Abstract
Involvement of excessive Th1 cell functions and heat shock protein expression in the pathogenesis of Behçet's disease (BD) has been reported. In this study we have characterized immune responses in intestinal lesions of BD. Peripheral blood lymphocytes (PBL) of BD and healthy controls (HC) and tissue specimens of intestinal Behçet's disease (intestinal BD), Crohn's disease (CD) and ulcerative colitis (UC) were analysed for mRNA and protein expression by reverse transcriptase-polymerase chain reaction (PCR) and immunohistochemistry, respectively. PBL of BD patients expressed the Th1-related chemokine receptor, CCR5 and CXCR3 preferentially compared with those of healthy controls. Intestinal lesions of BD expressed interferon (IFN)-γ, tumour necrosis factor (TNF)-α and interleukin (IL)-12 mRNA, indicating Th1 skewed responses in vivo. mRNA of Txk, a Tec family tyrosine kinase specific to Th1 cells, was expressed in the lesions, suggesting its contribution to the Th1-dominant responses. In the intestinal samples, CCR5 was detected in all the cases with BD, whereas Th2-related CCR3 and CCR4 were detected randomly, mainly in the cases with inactive BD and those receiving large amounts of prednisolone, indicating the Th1-dominant immune responses in the intestinal lesions. As the ligands of CCR5, MIP1α and MIP1β were detected, whereas RANTES was not. Heat shock protein (HSP) 60 was expressed in PBL and intestinal tissues of BD. Th1-dominant immune responses and HSP60 expression may induce the inflammatory responses and thus be associated with the pathogenesis of intestinal BD.
Keywords: Behçet's disease, CCR5, HSP, MIP1, Txk
Introduction
Behçet's disease (BD) is a multi-systemic inflammatory disease characterized by recurrent attacks of uveitis, oral aphtha, genital ulcers and erythema nodosum [1]. The aetiology and pathogenesis of this disease have been explored extensively. Both genetic factors and environmental factors are thought to play a role in the pathogenesis of this disease [1,2]. A relatively higher prevalence rate has been noted in middle and eastern Asian countries, especially the areas along the ‘Silk Road’. BD is not a common disease in western countries. Recent studies have disclosed the involvement of excessive Th1 cell functions and heat shock protein (HSP) expression in the pathogenesis of BD [3–6]. We and others have shown previously that ectopic expression of self-HSP led to the excessive activation of Th1 cells that were reactive with the HSP. The self-HSP-reactive lymphocytes expressed Txk, a Tec family tyrosine kinase specific to Th1 cells, and contributed to the development of disease manifestations (Nagafuchi et al., submitted).
Intestinal BD is a subtype of BD, accompanying intestinal ulcers associated with abdominal pain and lower gastrointestinal bleeding. Intestinal BD recurs frequently and there is no definitive therapy. The precise prevalence rate of intestinal BD is obscure; none the less, the prevalence of intestinal BD seems relatively high in Far Eastern countries, especially in Japan. Much remains unanswered about the pathogenesis of intestinal BD. Activated CD8+ T cell participation in its pathogenesis was reported by using peripheral blood lymphocytes (PBL) of intestinal BD [7]. Recently, the successful application of anti-tumour necrosis factor (TNF)-α antibody for intestinal BD has been reported [8–10]. The aims of this study were to elucidate whether T cell immune responses were skewed toward Th1 dominance in the intestinal lesions and if so, and to determine ectopic HSP expression and which chemokines were involved in the Th1 cell accumulation in the intestinal lesions of BD. We found that HSP expression and accumulation of Txk expressing Th1 cells in the lesions of intestinal BD, and MIP1α and MIP1β, seemed responsible for the Txk+ CCR5+ Th1 cell accumulation in the intestinal lesions.
Materials and methods
Patients
Peripheral blood was collected from 10 patients with BD. The mean age (± s.d.) of these patients was 34·5 + 8·3 years (range 24–55 years). BD patients fulfilled the diagnostic criteria proposed by both the BD Research Committee of Japan and the International Study Group of BD. The 10 BD patients donating PBL were treated with less than 5 mg prednisolone per day, and/or less than 3 mg colchicine per day. They received no immunosuppressant or anti-TNF-α agent. Ten healthy volunteer blood donors served as control subjects. Their mean age (± s.d.) was 36·2 + 6·3 years (range 25–52 years). Approval from the Human Studies Committee and individual informed consent from each patient were obtained before we conducted the present study. Four BD patients who underwent surgical resection and diagnostic biopsy of intestines provided the intestinal tissue. Patients with Crohn's disease (CD) and ulcerative colitis (UC) served as controls. The clinical data of the patients whose intestinal specimens were used in reverse transcription-polymerase chain reaction (RT-PCR) and immunohistochemical study are summarized in Table 1.
Table 1.
