of IgG and its subclasses in the glomeruli was reduced by PSL and/or ATRA, but it was difficult to discriminate the effect of PSL or ATRA. However, as deposition of IgG around the vessels was more reduced by the combination of PSL and ATRA than by single administration of these, PSL and ATRA might have acted synergistically.
In addition to the deposition of IgG, inflammatory cell infiltration was observed in and around the glomeruli, as well as in the interstitial areas, especially in the perivascular areas of the kidney. It was demonstrated that these cells were CD4+ T cells and macrophages. This finding was thought to be compatible with the previous report that mice deficient in CD4+ T cells had reduced levels of the serum IgG and autoantibodies and were protected from lupus nephritis [29]. In this study, the difference in the extent of inflammatory cell infiltration among the groups of treatment with PSL and/or ATRA was unremarkable, but it might be more remarkable after a period of histological study.
Gene expression of MCP-1 increases along with the progression of nephritis in NZB/W F1 mice [10], although the precise mechanism governing inflammatory cell infiltration in these mice is not clear. The finding that mRNA expression of MCP-1 was reduced by ATRA might be associated with the inhibition of infiltration of CD4+ T cells and macrophages leading to the synergism in the improvement of lupus nephritis by the combination of PSL and ATRA in this study.
Similarly, mRNA expression of iNOS was reduced by ATRA, and these findings appeared to be compatible with the previous report that retinoids inhibit the synthesis and mRNA of iNOS in the mesangial cells [30] and vascular smooth muscles [31]. This finding might be also associated with the synergism of PSL and ATRA in the treatment of lupus nephritis.
Retinoids, including ATRA, are known to bind to two distinct nuclear receptors, retinoic acid receptors (RARs) and retinoid x receptors (RXRs) [32], and to form transcription factors. Heterodimers of RAR and RXR or homodimers of RXRs bind to retinoid receptor-responsive elements present in the promoters of the retinoid target genes to regulate their expression [33,34]. This RAR is known to bind directly to the transcription factor AP-1 to antagonize AP-1-dependent transcription [35,36]. On the other hand, it has been reported that glucocorticoids bind to glucocorticoid receptor (GR), which inactivates various transcription factors including AP-1, NF-κB and NFIL-6, to exhibit their multiple actions such as anti-inflammation, immunosuppression and inhibition of cell growth [37,38]. Our results suggest the possibility of new therapeutic strategies for human SLE, which might serve to reduce the adverse reactions of two agents and to induce a synergistic effect. In order to investigate whether ATRA can serve as a steroid-sparing agent for the treatment of lupus nephritis, we are due to design experiments using the combination of ATRA and PSL in NZB/W F1 mice in which some diseases have already developed.
Acknowledgments
We thank K. Niki, K. Furukawa and J. Honda for technical assistance.
References
- 1.Kinoshita K, Funauchi M, Kanamaru A, et al. Retinoic acid reduces autoimmune renal injury and increases survival in NZB/W F(1) mice. J Immunol. 2003;170:5793–8. doi: 10.4049/jimmunol.170.11.5793. [DOI] [PubMed] [Google Scholar]
- 2.Orfanos CE, Bauer R. Evidence for antiinflammatory activities for oral and synthetic retinoids. Br J Dermatol. 1983;25:55–60. [PubMed] [Google Scholar]
- 3.Brinckerhoff CE, Coffey JW, Sullivan AC. Inflammation and collagenase production in rats with adjuvant arthritis reduced with 13-cis-retioic acid. Science. 1983;221:756–8. doi: 10.1126/science.6308759. [DOI] [PubMed] [Google Scholar]
- 4.Fumarulo R, Conese M, Semeraro N, et al. Retinoids inhibit the respiratory burst and degranulation of stimulated human polymorphonuclear leukocytes. Agent Actions. 1991;34:339–44. doi: 10.1007/BF01988726. [DOI] [PubMed] [Google Scholar]
