Abstract
Coeliac disease (CoD) is a small intestinal disorder characterized by villous atrophy, crypt cell hyperplasia and an increased production of T helper cell type 1 (Th1) cytokines. Interleukin (IL)-18 is a pro-inflammatory cytokine that has a crucial role in maintaining the Th1 response. In this study, the serum levels of IL-18 were measured in children with CoD or other gastrointestinal diseases in order to evaluate the possibility of using IL-18 as a disease activity marker. IL-18 levels were higher in samples from CoD patients [median 443 pg/ml (148–885)] compared to healthy controls [median 205 pg/ml (11–379)], P < 0·05. In contrast, the levels of IL-18 were not enhanced significantly in the serum from patients with inflammatory bowel disease (IBD) [median 324 pg/ml (207–546)] or in the disease control group [median 303 pg/ml (2–689)]. In CoD patients, after 2 weeks of gluten challenge (GC), serum IL-18 was unchanged [median 268 pg/ml (59–458)] compared to patients on a gluten-free diet [median 220 pg/ml (53–600)], while IL-18 was increased after 12 weeks of GC [median 551 pg/ml (94–952)], P < 0·01. The IL-18 levels correlated with IgA anti-transglutaminase antibody levels (rs = 0·59, P = 0·016) in serum from untreated CoD patients, and IL-18 also followed the degree of small intestinal villous atrophy in 12 out of 19 CoD patients. Our results support the view that serum IL-18 concentrations in children with CoD follow disease activity, suggesting a role for IL-18 in the induction of an inflammatory Th1-response after gluten exposure.
Keywords: coeliac, disease, cytokines, IL-18
Introduction
Coeliac disease (CoD) is a proximal small intestinal disorder characterized by various degrees of crypt cell hyperplasia, villous atrophy and increased numbers of intraepithelial lymphocytes [1]. An abnormal immune response to dietary gluten is crucial for the development of CoD in genetically susceptible individuals. Thus, modification of gluten peptides by the enzyme tissue transglutaminase (tTG) can enhance the binding of those peptides to HLA-DQ2 or -DQ8, thereby potentiating T cell stimulation [2,3]. CoD patients develop disease-specific IgA antibodies to tTG [4], which can be used in the screening and diagnosis of CoD [5,6] in addition to the standard diagnostic procedure with histological evaluation of small intestinal biopsies.
There is evidence that CD4+ T cells play a major role in tissue injury [7] and lamina propria CD4+ T cells have an active phenotype and produce large amounts of T helper cell type 1 (Th1) cytokines in response to gluten stimulation [8]. HLA-DQ2 or -DQ8 restricted T cell clones from the intestinal lesion, reacting with various gliadin epitopes, have shown a Th1 pattern, primarily by producing interferon (IFN)-γ upon stimulation with relevant gliadin-derived peptides [9]. It has also been shown that patients with untreated CoD have an increased production of cytokines such as interleukin (IL)-2, IFN-γ, IL-6 and tumour necrosis factor (TNF)-α from inflammatory cells, especially T cells and macrophages [10–14].
IL-18 is a proinflammatory cytokine that is a potent inducer of IFN-γ production and has a crucial role in maintaining the Th1 response, particularly in the presence of IL-12 [15,16]. IL-18 acts as a co-stimulant for Th1 cells to augment IL-2, granulocyte macrophage-colony stimulating factor (GM-CSF) and IL-2Rα production, induces cell proliferation and enhances T and natural killer (NK) cell maturation and cytotoxicity [17]. Because the receptors for IL-18 are a part of the IL-1 receptor family, many of the biological properties of IL-18 are similar to those of IL-1 [16].
Elevated IL-18 expression has been found in inflammatory disorders of the gut. Thus, IL-18 has been detected in mucosal lesions and serum of patients with Crohn's disease (CD) [18–20] and both IL-18 RNA and mature protein has been detected in duodenal mucosa of CoD patients [21]. In addition, CoD patients have enhanced IL-18 levels in serum, which are reduced after a period of gluten-free diet (GFD) [22].