Characteristics of patients with inflammatory bowel diseases in this study.
| Patients | Gender | Age (years) | Disease duration (years) | Location of the lesion | Procedure | Treatment |
|---|---|---|---|---|---|---|
| BD5 | F | 37 | 2 | IL | Resection | PSL 50 mg/day, AZP 100 mg/day |
| BD6 | M | 31 | 5 | CO | Biopsy | None |
| BD7 | M | 55 | 22 | CO | Biopsy | PSL10 mg/day, SASP 3 g/day |
| BD8 | F | 40 | 1 | CO | Biopsy | PSL 30 mg/day |
| CD1 | F | 32 | 5 | IL/CO | Biopsy | Naproxen 300 mg/day |
| CD2 | M | 26 | 7 | CO | Biopsy | AZP50 mg/day, mesalazine 3 g/day |
| CD3 | M | 32 | 1 | IL/CO | Biopsy | Mesalazine 3 g/day |
| CD4 | M | 29 | 1 | IL/CO | Biopsy | Mesalazine 1·5 g/day |
| UC1 | F | 35 | 3 | CO | Resection | Mesalazine 3 g/day |
IL, ileum; CO, colon; PSL, prednisolone; AZP, azathioprine; SASP, salazosulphapyridine.
Separation of PBL
PBL were obtained by Ficoll-Hypaque centrifugation of heparinized blood from normal healthy donors and patients with BD. Freshly isolated PBL were suspended in RPMI-1640 medium containing 10% fetal calf serum (FCS), penicillin and streptomycin (Sigma-Aldrich, St. Louis, MO). T lymphocytes were separated by rosette formation with neuraminidase-treated sheep red blood cells (SRBC), followed by Ficoll-Hypaque centrifugation.
RT-PCR
Total RNA extraction and cDNA synthesis from PBL and intestinal specimens have been reported [11]. Cycling parameters were: denaturing at 94°C for 30 s, annealing at 60°C for 30 s and elongation at 72°C for 60 s. The reaction was repeated 30 times, preceded by hot starting at 94°C for 2 min and followed by elongation at 72°C for 10 min. Nested RT-PCR was used to amplify interferon (IFN)-γ, TNF-α, interleukin (IL)-12 p40, IL-12 receptor (IL-12R)β2, Txk and CXCR3 mRNA. The first PCR was conducted using a primer set of sense 1 and antisense 1, and the second PCR used sense 2 and antisense 2. Sequences of primers were as follows:
HSP60 [309 base pairs (bp)], sense TGTTTTGGGAGGGGG TTGTGC, antisense AACAGAGAGGCCACACCAGCA;
Txk (189 bp), sense 1 and sense 2 GTACGGAGGCTGCCAT AAAA, antisense 1 TGGCCTCTTCAATGAAATCC, antisense 2 CAGCTGTGGCTGGTAAACAA;
IFN-γ (129 bp), sense 1 TGACCAGAGCATCCAAAAGA, sense 2 CGAGATGACTTCGAAAAGCTGAC, antisense 1 and 2 CCTTTTTCGCTTCCCTGTTTTA;
IL-12 p40 (203 bp), sense 1 AACTTGCAGCTGAAGCCATT, sense 2 CATGGGCCTTCATGCTATTT, antisense 1 and 2 TGATGTACTTGCAGCCTTGC;
IL-12Rβ2 (204 bp), sense 1 GAGAGGCGATGTGACTGTGA, sense 2 GAATCAACCTCACCCCTGAA, antisense 1 and 2 CCAGTCCCTCATCTCTCCAA;
TNF-α (190 bp), sense 1 ATGAGCACTGAAAGCATGATC, sense 2 AGGGACCTCTCTCTAATCAG, antisense 1 GGCGATGCGGCTGATGGT, antisense 2 ATGGCAC CACCAGCTGGTTAT;
CXCR1 (149 bp), sense CCTCAACCCCATCATCTAC, antisense TGGAAGAGACATTGACAGAC;
CXCR2 (131 bp), sense CATGGAGAGTGACAGCTTTGAA, antisense CTTGTTGATTTCCAGGGATTCTG;
CXCR3 (401 bp, 181 bp), sense 1 CCACTGCCAATACAA CTTCC, sense 2 GCCTACTGCTATGCCCACAT, antisense 1 GCAAGAGCAGCATCCACATC, antisense 2 ACGTCTACCCTGCTTTCTCG;
CX3CR1 (284 bp), sense CAGACGCTGTTTTCCTGCAA, antisense ACACAGGACAGCCAGGCATT; CCR3 (216 bp), sense CCATCTTCTGTCTCGTTCTCC, antisense TCCGCTCACAGTCATTTCC;
CCR4 (269 bp), sense AAGAAGAACAAGGCGGTGAA GATG, antisense AGGCCCCTGCAGGTTTTGAAG;
CCR5 (157 bp), sense GCTCTCATTTTCCATACAGTC, antisense TGCCTCTTCTTCTCATTTC;
MIP1α (195 bp), sense CGCCTGCTGCTTCAGCTACACCT CCCGGCA-3′, antisense 5′-TGGACCCCTCAGGCACT CAGCTCCAGGTCG-3′;
MIP1β (186 bp), sense ACCCTCCCACCGCCTGCTGCTTT TCTTACA, antisense GTTCCAGGTCATACACGTACTC CTGGACCC;
RANTES (130 bp), sense GGCAGCCCTC GCTGTCATCC TCA, antisense CTTGATGTGGGCACG GGGCAGTG;
β-actin (314 bp), sense TCCTGTGGCATCCACGAAACT, antisense GAAGCATTTGCGGTGGACGAT.