- 5.Huang ME, Ye YC, Wang ZY, et al. Use of all-trans retinoic acid in the treatment of acute promyelocytic leukemia. Blood. 1988;72:567–72. [PubMed] [Google Scholar]
- 6.Ward A, Brogden RN, Avery GS, et al. Etretinate. A review of its pharmacological properties and therapeutic efficacy in psoriasis and other skin disorders. Drugs. 1983;26:9–43. doi: 10.2165/00003495-198326010-00002. [DOI] [PubMed] [Google Scholar]
- 7.Peck GL, Olsen TG, Cheripko J, et al. Isotretinoin versus placebo in the treatment of cystic acne. A randomized double-blind study. J Am Acad Dermatol. 1982;6:735–45. doi: 10.1016/s0190-9622(82)70063-5. [DOI] [PubMed] [Google Scholar]
- 8.Brinckerhoff CE. Retinoids and rheumatoid arthritis: modulation of extracellular matrix by controlling expression of collagenase. Meth Enzymol. 1990;190:175–88. doi: 10.1016/0076-6879(90)90022-s. [DOI] [PubMed] [Google Scholar]
- 9.Kang BY, Chung SW, Kim TS, et al. Retinoid-mediated inhibition of interleukin-12 production in mouse macrophages suppresses Th1 cytokine profile in CD4+ T cells. Br J Pharmacol. 2000;130:581–6. doi: 10.1038/sj.bjp.0703345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carr MW, Roth SJ, Springer TA, et al. Monocyte chemoattractant protein 1 acts as a T-lymphocyte chemoattractant. Proc Natl Acad Sci USA. 1994;11:3652–6. doi: 10.1073/pnas.91.9.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Furusu A, Miyazaki M, Kohno S, et al. Expression of endothelial and inducible nitric oxide synthase in human glomerulonephritis. Kidney Int. 1993;53:1760–8. doi: 10.1046/j.1523-1755.1998.00907.x. [DOI] [PubMed] [Google Scholar]
- 12.Kelley VE, Winkelstein A, Dixon FJ, et al. Prostaglandin E1 inhibits T-cell proliferation and renal disease in MRL/1 mice. Clin Immunol Immunopathol. 1981;21:190–203. doi: 10.1016/0090-1229(81)90208-7. [DOI] [PubMed] [Google Scholar]
- 13.Kinoshita K, Tesch G, Kelley VR, et al. Costimulation by B7-1 and B7-2 is required for autoimmune disease in MRL-Faslpr mice. J Immunol. 2000;164:6046–56. doi: 10.4049/jimmunol.164.11.6046. [DOI] [PubMed] [Google Scholar]
- 14.Hirose S, Kinoshita K, Shirai T, et al. Effects of major histocompatibility complex on autoimmune disease of H-2-congenic New Zealand mice. Int Immunol. 1990;2:1091–5. doi: 10.1093/intimm/2.11.1091. [DOI] [PubMed] [Google Scholar]
- 15.Romagnani S, Parronchi P, Del Prete GF, et al. An update on human Th1 and Th2 cells. Int Arch Allergy Immunol. 1997;113:153–6. doi: 10.1159/000237532. [DOI] [PubMed] [Google Scholar]
- 16.Theofilopoulos AN, Dixon FJ. Murine models of systemic lupus erythematosus. Adv Immunol. 1985;37:269–390. doi: 10.1016/s0065-2776(08)60342-9. [DOI] [PubMed] [Google Scholar]
- 17.Gately MK, Desai BB, Gubler U, et al. Regulation of human lymphocyte proliferation by a heterodimeric cytokine, IL-12 (cytotoxic lymphocyte maturation factor) J Immunol. 1991;147:874–82. [PubMed] [Google Scholar]
- 18.Perussia B, Chan SH, Trinchieri G, et al. Natural killer (NK) cell stimulatory factor or IL-12 has differential effects on the proliferation of TCR-alpha beta+, TCR-gamma delta+ T lymphocytes, and NK cells. J Immunol. 1992;149:3495–502. [PubMed] [Google Scholar]
- 19.Seder RA, Gazzinelli R, Paul WE, et al. Interleukin 12 acts directly on CD4+ T cells to enhance priming for interferon gamma production and diminishes interleukin 4 inhibition of such priming. Proc Natl Acad Sc USA. 1993;90:10188–92. doi: 10.1073/pnas.90.21.10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fan X, Oertli B, Wuthrich RP. Up-regulation of tubular epithelial interleukin-12 in autoimmune MRL-Fas(lpr) mice with renal injury. Kidney Int. 1997;51:79–86. doi: 10.1038/ki.1997.10. [DOI] [PubMed] [Google Scholar]