Although there are indications that serum levels of anti-tTG antibodies correlate to the mucosal lesion [23,24], there are a few patients with increased anti-tTG levels and not yet manifest villous atrophy. Patients with normal mucosa and latent CoD can subsequently develop villous atrophy after several years of follow-up [25] and there is therefore a need for a non-invasive disease activity marker to further improve the diagnosis and avoid unnecessary successive intestinal biopsies.
The aim of this study was to elucidate if the serum levels of IL-18 correspond to gluten exposure or to the condition of the small intestinal mucosa. Furthermore, we wanted to investigate if IL-18 could be used as a disease activity marker in children with CoD.
Materials and methods
Patients
Included in this retrospective study were serum samples from 55 children (31 girls, 24 boys, median age 4 years, range 1–16 years) with biopsy-verified CoD and the diagnosis were confirmed according to the original European Society for Pediatric Gastroenterology, Hepatology and Nutrition criteria [26]. Analysed were 16 serum samples from untreated patients (UT), 42 samples collected after 1–3 years on a GFD, 38 samples after 2 weeks of gluten challenge (GC) and 27 serum samples after 12 weeks of GC. Also included were serum samples from 28 children (11 girls, 17 boys, median age 14 years, range 1–18 years) with IBD [four with CD and 24 with ulcerative colitis (UC)] and 22 children (11 girls, 11 boys, median age 4 years, range 1–16 years) with other gastrointestinal diseases [disease controls (DC)]. All children in the DC group had a small intestinal biopsy performed; seven had cow's milk protein intolerance, three had other food hypersensitivity and 12 had transient gastrointestinal disorders. Serum samples from 22 children (10 girls, 12 boys, median age 6 years, range 1–16 years) without any gastrointestinal symptoms, and no relatives with CoD, served as healthy controls (HC). All studies on patients and HC subjects were performed in accordance with the ethical rules of the Uppsala University Hospital and the University Hospital of Turku.
IL-18 immunoassay
Serum IL-18 levels were measured using a human IL-18 enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems, Abingdon, UK) according to the instructions from the manufacturer.
Intestinal biopsy
All small intestinal biopsies were previously studied histologically by one pathologist (Dr Wolfgang Kraaz, Uppsala University Hospital) and normality for the children was defined as a villous/crypt ratio of 2 : 1 or more and an IEL count within normal limits (one or less IEL/five epithelial cells). The histopathological changes were classified as: normal mucosa, partial/subtotal villous atrophy (a villous/crypt ratio distinctly less than 2 : 1) and total villous atrophy (flat mucosa) [27].
Coeliac disease autoantibodies
IgA and IgG autoantibodies to tTG in serum were determined with an ELISA (Research prototype, Pharmacia Diagnostics, Freiburg, Germany), as described elsewhere [24]. Microplate strips were coated with recombinant human tTG at optimal concentration. Patient sera were diluted 1/100 and the antibody concentrations were expressed as arbitrary units per millilitre (U/ml).
Statistical analysis
Cytokine levels are expressed as median (10th and 90th percentiles) values. The Kruskal–Wallis test was used to estimate differences in IL-18 levels between groups. The Wilcoxon rank test was used to compare values before and during treatment, and the Friedman's test with Dunn's post-test were used to compare the values before and after GC. Spearman's rank method was used to calculate the correlation between IL-18 and the anti-tTG antibody levels. All calculations were performed using the Graphpad Prism® software.