Immunohistochemical staining
Cryostat sections (5 µm thick) of intestinal lesions were fixed in 4% paraformaldehyde for 15 min. All subsequent procedures were performed using Dako LSAB®2 Kit (Dako, Carpinteria, CA, USA). The samples were incubated with primary antibodies and followed by peroxidase conjugated secondary antibodies. The primary antibodies included anti-CD3 (Dako), anti-CD4 (Dako), anti-CD8 (Dako), anti-CD20 (Dako), anti-CD68 (Dako), anti-HSP60 (Affinity BioReagents, Golden, CO, USA), anti-Txk (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and anti-CCR5 (Capralogics, Hardwick, MA, USA). Anti-MIP1α antibody and anti-MIP1β antibody were kind gifts from Dr Tanaka (University of Occupational and Environmental Health, Japan) [12]. Appropriate control antibodies were included in all the staining. After colour development, tissue specimens were counterstained with haematoxylin.
Results
Infiltration of mononuclear cells to the affected site of intestinal BD
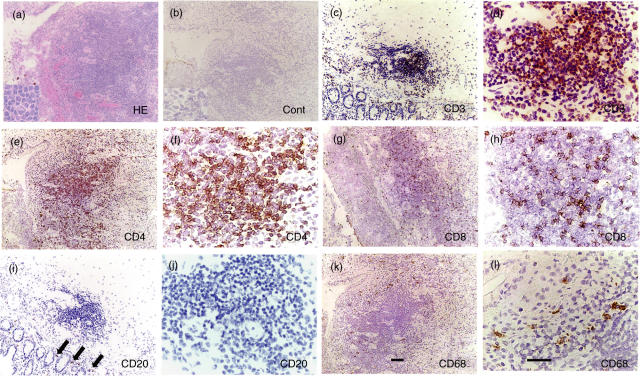
It has been reported that nodular cutaneous lesions of BD were infiltrated by mononuclear cells and neutrophils [13,14]. We performed a pathological examination of the intestinal lesions in four patients with BD. All specimens showed variable dense focal mononuclear cell infiltration in haematoxylin and eosin (H&E)-stained frozen sections. Mononuclear cell infiltration was observed in mucosal and submucosal tissue in intestinal lesions in BD (Fig. 1). We first characterized the cells infiltrating the intestinal lesions by immunohistochemistry. Almost all of them were mononuclear cells (Fig. 1a). The mononuclear cells accumulating to the ileocaecal ulcer of BD patients were mainly T cells (Fig. 1b–d), consisting of mainly CD4+ cells (Fig. 1e,f) and a small number of CD8+ cells (Fig. 1g,h). In contrast, a few B cells were found around the accumulating mononuclear cells (Fig. 1i,j). A small number of CD68+ macrophages resided mainly on the outer side of the aggregate (Fig. 1k,l). These findings indicate that the infiltrating cells consisted mainly of CD4+ T cells.
Fig. 1.
Infiltration of mononuclear cells to intestinal lesions of BD. (a) An intestinal ulcer of BD was infiltrated by inflammatory cells; a vast majority were mononuclear cells. A very small number of neutrophils also infiltrated to the site. Inset, higher magnification. (b) Result of staining with control antibody, mouse IgG, is shown. (c) Almost all the mononuclear cells were found to be CD3+ T cells. (d) Higher magnification of (c). (e) A majority of the infiltrated cells to the intestinal ulcer were CD4+ cells. (f) Higher magnification of (e). (g) CD8+ cells constituted a minor population infiltrating to the lesions. (h) Higher magnification of (g). (i) A few CD20+ B cells were found in the lesions (arrows). (j) Higher magnification of (i). (k) CD68+ macrophages resided mainly on the outer side of the aggregate. (l) Higher magnification of (k). Scale bars represent 100 µm for (a) CEGIK and 50 µm for (b) DFHJL. Results of a representative case of BD5 are shown.
