Abstract
Interleukin (IL)-2 immunotherapy is used for the treatment of metastatic melanoma and renal cell carcinoma and mediates its effects through the clonal expansion of lymphocytes. Although IL-2 remains the most effective form of therapy for these cancers, response rates are poor and dose escalation is hampered by side effects, which include vascular leak and lymphopenia. The mechanism underlying T cell loss is currently unidentified but could be the induction of activation-induced cell death (AICD) mediated by FasL. Our previous studies have shown that the amino acid taurine can attenuate apoptosis induced by a number of factors in different cell types. Here, we induced T cell AICD via CD3 and IL-2 stimulation and investigated the effect of taurine on lymphocyte apoptosis. Anti-CD3-activated Jurkat T cells treated with IL-2 significantly increased FasL expression, which was associated with increased apoptosis. Treatment with taurine prior to stimulation down-regulated FasL protein expression and partially inhibited apoptosis. Inhibition of FasL-signalling resulted in an identical reduction in apoptosis. As the kinetics of AICD are completely different in circulating T cells, we repeated these experiments in such cells to confirm our finding. Stimulation of CD4+ circulating T cells induced apoptosis in sensitized, but not freshly isolated T cells, which was abrogated partially by taurine. In Jurkat cells it was determined that taurine-mediated down-regulation of FasL protein expression was associated with decreased FasL mRNA expression and reduced NFκB activation. These results reveal one possible mechanism underlying the lymphopenia observed with IL-2 immunotherapy, involving increased FasL expression leading to apoptosis. Taurine may be of use in reversing the lymphopenia associated with IL-2, thereby augmenting its immunotherapeutic potential.
Keywords: FasL, interleukin-2, nuclear factor kappa-B, T cell, taurine
Introduction
Redirection of immune responses is the primary goal of immunotherapy and the key to an effective fight against cancer. Achieving this goal is hampered by a lack of antigen-driven responses, insufficient trafficking of immune effectors to tumour, self-tolerance and negative regulatory mechanisms [1]. Non-specific immunotherapies such as interleukin (IL)-2 have been moderately successful; however, the activation of T cells by cytokines in the absence of antigenic stimulation encourages the immune system to down-regulate immune effectors [2] − a response that prevents the development of autoimmunity during health. Among the mechanisms which limit inappropriate T cell activation are negative regulation by CTLA-4 [3], the emergence of CD4+CD25+ regulatory T cells and activation-induced cell death (AICD) [3–5]. The model of cellular activation by non-specific immunotherapies such as IL-2 is therefore flawed, as treatment may perpetuate tumour tolerance. AICD is mediated via a number of receptor/ligand pathways such as FasL, tumour necrosis factor (TNF) and TNF-R-related apoptosis-inducing ligand (TRAIL) [5]. FasL, a cell death-inducing cytokine, is expressed on the surface of T cells and is regulated by the transcription factor nuclear factor (NF) κB [4]. The FasL receptor, Fas is expressed ubiquitously in many tissues with particularly high expression in the thymus, liver, heart and kidney. FasL is expressed on T cells and natural killer cells, and has shown to be expressed in immunologically privileged sites such as the eye and testis and can be involved in tumour-mediated T cell death.
The T cell growth factor and cytokine interleukin-2 plays a pivotal role in AICD [6]. IL-2 therapy is limited by its toxicity, the most serious manifestation of which is vascular leak syndrome [7–10]. Another side effect is lymphopenia, which occurs shortly after treatment commences [11]. The reason for this transient loss of circulating lymphocytes has not been identified. Given that IL-2 plays a central role in AICD, it may be possible that administration of IL-2 during therapy results in the generation of large numbers of T cells that recognize tumour cells as self, and are subsequently deleted by AICD resulting in lymphopenia.
Given the limitations of IL-2, due to its adverse side effects, many investigators are tending towards reducing IL-2 dosage while attempting to maintain its antitumour therapeutic potential through co-administration with other cytokines such as IL-18 [12]. In contrast, an alternative approach may be to focus on effectively reducing the side effects of therapy while maintaining the high doses of IL-2 required for maximum antitumour efficacy.
We have shown previously that the amino acid taurine, when given in combination with IL-2, reduces vascular leak, enhances lymphocyte cytotoxicity, increases survival and reduces tumour burden, while not generating an autoimmune response, in a model of metastatic melanoma [13].
Physiologically, taurine is a semi-essential amino acid synthesized from the amino acids methionine and cysteine [14] and accounts for approximately 44% of the total free amino acid pool in lymphocytes [15]. The properties of taurine are well-described and include roles in antioxidation, osmoregulation, membrane stabilization and Ca2+ flux regulation. We have shown previously that taurine attenuates apoptosis in a number of different cell types [16–18]. In the neutrophil, taurine inhibits apoptosis induced by sodium-arsenite through an oxygen-dependent mechanism [16], while in the hepatocyte taurine attenuates nitric oxide- and reactive oxygen intermediate-induced apoptosis [17]. In endothelial cells, taurine attenuates sodium arsenite and/or TNF-α-induced apoptosis [18]. It has also been shown that preloading Jurkat T cells with 40 m m taurine decreases apoptotic DNA fragmentation induced through Fas receptor stimulation [19].