Results
Enhanced IL-18 levels in serum from patients with CoD
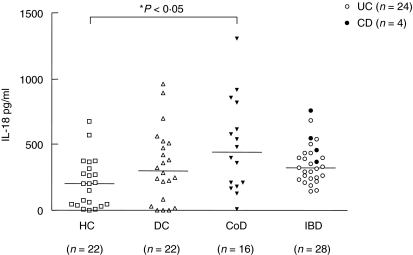
In Fig. 1, it is shown that the levels of IL-18 were higher in serum from patients with CoD [median 443 pg/ml (148–885)] compared to HC [median 205 pg/ml (11–379)], P < 0·05. In contrast, the levels of IL-18 were not enhanced in serum from IBD patients [median 324 pg/ml (207–546)] or in the DC group [median 303 pg/ml (2–689)] compared to HC. The two samples with the highest IL-18 values in the DC group were from patients with food hypersensitivity. The IL-18 levels in serum of the four patients with CD were all higher (372, 459, 551 and 762 pg/ml) than the median value for the UC samples [308 pg/ml (198–483)].
Fig. 1.
Comparison of serum IL-18 concentrations in healthy controls (HC), disease controls (DC), children with untreated coeliac disease (CoD) and children with inflammatory bowel disease (IBD). Filled circles represent Crohn's disease (CD) and open circles ulcerative colitis (UC). The horizontal lines represent median values.
Effect of GFD and GC on IL-18 levels in serum from CoD patients
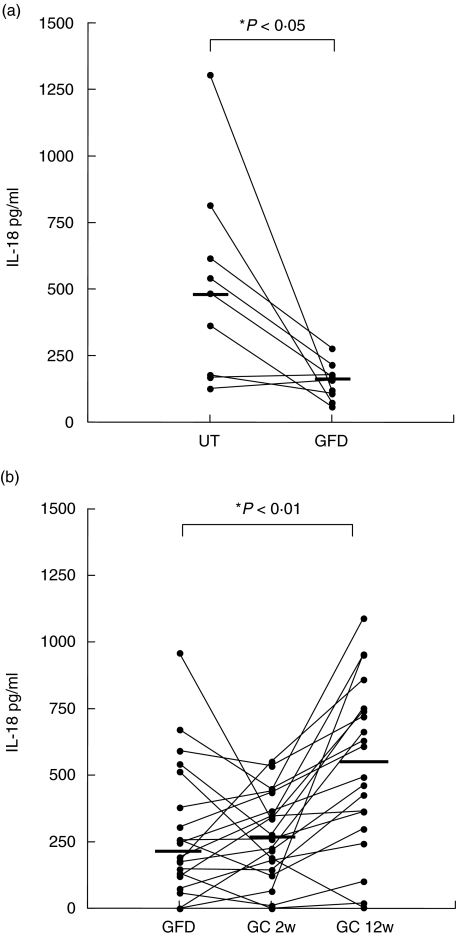
In order to study if the level of IL-18 is affected by gluten intake, serum samples collected after 10–36 months on a GFD and samples collected after 12 weeks of GC were analysed. Samples were obtained from nine CoD patients before and during GFD. Figure 2a shows that during the GFD the IL-18 levels in serum decreased for seven of the nine patients, while two remained at an already low level. The median value for the untreated group was 485 pg/ml (161–914) and for the GFD group 160 pg/ml (72–229), P < 0·05.
Fig. 2.
Comparison of serum levels of IL-18 in (a) CoD patients (n = 9) before treatment (UT) and during gluten-free diet (GFD), and (b) during GFD, after 2 weeks and after 12 weeks of gluten-challenge (GC), n = 20. The horizontal lines represent median values.
Serum samples were obtained from CoD patients during GFD, after 2 weeks and 12 weeks of GC. From 20 of the 55 CoD patients in this study, samples from all three occasions were analysed, while there were only samples from one or two occasions available from the remaining 35 patients. Figure 2 shows that for the 20 patients, after 2 weeks of GC, serum IL-18 levels were unchanged [median 268 pg/ml (59–458)] compared to patients on GFD [median 220 pg/ml (53–600)], while IL-18 levels were increased after 12 weeks of GC [median 551 pg/ml (94–952)], P < 0·01. When the samples from the remaining 35 CoD patients were included, the median IL-18 levels were 252 pg/ml (77–551) during GFD (n = 42), 235 pg/ml (16–443) after 2 weeks of GC (n = 38) and 463 pg/ml (102–896) after 12 weeks of GC (n = 27).