Th1 lymphocytes accumulating in the intestinal lesions in patients with BD
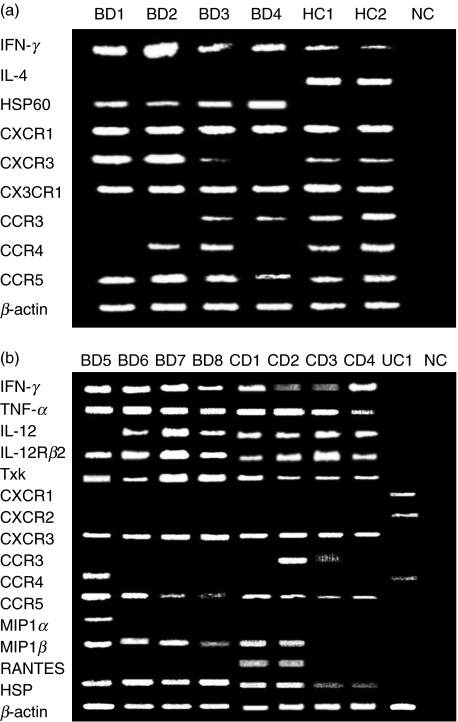
The importance of HSP-reactive Th1 cells in the pathogenesis of BD has been reported [15,16]. It is interesting to test whether the Th1 response is also important in the development of intestinal lesions in patients with BD. We first analysed mRNA expression of IFN-γ and IL-4 in PBL from BD and healthy controls (HC) by RT-PCR (Fig. 2a). PBL of BD patients expressed more IFN-γ mRNA than those of HC. In accordance with the findings of the PCR study, the IFN-γ protein production measured by enzyme-linked immunosorbent assay (ELISA) was increased in BD patients (Nagafuchi et al., submitted). In contrast, in this PCR condition, expression of IL-4 mRNA was not detected in BD compared with HC; HSP60 was found in BD but not in HC.
Fig. 2.
mRNA expression of HSP60 and Th1-type cytokines, chemokines and chemokine receptors in the affected sites of intestinal BD. (a) PBL from four BD patients and two HC were analysed for mRNA expression of HSP60 and chemokine receptors. All the BD patients expressed IFN-γ mRNA but did not express IL-4 mRNA. HSP60 was detected in BD PBL but not in HC PBL. The Th1-related chemokine receptor, CCR5, was expressed in all the BD PBL. Another Th1-related chemokine receptor, CXCR3, was detected only in PBL from the patients in the active phase (BD1, BD2). These two patients lacked expression of CCR3, a Th2-related chemokine receptor, whereas it was expressed in BD3 and BD4 in the inactive BD. Another Th2-related chemokine receptor, CCR4, was not expressed in patients BD1 and BD4, whereas it was expressed in BD2 and BD3. (b) Intestinal lesions of four BD patients, four CD patients and one UC patient were analysed. BD patients and CD patients expressed all the Th1-associated mRNAs tested here. In contrast, the UC patient showed CCR4 expression and did not express Th1-associated mRNAs. NC, negative control studies using H2O as templates. Patient BD5 lacked IL-12 mRNA expression probably because she had received high-dose corticosteroid administration at the time of resection.
We next analysed the expression of chemokine receptors, including CXCR3 and CCR5, Th1-specific chemokine receptors, by using BD PBL. CCR5 was expressed in all the samples of PBL from patients with BD, whereas CXCR3 was detected in three of the four tested patients with BD (Fig. 2a). In contrast, CCR3, a Th2-specific chemokine receptor, was detected only in patients in the inactive phase (BD3/4). CCR4, another Th2-specific chemokine receptor, was detected in BD2 and BD3. All the chemokine receptors examined were detected from the PBL of HC.
We next investigated mRNA of cytokines and chemokine receptors expressed in intestinal lesions of BD. mRNAs of IFN-γ, TNF-α and IL-12Rβ2 were expressed in all the BD samples (Fig. 2b). IL-12 was detected in all the BD samples except BD1 (Fig. 2b). Expression of Txk, a Th1 cell-specific Tec family tyrosine kinase that contributes to the up-regulation of IFN-γ gene transcription, was analysed [11,17]. Txk mRNA was expressed in the ileocaecal lesions (Fig. 2b). Expression of Txk protein in mononuclear cells accumulating to the ileocaecal ulcer was confirmed by immunohistochemistry (Fig. 3a,b). Similarly, we detected CCR5 mRNA expression in all intestinal tissue samples from patients with BD (Fig. 2a). Thus, in patients with BD, lymphocytes infiltrating to the intestine were CCR5+ Th1 cells.
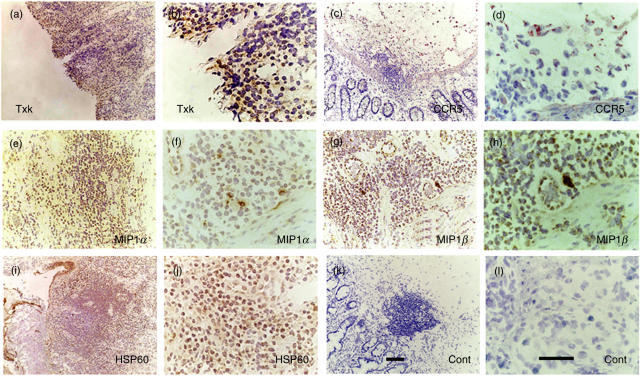
Fig. 3.