- 21.Jacob CO, van der Meide PH, McDevitt HO. In vitro treatment of (NZB × NZW) F1 lupus-like nephritis with monoclonal antibody to γ interferon. J Exp Med. 1987;166:798–803. doi: 10.1084/jem.166.3.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balomenos D, Rumold R, Theofilopoulos AN. Interferon-gamma is required for lupus-like disease and lymphoaccumulation in MRL-lpr mice. J Clin Invest. 1998;101:364–71. doi: 10.1172/JCI750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haas C, Ryffel B, Le HM. IFN-γ receptor deletion prevents autoantibody production and glomerulonephritis in lupus-prone (NZB × NZW) F1 mice. J Immunol. 1998;160:3713–8. [PubMed] [Google Scholar]
- 24.Schwarting A, Wada T, Kelley VR, et al. IFN-gamma receptor signaling is essential for the initiation, acceleration, and destruction of autoimmune kidney disease in MRL-Fas(lpr) mice. J Immunol. 1998;161:494–503. [PubMed] [Google Scholar]
- 25.Chang TL, Shea CM, Abbas AK, et al. Heterogeneity of helper/inducer T lymphocytes. III. Responses of IL-2- and IL-4-producing (Th1 and Th2) clones to antigens presented by different accessory cells. J Immunol. 1990;145:2803–8. [PubMed] [Google Scholar]
- 26.Funauchi MYuH, Kanamaru A, et al. Increased interleukin-4 production by NK T cells in systemic lupus erythematosus. Clin Immunol. 1999;92:197–202. doi: 10.1006/clim.1999.4742. [DOI] [PubMed] [Google Scholar]
- 27.Schorlemmer HU, Dickneite G, Enssel KH, et al. Modulation of the immunoglobulin dysregulation in GVH- and SLE-like diseases by the murine IL-4 receptor (IL-4R) Inflamm Res. 1995;44:194–6. doi: 10.1007/BF01778328. [DOI] [PubMed] [Google Scholar]
- 28.Faquim-Mauro EL, Coffman RL, Macedo MS, et al. Cutting edge: mouse IgG1 antibodies comprise two functionally distinct types that are differentially regulated by IL-4 and IL-12. J Immunol. 1999;163:3572–6. [PubMed] [Google Scholar]
- 29.Chesnutt MS, Finck BK, Wofsy D, et al. Enhanced lymphoproliferation and diminished autoimmunity in CD4-deficient MRL/lpr mice. Clin Immunol Immunopathol. 1998;87:23–32. doi: 10.1006/clin.1997.4492. [DOI] [PubMed] [Google Scholar]
- 30.Datta PK, Lianos EA. Retinoic acids inhibit inducible nitric oxide synthase expression in mesangial cells. Kidney Int. 1999;56:486–3. doi: 10.1046/j.1523-1755.1999.00576.x. [DOI] [PubMed] [Google Scholar]
- 31.Hirokawa K, O'Shaughnessy KM, Wilkins MR, et al. Inhibition of nitric oxide synthesis in vascular smooth muscle by retinoids. Br J Pharmacol. 1994;113:1448–54. doi: 10.1111/j.1476-5381.1994.tb17159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jonat C, Rahmsdorf HJ, Herrlich P, et al. Antitumor promotion and antiinflammation: down-modulation of AP-1 (Fos/Jun) activity by glucocorticoid hormone. Cell. 1990;62:1189–204. doi: 10.1016/0092-8674(90)90395-u. [DOI] [PubMed] [Google Scholar]
- 33.Mangelsdorf DJ, Kliewer SA, Evans RM, et al. Retinoid receptors. Recent Prog Horm Res. 1993;48:99–121. doi: 10.1016/b978-0-12-571148-7.50008-7. [DOI] [PubMed] [Google Scholar]
- 34.Zhang XK, Lehmann J, Pfahl M, et al. Homodimer formation of retinoid X receptor induced by 9-cis retinoic acid. Nature. 1992;358:587–91. doi: 10.1038/358587a0. [DOI] [PubMed] [Google Scholar]
- 35.Shultz PJ, Tayeh MA, Raij L, et al. Synthesis and action of nitric oxide in rat glomerular mesangial cells. Am J Physiol. 1991;261:600–6. doi: 10.1152/ajprenal.1991.261.4.F600. [DOI] [PubMed] [Google Scholar]
- 36.Schule R, Rangarajan P, Evans RM, et al. Retinoic acid is a negative regulator of AP-1-responsive genes. Proc Natl Acad Sci USA. 1991;88:6092–6. doi: 10.1073/pnas.88.14.6092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nicholson RC, Mader S, Chambon P, et al. Negative regulation of the rat stromelysin gene promoter by retinoic acid is mediated by an AP1 binding site. EMBO J. 1990;9:4443–54. doi: 10.1002/j.1460-2075.1990.tb07895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ray A, Prefontaine KE. Physical association and functional antagonism between the p65 subunit of transcription factor NF-kappa B and the glucocorticoid receptor. Proc Natl Acad Sci USA. 1994;91:752–6. doi: 10.1073/pnas.91.2.752. [DOI] [PMC free article] [PubMed] [Google Scholar]