Correlation of IL-18 serum levels with small intestinal villous atrophy
In order to investigate if IL-18 correlate to intestinal damage, the IL-18 levels were compared with the degree of villous atrophy in small intestinal biopsies from the CoD patients (n = 19), from whom more than one biopsy specimen and serum sample was available (Table 1). The amount of IL-18 followed the degree of villous flattening in 12 of the 19 patients, i.e. there were enhanced IL-18 levels in patients with villous atrophy and low levels in patients with normal mucosa. However, two of the patients with normal mucosa (patients 26 and 31) had increased IL-18 in serum, and the reverse was true for three untreated patients with total villous atrophy (patients 15, 24 and 46), in whom low amounts of IL-18 (below 250 pg/ml) were detected. After GFD, two of the children (patients 19 and 33) had a partial/subtotal villous atrophy and increased serum levels of IL-18 (426 and 543 pg/ml).
Table 1.
Serum IL-18 concentrations, IgA and IgG anti-tissue transglutaminase (anti-tTG) levels and biopsy results from individual CoD patients at diagnosis (UT), during gluten-free diet (GFD) and after 12 weeks of gluten challenge (GC).
| UT | GFD | GC 12w | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient no. | IL-18 (pg/ml) | Biopsy | IgA tTG (U/ml) | IgG tTG (U/ml) | IL-18 (pg/ml) | Biopsy | IgA tTG (U/ml) | IgG tTG (U/ml) | IL-18 (pg/ml) | Biopsy | IgA tTG (U/ml) | IgG tTG (U/ml) |
| 1 | 1305 | P/S | 7658 | 7·9 | 121 | N | 4·4 | 2·7 | 952 | P/S | 8512 | 21 |
| 9 | 542 | P/S | 1369 | 5·3 | 217 | N | 3·6 | 1·3 | – | – | – | – |
| 15 | 170 | T | 625 | 36 | 179 | N | 2·0 | 1·0 | 859 | T | 711 | 34 |
| 16 | 816 | T | 958 | 9·5 | 75 | N | 0·9 | 0·4 | 244 | P/S | 242 | 6·7 |
| 19 | – | – | – | – | 426 | P/S | 35 | 7·4 | 637 | P/S | 807 | 21 |
| 22 | 485 | T | 545 | 11 | 172 | N | 1·9 | 0·2 | – | – | – | – |
| 23 | 617 | T | 1363 | 156 | 278 | N | 0·8 | 2·6 | 326 | P/S | 1026 | 113 |
| 24 | 178 | T | 4·7 | 1·4 | 108 | N | 0·4 | 0·1 | – | – | – | – |
| 25 | – | – | – | – | 193 | N | 1·4 | 0·5 | 365 | P/S | 503 | 56 |
| 26 | – | – | – | – | 960 | N | 5·7 | 1·6 | 742 | P/S | 420 | 64 |
| 31 | – | – | – | – | 791 | N | 2·6 | 0·3 | 398 | P/S | 135 | 3·4 |
| 32 | – | – | – | – | 0 | N | 1·6 | 0·2 | 463 | P/S | 561 | 2·7 |
| 33 | – | – | – | – | 543 | P/S | 25 | 51 | 752 | P/S | 392 | 122 |
| 35 | – | – | – | – | 306 | N | 135 | 0·3 | 609 | P/S | 743 | 0·6 |
| 38 | – | – | – | – | 246 | N | 1·8 | 0·4 | 493 | T | 710 | 1·9 |
| 40 | 210 | P/S | 643 | 104 | – | – | – | – | 102 | P/S | 218 | 2·1 |
| 41 | 364 | P/S | 257 | 1·2 | 59 | – | 3·4 | 0·1 | 102 | P/S | 718 | 2·2 |
| 45 | – | – | – | – | 0 | N | 0·9 | 0·5 | 955 | P/S | 200 | 4·4 |
| 46 | 127 | T | 5481 | 79 | 160 | N | 19 | 3·0 | – | – | – | – |
T = total villous atrophy; P/S = partial/subtotal villous atrophy; N = normal mucosa.