Expression of Txk, CCR5, MIP1α, MIP1β and HSP60 in the intestinal ulcer of BD. (a) Txk was expressed in the lymphocytes accumulating to the lesions. (b) Higher magnification of (a). (c) CCR5 was expressed in the mononuclear cell aggregate. (d) Higher magnification of (c). (e) MIP1α was expressed in mononuclear cells. (f) Higher magnification of (e). (g) MIP1β was expressed in mononuclear cells. (h) Higher magnification of (g). (i) HSP60 expressing mononuclear cells located within and outside of the cell aggregates. (j) Higher magnification of (i). (k) Result of a control antibody, mouse IgG, is shown. (l) Higher magnification of (k). Scale bars represented 100 µm for (a) CEGIK and 50 µm for (b) DFHJL. Results of a representative case of BD5 are shown.
MIP1α, MIP1β and RANTES are able to bind to CCR5. We then determined the expression of the chemokines by RT-PCR in the intestinal tissue samples of BD to elucidate the chemokine responsible for focal accumulation of CCR5+ Th1 cells. MIP1β was expressed in all the samples, whereas MIP1α was detected from only one patient (Fig. 2a). RANTES was not expressed at all. Immunohistochemistry disclosed that CCR5-expressing cells accumulated in the lesions, almost corresponding to the site of CD4+ cells (Fig. 3c,d). In contrast, MIP1α- and MIP1β-expressing cells were located on the outer side of the mononuclear cell aggregate (Fig. 3e–h).
To investigate whether these immune responses were specific or not for BD, intestinal lesions of patients with CD and UC were analysed similarly by RT-PCR. The CD lesions showed almost similar results, Th1-dominant immune responses and production of proinflammatory cytokines (Fig. 2b). However, the UC lesions did not express Th1-specific mRNAs but did express CCR4, suggesting that the immune responses of the affected UC sites may differ from those of intestinal BD and CD. Collectively, Th1-dominant immune responses occurred in the affected intestinal BD sites.
Expression of HSP60 in PBL and in the intestinal lesions in patients with BD
Several reports have mentioned the expression of self-HSP in BD [18]. We have detected the expression of self-HSP60 in the skin lesions of the erythema nodosa of BD patients (Nagafuchi et al., submitted). We then studied the self-HSP60 mRNA expression of PBL by RT-PCR. HSP60 mRNA was not expressed in the freshly isolated PBL from normal individuals (Fig. 2b). However, PBL from patients with BD spontaneously expressed HSP60 mRNA. Thus, aberrant HSP60 expression was evident in PBL from patients with BD. We found that HSP60 mRNA was expressed in the lesions of intestinal BD (Fig. 2b). It was also expressed in the affected sites of CD, but not in those of UC. Human HSP60 protein expression was confirmed in the intestinal lesions of BD (Fig. 3k,l). The HSP60 may become a trigger and a target of the Th1-dominant immune responses leading to the inflammatory responses of the intestine.
Discussion
HSPs are induced by variable stress factors and are possible (auto)antigens of autoimmune responses in disease. It has been reported that HSP60 was expressed in tissues from healthy individuals and patients with inflammatory bowel diseases, collagen diseases and other diseases [18–20]. In inflammatory bowel diseases, including CD and UC, enhanced expression of HSPs has been reported [19,20]. HSP60 was increased in the inflamed colorectal mucosa of UC patients and a small group of active CD patients [19]. In this study, we found HSP60 expression in PBL from BD and intestinal lesions of BD and CD; however, it was not found in the PBL of HC patients and intestinal lesions of UC patients (Fig. 2a,b): we do not know the reason for this. One possible explanation is that we used a relatively low number of PCR cycles that may have contributed to detect quantitative differences among different samples. Thus, our experimental results suggest that HSP60 may be expressed more in the periphery of BD and intestinal lesions of BD and CD than in the periphery of HC and intestinal lesions of UC. In any event, we need to compare more precisely the expression levels of HSP60 in PBL in the near future by using quantitative methods, including real-time PCR.
We have found previously that specific epitopes of human HSP60, especially peptide 336–351-stimulated PBL of patients with BD, resulted in excess amounts of Th1 cytokine production [16,21]. Thus, self-HSP-reactive T cells were involved in the pathogenesis of BD. We and others have observed enhanced expression of HSP60 in mucocutaneous lesions of BD [18]. Specific epitopes of self-HSP60 may also play an important role in the immunological aberration and ileocaecal pathogenesis of intestinal BD. The specific epitopes of HSP60 show homology with bacterial HSP65, indicating a molecular mimicry mechanism between bacterial HSP and self-HSP as a possible pathogenic mechanism of BD [16,21]. Recently, human HSP60 has been reported to induce a potent inflammatory response in the innate immune system via its ligands, toll-like receptors (TLRs), and operates in a similar manner to that of classical pathogen-derived ligands [22,23]. Human HSP60 can thus activate non-specifically the innate immune system and stimulates the maturation of dendritic cells, resulting in the release of proinflammatory cytokines and IL-12, which primes Th1-type immune responses [24]. Moreover, HSP60 induces T cell activation directly via TLR2 [25]. Taken together, these findings strongly support that the overexpression of HSP60 in ileocaecal lesions correlates closely with the antigen-specific and non-specific pathogenesis of intestinal BD.
We observed intensive inflammatory infiltration of CD4+ T cells and to a lesser degree of CD8+ T cells and macrophages into the ileocaecal mucosa of intestinal BD. Atari et al. reported that intestinal lesions of BD were found at sites coinciding with intramucosal lymphoid tissue [26]; our finding coincided with theirs (Fig. 1). The migration of inflammatory cells is mediated by chemokines specific to each population of inflammatory cells. We detected Txk, a Th1-specific transcription factor, and CCR5, a Th1-dominant chemokine receptor, in the intestinal lesions, suggesting that the majority of infiltrating cells of intestinal BD were Th1-type cells. We also found enhanced expression of MIP1α and MIP1β, specific ligands for CCR5, in the inflammatory sites. There is considerable evidence that activated macrophages produce higher levels of both MIP1α and MIP1β. The enhanced expression of HSP60 may play a bipolar role in innate immunity and antigen-specific cellular immunity, and can be shared by them and connect to each other. Although the true mechanisms by which enhanced MIP1 expression is induced in ileocaecal legions of intestinal BD remain to be elucidated, our findings provide evidence that enhanced expression of HSP60 may be one of the causes of the overexpression of MIP1α and MIP1β.
Three of the four intestinal BD patients analysed in this study were treated with prednisolone. It is thus possible that the expression of cytokines and chemokine receptors was modulated by the treatment (Table 1). In fact, patient BD5, who was treated with 50 mg prednisolone per day, did not produce IL-12 but expressed CCR4 and MIP1α, showing the different mRNA expression pattern from the other BD patients (Fig. 2b). However, the mRNA expression pattern of patients BD7 and BD8 seemed unaltered by the prednisolone, showing a similar mRNA expression pattern to that of patient BD6 with no treatment (Table 1, Fig. 2b).
We detected CCR5 as well as MIP1α and MIP1β in the ileocaecal lesions of intestinal BD, suggesting infiltration of CCR5+ Th1 cells, which is also confirmed by the expression of Th1-specific Txk and IFN-γ, chemoattracted by MIP1α and MIP1β. The involvement of chemokines and chemokine receptors, especially that of CCR5, in the pathogenesis of BD have been suspected [27]. In diseases of the digestive system, CCR5 and its ligands play critical roles in local and systemic complications in acute pancreatitis and fulminant hepatic failure [28,29]. Th1-type cytokine production, including IL-12 and IFN-γ in addition to TNF-α production, have been reported to play a major role in CD [30–32]. Anti-TNF-α antibody is used to treat patients with CD and also patients with intestinal BD, suggesting partial common pathogenic pathways between the two diseases [8–10,33,34]. The triggering antigens may be different between BD and CD. It is possible that HSP60 is a pathogenic antigen in CD because of the excess expression in the disease-affected sites. We have not examined T cell responses to HSP60 in CD directly; however, our present results suggest that a predominant Th1 cell response may have occurred in patients with CD. As mentioned above, T cells from BD patients proliferated against the 336–351 peptide of HSP60, but those from RA patients did not react to the same peptide [16]. T cells from CD may respond to different HSP60 epitopes from those of BD, and if so this difference would contribute to the distinct pathological responses to HSP60 between BD and CD.
In this study, we have shown the Th1-dominant infiltration of CD4+ T cells in ileocaecal mucosa. HSP60, MIP1α, MIP1β and CCR5 may act as key molecules in the pathogenesis and progression of ileocaecal inflammation of BD. Modalities inhibiting self-HSP60 expression may be applicable for intestinal BD. Indeed, certain immunosuppressants were reported to suppress HSP60 expression effectively [35]. An inhibitor of interactions of CCR5 with MIP1α and MIP1β may lead to reduced infiltration of CCR5+ inflammatory cells into intestinal lesions and progressive inflammation [36]. We hope that our findings for the pathogenesis of intestinal BD may contribute to development of a new therapeutic strategy for this disease in the near future.