Comparison of IL-18 levels with antibodies to tTG
There was a correlation (rs = 0·59, P = 0·016) between IL-18 and IgA anti-tTG antibody levels in serum from the 16 untreated CoD patients (data not shown). As anti-tTG levels correspond to the gluten exposure, we also compared IL-18 with the amount of IgA and IgG anti-tTG in individual samples (Table 1) from patients before treatment, on a GFD and after GC. During the period of GFD, the IL-18 and IgA anti-tTG levels decreased in 7/9 samples and the IgG anti-tTG levels in 6/9 patients. In addition, after 12 weeks of GC, 10 of 14 samples had increased amounts of both IL-18, IgA anti-tTG and IgG anti-tTG levels. Thus, in most patients, there was a good correlation between IL-18 and anti-tTG antibodies throughout the study period.
Discussion
IL-18 is a potent inducer of IFN-γ production and has a crucial role in maintaining the Th1 response. In the present study, the serum levels of IL-18 were enhanced in children who were consuming gluten-containing food. This is in accordance with other recent studies, where similar amounts of IL-18 were found in adult CoD patients [22], and where cells expressing both mRNA and protein of Th1 cytokines were found in the periphery of adults and children with CoD [28,29]. The fact that serum IL-18 and anti-tTG antibody levels decreased during GFD and increased after GC in this study fits well with our previous findings of enhanced levels of IFN-γ-producing cells and presence of IgA anti-tTG in the peripheral blood from children with untreated CoD, that decreased during GFD, and increased again after GC [24,29]. Thus, the presence of IL-18 coincides with Th1-producing cells in peripheral blood, supporting the idea that IL-18 may contribute to the induction of a Th1-response to gluten in patients with CoD.
Previous studies have shown expression of IL-18 in the gastrointestinal tract, mainly by activated macrophages, dendritic and epithelial cells [18,19]. IFN-γ and IL-18 can stimulate macrophages to release TNF-α, FasL and IL-1β which result in a potent inflammatory response [16], indicating that IL-18 may serve as a potent regulatory factor for intestinal mucosal lymphocytes, thereby contributing to intestinal inflammation. There is a possibility that IL-18 in serum is a result of leakage from sites of local inflammation in the gut. The fact that high serum levels of IL-18 corresponded to villous atrophy in the majority of patients supports this hypothesis. However, two patients had high levels of IL-18 even though their mucosa was normal, and three patients with total villous atrophy had low IL-18 serum levels, indicating that serum IL-18 levels are not strictly correlated to the status of the intestinal mucosa. IL-18 is synthesized as an inactive precursor protein (proIL-18) that is processed by the IL-1β-converting enzyme, also called caspase-1 [30], or by proteinase-3, which has been proposed as an alternative enzyme necessary for IL-18 processing [31]. It has been shown that freshly isolated peripheral blood mononuclear cells (PBMCs) from healthy blood donors can express proIL-18 [32], suggesting a possibility that IL-18 found in peripheral blood could be derived from PBMC-produced proIL-18, that has been cleaved into mature IL-18 extracellularly during the course of an inflammatory reaction [31].
It has also been reported that IBD patients, particularly those with CD, have enhanced expression of IL-18 in intestinal mucosa and in serum [18–20]. In the inflamed colonic mucosa of CD patients, many IL-18 positive macrophages had infiltrated the lamina propria [20] and in a mouse model of IBD anti-IL-18 antibodies reduced the intestinal inflammation as well as the IFN-γ production [33]. However, in the present study the serum levels of IL-18 in IBD patients were not increased compared to healthy subjects, possibly because the majority of the IBD patients had UC.