References
- 1.Sakane T, Takeno M, Suzuki N, Inaba G. Behçet's disease. N Engl J Med. 1999;341:1284–91. doi: 10.1056/NEJM199910213411707. [DOI] [PubMed] [Google Scholar]
- 2.Lehner T. Immunopathogenesis of Behçet's disease. Ann Med Interne. 1999;150:483–7. [PubMed] [Google Scholar]
- 3.Esin S, Gul A, Hodara V, et al. Peripheral blood T cell expansions in patients with Behçet's disease. Clin Exp Immunol. 1997;107:520–7. doi: 10.1046/j.1365-2249.1997.d01-947.x. [DOI] [PubMed] [Google Scholar]
- 4.Balabanova M, Calamia KT, Perniciaro C, O'Duffy JD. A study of the cutaneous manifestations of Behçet's disease in patients from the United States. J Am Acad Dermatol. 1999;41:540. [PubMed] [Google Scholar]
- 5.Mantas C, Direskeneli H, Oz D, Yavuz S, Akoglu T. IL-8 producing cells in patients with Behçet's disease. Clin Exp Rheumatol. 2000;18:249–51. [PubMed] [Google Scholar]
- 6.Frassanito MA, Dammacco R, Cafforio P, Dammacco F. Th1 polarization of the immune response in Behçet's disease: a putative pathogenetic role of interleukin-12. Arthritis Rheum. 1999;42:1967–74. doi: 10.1002/1529-0131(199909)42:9<1967::AID-ANR24>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 7.Ormala T, Rintala R, Savilahti E. T cells of the colonic mucosa in patients with infantile colitis. J Pediatr Gastroenterol Nutr. 2001;33:133–8. doi: 10.1097/00005176-200108000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Travis SP, Czajkowski M, McGovern DP, Watson RG, Bell AL. Treatment of intestinal Behçet's syndrome with chimeric tumour necrosis factor alpha antibody. Gut. 2001;49:725–8. doi: 10.1136/gut.49.5.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hassard PV, Binder SW, Nelson V, Vasiliauskas EA. Anti-tumor necrosis factor monoclonal antibody therapy for gastrointestinal Behçet's disease: a case report. Gastroenterology. 2001;120:995–9. doi: 10.1053/gast.2001.22556. [DOI] [PubMed] [Google Scholar]
- 10.Kram MT, May LD, Goodman S, Molinas S. Behçet's ileocolitis: successful treatment with tumor necrosis factor-alpha antibody (infliximab) therapy: report of a case. Dis Colon Rectum. 2003;46:118–21. doi: 10.1007/s10350-004-6506-4. [DOI] [PubMed] [Google Scholar]
- 11.Kashiwakura J, Suzuki N, Nagafuchi H, et al. Txk, a nonreceptor tyrosine kinase of the Tec family, is expressed in T helper type 1 cells and regulates interferon gamma production in human T lymphocytes. J Exp Med. 1999;190:1147–54. doi: 10.1084/jem.190.8.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanaka Y, Minami Y, Mine S, et al. H-Ras signals to cytoskeletal machinery in induction of integrin-mediated adhesion of T cells. J Immunol. 1999;163:6209–16. [PubMed] [Google Scholar]
- 13.Demirkesen C, Tuzuner N, Mat C, et al. Clinicopathologic evaluation of nodular cutaneous lesions of Behçet's syndrome. Am J Clin Pathol. 2001;116:341–6. doi: 10.1309/GCTH-0060-55K8-XCTT. [DOI] [PubMed] [Google Scholar]
- 14.Kim B, LeBoit PE. Histopathologic features of erythema nodosum-like lesions in Behçet disease: a comparison with erythema nodosum focusing on the role of vasculitis. Am J Dermatopathol. 2000;22:379–90. doi: 10.1097/00000372-200010000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Direskeneli H, Saruhan-Direskeneli G. The role of heat shock proteins in Behçet's disease. Clin Exp Rheumatol. 2003;21:S44–8. [PubMed] [Google Scholar]
- 16.Kaneko S, Suzuki N, Yamashita N, et al. Characterization of T cells specific for an epitope of human 60-kD heat shock protein (hsp) in patients with Behçet's disease (BD) in Japan. Clin Exp Immunol. 1997;108:204–12. doi: 10.1046/j.1365-2249.1997.3611265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takeba Y, Nagafuchi H, Takeno M, Kashiwakura J, Suzuki N. Txk, a member of nonreceptor tyrosine kinase of Tec family, acts as a Th1 cell-specific transcription factor and regulates IFN-gamma gene transcription. J Immunol. 2002;168:2365–70. doi: 10.4049/jimmunol.168.5.2365. [DOI] [PubMed] [Google Scholar]
- 18.Ergun T, Ince U, Eksioglu-Demiralp E, et al. HSP 60 expression in mucocutaneous lesions of Behçet's disease. J Am Acad Dermatol. 2001;45:904–9. doi: 10.1067/mjd.2001.117728. [DOI] [PubMed] [Google Scholar]
- 19.Winrow VR, Mojdehi GM, Ryder SD, Rhodes JM, Blake DR, Rampton DS. Stress proteins in colorectal mucosa. Enhanced expression in ulcerative colitis. Dig Dis Sci. 1993;38:1994–2000. doi: 10.1007/BF01297075. [DOI] [PubMed] [Google Scholar]
- 20.Ludwig D, Stahl M, Ibrahim ET, et al. Enhanced intestinal expression of heat shock protein 70 in patients with inflammatory bowel diseases. Dig Dis Sci. 1999;44:1440–7. doi: 10.1023/a:1026616221950. [DOI] [PubMed] [Google Scholar]
- 21.Direskeneli H, Eksioglu-Demiralp E, Yavuz S, et al. T cell responses to 60/65 kDa heat shock protein derived peptides in Turkish patients with Behçet's disease. J Rheumatol. 2000;27:708–13. [PubMed] [Google Scholar]
- 22.Ohashi K, Burkart V, Flohe S, Kolb H. Heat shock protein 60 is a putative endogenous ligand of the toll-like receptor-4 complex. J Immunol. 2000;164:558–61. doi: 10.4049/jimmunol.164.2.558. [DOI] [PubMed] [Google Scholar]
- 23.Vabulas RM, Ahmad-Nejad P, da Costa C, et al. Endocytosed HSP60s use toll-like receptor 2 (TLR2) and TLR4 to activate the toll/interleukin-1 receptor signaling pathway in innate immune cells. J Biol Chem. 2001;17:31332–9. doi: 10.1074/jbc.M103217200. [DOI] [PubMed] [Google Scholar]
- 24.Flohe SB, Bruggemann J, Lendemans S, et al. Human heat shock protein 60 induces maturation of dendritic cells versus a Th1-promoting phenotype. J Immunol. 2003;170:2340–8. doi: 10.4049/jimmunol.170.5.2340. [DOI] [PubMed] [Google Scholar]
- 25.Zanin-Zhorov A, Nussbaum G, Franitza S, Cohen IR, Lider O. T cells respond to heat shock protein 60 via TLR2: activation of adhesion and inhibition of chemokine receptors. FASEB J. 2003;17:1567–9. doi: 10.1096/fj.02-1139fje. [DOI] [PubMed] [Google Scholar]
- 26.Takada Y, Fujita Y, Igarashi M, et al. Intestinal Behçet's disease. Pathognomonic changes in intramucosal lymphoid tissues and effect of a ‘rest cure’ on intestinal lesions. J Gastroenterol. 1997;32:598–604. doi: 10.1007/BF02934108. [DOI] [PubMed] [Google Scholar]
- 27.Onder M, Kokturk N, Poyraz A, Oztas MO. Expression of CCR5 in Behçet's disease. Adv Exp Med Biol. 2003;528:245–7. doi: 10.1007/0-306-48382-3_49. [DOI] [PubMed] [Google Scholar]
- 28.Rau B, Baumgart K, Kruger CM, Schilling M, Beger HG. CC-chemokine activation in acute pancreatitis: enhanced release of monocyte chemoattractant protein-1 in patients with local and systemic complications. Intensive Care Med. 2003;29:622–9. doi: 10.1007/s00134-003-1668-4. [DOI] [PubMed] [Google Scholar]
- 29.Leifeld L, Dumoulin FL, Purr I, et al. Early up-regulation of chemokine expression in fulminant hepatic failure. J Pathol. 2003;199:335–44. doi: 10.1002/path.1298. [DOI] [PubMed] [Google Scholar]
- 30.Mariani P, Bachetoni A, D’Alessandro M, Lomanto D, Mazzocchi P, Speranza V. Effector Th-1 cells with cytotoxic function in the intestinal lamina propria of patients with Crohn's disease. Dig Dis Sci. 2000;45:2029–35. doi: 10.1023/a:1005516730754. [DOI] [PubMed] [Google Scholar]
- 31.Kakazu T, Hara J, Matsumoto T, et al. Type 1 T-helper cell predominance in granulomas of Crohn's disease. Am J Gastroenterol. 1999;94:2149–55. doi: 10.1111/j.1572-0241.1999.01220.x. [DOI] [PubMed] [Google Scholar]
- 32.Colpaert S, Vastraelen K, Liu Z, et al. In vitro analysis of interferon gamma (IFN-gamma) and interleukin-12 (IL-12) production and their effects in ileal Crohn's disease. Eur Cytokine Netw. 2002;13:431–7. [PubMed] [Google Scholar]
- 33.Sands BE, Anderson FH, Bernstein CN, et al. Infliximab maintenance therapy for fistulizing Crohn's disease. N Engl J Med. 2004;350:876–85. doi: 10.1056/NEJMoa030815. [DOI] [PubMed] [Google Scholar]
- 34.Gordon JN, MacDonald TT. Biological therapy in Crohn's disease. Hosp Med. 2003;64:708–12. doi: 10.12968/hosp.2003.64.12.2361. [DOI] [PubMed] [Google Scholar]
- 35.Itoh H, Komatsuda A, Wakui H, Miura AB, Tashima Y. Mammalian HSP60 is a major target for an immunosuppressant mizoribine. J Biol Chem. 1999;274:35147–51. doi: 10.1074/jbc.274.49.35147. [DOI] [PubMed] [Google Scholar]
- 36.Gao P, Zhou XY, Yashiro-Ohtani Y, et al. The unique target specificity of a nonpeptide chemokine receptor antagonist: selective blockade of two Th1 chemokine receptors CCR5 and CXCR3. J Leukoc Biol. 2003;73:273–80. doi: 10.1189/jlb.0602269. [DOI] [PubMed] [Google Scholar]