Under certain conditions IL-18 is also capable of generating a Th2 response, as measured by Th2 cytokines and initiation of allergic manifestations. IL-18 has been shown to directly stimulate IL-4 production and histamine release from basophils, enhance IL-4 and IL-13 production from both NK and T cells in synergy with IL-2 and induce IgE expression by B cells [17]. In addition, several studies have demonstrated that increased IL-18 expression and serum levels are associated with allergic diseases, for example allergic asthma, atopic dermatitis and allergic rhinitis [34–37]. These findings might be a possible explanation for our results with high IL-18 levels in serum from two of the HC and two of the DC subjects, as the atopy status of the HC subjects is unknown and the DC children were diagnosed as having food hypersensitivity.
The findings in our study support the view that serum IL-18 concentrations in children with CoD follow the disease activity, suggesting a role for IL-18 in the induction of an inflammatory Th1-response after gluten exposure. Further studies are needed to investigate the cellular source of peripheral IL-18 and if IL-18 can be used as a marker of the inflammatory status of the small intestine.
Acknowledgments
The authors thank Sonja Gertz for invaluable help in recruiting the children for this study and Ingrid Dahlbom for reviewing the manuscript critically.
References
- 1.Sollid LM. Coeliac disease: dissecting a complex inflammatory disorder. Nat Rev Immunol. 2002;2:647–55. doi: 10.1038/nri885. [DOI] [PubMed] [Google Scholar]
- 2.Molberg Ø, McAdam SN, Körner R, et al. Tissue transglutaminase selectively modifies gliadin peptides that are recognized by gut-derived T cells in celiac disease. Nat Med. 1998;41:713–7. doi: 10.1038/nm0698-713. [DOI] [PubMed] [Google Scholar]
- 3.van de Wal Y, Kooy Y, van Veelen P, et al. Selective deamidation by tissue transglutaminase strongly enhances gliadin-specific T cell reactivity. J Immunol. 1998;161:1585–8. [PubMed] [Google Scholar]
- 4.Dieterich W, Laag E, Schopper H, et al. Autoantibodies to tissue transglutaminase as predictors of celiac disease. Gastroenterology. 1998;115:1317–21. doi: 10.1016/s0016-5085(98)70007-1. [DOI] [PubMed] [Google Scholar]
- 5.Bürgin-Wolff A, Dahlbom I, Hadziselimovic F, Petersson CJ. Antibodies against human tissue transglutaminase and endomysium in diagnosing and monitoring coeliac disease. Scand J Gastroenterol. 2002;37:685–91. doi: 10.1080/00365520212496. [DOI] [PubMed] [Google Scholar]
- 6.Mäki M, Mustalahti K, Kokkonen J, et al. Prevalence of celiac disease among children in Finland. N Engl J Med. 2003;348:2517–24. doi: 10.1056/NEJMoa021687. [DOI] [PubMed] [Google Scholar]
- 7.MacDonald TT, Bajaj-Elliott M, Pender SL. T cells orchestrate intestinal mucosal shape and integrity. Immunol Today. 1999;20:505–10. doi: 10.1016/s0167-5699(99)01536-4. [DOI] [PubMed] [Google Scholar]
- 8.Nilsen EM, Jahnsen FL, Lundin KE, et al. Gluten induces an intestinal cytokine response strongly dominated by interferon gamma in patients with celiac disease. Gastroenterology. 1998;115:551–63. doi: 10.1016/s0016-5085(98)70134-9. [DOI] [PubMed] [Google Scholar]
- 9.Nilsen EM, Lundin KE, Krajci P, Scott H, Sollid LM, Brandtzaeg P. Gluten specific, HLA-DQ restricted T cells from coeliac mucosa produce cytokines with Th1 or Th0 profile dominated by interferon gamma. Gut. 1995;37:766–76. doi: 10.1136/gut.37.6.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Westerholm-Ormio M, Garioch J, Ketola I, Savilahti E. Inflammatory cytokines in small intestinal mucosa of patients with potential coeliac disease. Clin Exp Immunol. 2002;128:94–101. doi: 10.1046/j.1365-2249.2002.01798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kontakou M, Przemioslo RT, Sturgess RP, Limb AG, Ciclitira PJ. Expression of tumour necrosis factor-alpha, interleukin-6, and interleukin-2 mRNA in the jejunum of patients with coeliac disease. Scand J Gastroenterol. 1995;30:456–63. doi: 10.3109/00365529509093307. [DOI] [PubMed] [Google Scholar]
- 12.Przemioslo RT, Kontakou M, Nobili V, Ciclitira PJ. Raised pro-inflammatory cytokines interleukin 6 and tumour necrosis factor alpha in coeliac disease mucosa detected by immunohistochemistry. Gut. 1994;35:1398–403. doi: 10.1136/gut.35.10.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kontakou M, Sturgess RP, Przemioslo RT, Limb GA, Nelufer JM, Ciclitira PJ. Detection of interferon gamma mRNA in the mucosa of patients with coeliac disease by in situ hybridisation. Gut. 1994;35:1037–41. doi: 10.1136/gut.35.8.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hansson T, Ulfgren AK, Lindroos E, Dannaeus A, Dahlbom I, Klareskog L. Transforming growth factor-beta (TGF-beta) and tissue transglutaminase expression in the small intestine in children with coeliac disease. Scand J Immunol. 2002;56:530–7. doi: 10.1046/j.1365-3083.2002.01157.x. [DOI] [PubMed] [Google Scholar]
- 15.Okamura H, Kashiwamura S, Tsutsui H, Yoshimoto T, Nakanishi K. Regulation of interferon-gamma production by IL-12 and IL-18. Curr Opin Immunol. 1998;10:259–64. doi: 10.1016/s0952-7915(98)80163-5. [DOI] [PubMed] [Google Scholar]
- 16.Dinarello CA. IL-18: a TH1-inducing, proinflammatory cytokine and new member of the IL-1 family. J Allergy Clin Immunol. 1999;103:11–24. doi: 10.1016/s0091-6749(99)70518-x. [DOI] [PubMed] [Google Scholar]
- 17.Nakanishi K, Yoshimoto T, Tsutsui H, Okamura H. Interleukin-18 regulates both Th1 and Th2 responses. Annu Rev Immunol. 2001;19:423–74. doi: 10.1146/annurev.immunol.19.1.423. [DOI] [PubMed] [Google Scholar]
- 18.Pizarro TT, Michie MH, Bentz M, et al. IL-18, a novel immunoregulatory cytokine, is up-regulated in Crohn's disease: expression and localization in intestinal mucosal cells. J Immunol. 1999;162:6829–35. [PubMed] [Google Scholar]
- 19.Monteleone G, Trapasso F, Parrello T, et al. Bioactive IL-18 expression is up-regulated in Crohn's disease. J Immunol. 1999;163:143–7. [PubMed] [Google Scholar]
- 20.Kanai T, Watanabe M, Okazawa A, et al. Interleukin 18 is a potent proliferative factor for intestinal mucosal lymphocytes in Crohn's disease. Gastroenterology. 2000;119:1514–23. doi: 10.1053/gast.2000.20260. [DOI] [PubMed] [Google Scholar]
- 21.Salvati VM, MacDonald TT, Bajaj-Elliott M, et al. Interleukin 18 and associated markers of T helper cell type 1 activity in coeliac disease. Gut. 2002;50:186–90. doi: 10.1136/gut.50.2.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Merendino RA, Di Pasquale G, Sturniolo GC, et al. Relationship between IL-18 and sICAM-1 serum levels in patients affected by coeliac disease: preliminary considerations. Immunol Lett. 2003;85:257–60. doi: 10.1016/s0165-2478(02)00243-2. [DOI] [PubMed] [Google Scholar]
- 23.Tursi A, Brandimarte G, Giorgetti GM. Prevalence of antitissue transglutaminase antibodies in different degrees of intestinal damage in celiac disease. J Clin Gastroenterol. 2003;36:219–21. doi: 10.1097/00004836-200303000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Hansson T, Dahlbom I, Rogberg S, et al. Recombinant human tissue transglutaminase for diagnosis and follow-up of childhood coeliac disease. Pediatr Res. 2002;51:700–5. doi: 10.1203/00006450-200206000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Collin P, Helin H, Mäki M, Hällström O, Karvonen AL. Follow-up of patients positive in reticulin and gliadin antibody tests with normal small-bowel biopsy findings. Scand J Gastroenterol. 1993;28:595–8. doi: 10.3109/00365529309096094. [DOI] [PubMed] [Google Scholar]
- 26.Meeuwisse GW. Diagnostic criteria in coeliac disease. Acta Paediatr Scand. 1970;59:461–3. [Google Scholar]
- 27.Hansson T, Dannaeus A, Kraaz W, Sjöberg O, Klareskog L. Production of antibodies to gliadin by peripheral blood lymphocytes in children with celiac disease: the use of an enzyme-linked immunospot technique for screening and follow-up. Pediatr Res. 1997;41:554–9. doi: 10.1203/00006450-199704000-00016. [DOI] [PubMed] [Google Scholar]
- 28.Nilsen EM, Gjertsen HA, Jensen K, Brandtzaeg P, Lundin KE. Gluten activation of peripheral blood T cells induces a Th0-like cytokine pattern in both coeliac patients and controls. Clin Exp Immunol. 1996;103:295–303. doi: 10.1046/j.1365-2249.1996.d01-611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hansson T, Dannaeus A, Klareskog L. Cytokine-producing cells in peripheral blood of children with coeliac disease secrete cytokines with a type 1 profile. Clin Exp Immunol. 1999;116:246–50. doi: 10.1046/j.1365-2249.1999.00882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gu Y, Kuida K, Tsutsui H, et al. Activation of interferon-gamma inducing factor mediated by interleukin-1beta converting enzyme. Science. 1997;275:206–9. doi: 10.1126/science.275.5297.206. [DOI] [PubMed] [Google Scholar]
- 31.Fantuzzi G, Dinarello CA. Interleukin-18 and interleukin-1 beta: two cytokine substrates for ICE (caspase-1) J Clin Immunol. 1999;19:1–11. doi: 10.1023/a:1020506300324. [DOI] [PubMed] [Google Scholar]
- 32.Puren AJ, Fantuzzi G, Dinarello CA. Gene expression, synthesis, and secretion of interleukin 18 and interleukin 1beta are differentially regulated in human blood mononuclear cells and mouse spleen cells. Proc Natl Acad Sci USA. 1999;96:2256–61. doi: 10.1073/pnas.96.5.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Siegmund B, Fantuzzi G, Rieder F, et al. Neutralization of interleukin-18 reduces severity in murine colitis and intestinal IFN-gamma and TNF-alpha production. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1264–73. doi: 10.1152/ajpregu.2001.281.4.R1264. [DOI] [PubMed] [Google Scholar]
- 34.Tanaka H, Miyazaki N, Oashi K, et al. IL-18 might reflect disease activity in mild and moderate asthma exacerbation. J Allergy Clin Immunol. 2001;107:331–6. doi: 10.1067/mai.2001.112275. [DOI] [PubMed] [Google Scholar]
- 35.Wong CK, Ho CY, Ko FW, C, et al. Proinflammatory cytokines (IL-17, IL-6, IL-18 and IL-12) and Th cytokines (IFN-gamma, IL-4, IL-10 and IL-13) in patients with allergic asthma. Clin Exp Immunol. 2001;125:177–83. doi: 10.1046/j.1365-2249.2001.01602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoshizawa Y, Nomaguchi H, Izaki S, Kitamura K. Serum cytokine levels in atopic dermatitis. Clin Exp Dermatol. 2002;27:225–9. doi: 10.1046/j.1365-2230.2002.00987.x. [DOI] [PubMed] [Google Scholar]
- 37.Verhaeghe B, Gevaert P, Holtappels G, et al. Up-regulation of IL-18 in allergic rhinitis. Allergy. 2002;57:825–30. doi: 10.1034/j.1398-9995.2002.23539.x. [DOI] [PubMed] [Google Scholar]