In this study we show that cellular activation through the T cell receptor and stimulation with IL-2 induces up-regulation of the death-inducing cytokine FasL on Jurkat T cells, which results in AICD. Additionally, we demonstrate that taurine prevents FasL-mediated AICD through modulation of FasL protein and mRNA expression, and a reduction in NFκB activation. This study supports our previous finding that taurine potentiates the antitumour effect of IL-2 therapy in an in vivo metastatic melanoma model [12]. Importantly, these results, which we describe initially in Jurkat T cells, are reproducible in isolated peripheral blood lymphocytes (PBLs).
Materials and methods
Cell culture
Jurkat T lymphocytes (clone E6·1) obtained from the ATCC (LGC Ltd, Bourn, UK) and CD4+ PBLs were maintained in RPMI-1640 medium (GibcoBRL, Paisley, UK). Growth medium was supplemented with 2 mm l-glutamine, 10% fetal bovine serum (FBS), 50 units/ml penicillin and 50 units/ml streptomycin and maintained in vented tissue culture flasks at 37°C, 5% CO2, 95% humidified air.
Isolation of CD4+ peripheral blood T lymphocytes
Venous blood was drawn in blood tubes containing a lithium–heparin anticoagulant (10 units/ml). Fifty µl RosetteSep cocktail (Stemcell Technologies, Vancouver, BC, Canada) was added per ml blood. The solution was incubated for 20 min at room temperature (18–25°C). Blood was diluted with an equal volume of sterile phosphate-buffered saline (PBS) + 2% FBS (GibcoBRL). Diluted blood was overlaid onto an equal volume of Ficoll-Paque PLUS (Amersham Pharmacia Biotech AB, Uppsala, Sweden) density gradient medium. Blood was centrifuged over the density gradient at 1200 g for 20 min. CD4+ T cells were removed, washed twice with PBS + 2% FBS and resuspended in supplemented growth medium.
Treatment of Jurkat and peripheral T cells with CD3, IL-2 and taurine
Cell viability was assessed via trypan blue exclusion. To sensitize cells to apoptosis CD4+ PBLs, maintained at 5 × 105/ml, were stimulated for 3 days with 0·5 µg/ml phytohaemagglutinin-P (PHA-P) (Sigma, Ireland) and 1 ng/ml phorbol 12-myristate 13-acetate (PMA) (Sigma) in RPMI-1640. After 3 days PBLs were washed and stimulated with 30 U/ml IL-2 (Chiron, Amesterdam, the Netherlands) in growth medium for a further 3 days (referred to hereafter as 6-day T cells) [20]. Jurkat cells, freshly isolated T cells and 6-day T cells were incubated with medium supplemented with or without 40 m m taurine (Sigma) for 64 h in a humidified CO2 incubator at 37°C. Stimulation was carried out in 24-well tissue culture plates (NUNC Brand Products, Denmark). For T cell receptor (TCR) stimulation wells were coated with 300 µl anti-CD3 MoAb (PharMingen, San Diego, CA, USA) (5 µg/ml), prepared in sterile PBS (Dulbecco's Ca2+, Mg2+ and NaHCO3 free) and incubated for 3 h at 37°C. After 3 h the CD3 MoAb-coated wells were washed gently with sterile PBS. T cells, some of which were preloaded with 40 m m taurine, were plated in 1 ml volumes at 1 × 106 cells/well in the presence or absence of immobilized anti-CD3 MoAb and 500 units/ml rhIL-2. Plates were then incubated for 18 h at 37°C, 5% CO2. Following 18 h of incubation cells were washed extensively with warm sterile PBS. The cells were then retreated for 18 h as before with rhIL-2 and taurine in the absence of anti-CD3 MoAb.
Flow cytometric analysis of surface receptor/ligand expression and apoptosis
Fas, FasL and IL-2r expression were assessed on Jurkat and freshly isolated peripheral T cells. Cells (1 × 105 cells/100 µl) were incubated on ice for 30 min with 10 µl anti-CD95-FITC MoAb (IQ Products, Groningen, the Netherlands), anti-FasL clone 8B8 (Oncogene, Boston, MA, USA) or anti-IL-2r (Dako, UK). The FasL clone 8B8 has been found to be specific for FasL when measured by flow cytometry (reviewed in [5]). FasL and IL-2r detection was carried out using a rabbit antimouse FITC-labelled secondary antibody (Dako). An appropriate FITC-labelled isotypically matched antibody was used as a negative control. Non-specific antibody binding to peripheral CD4+ T cells was prevented by washing cells with 1 µg human IgG (Sigma). After 30 min the samples were washed thoroughly with ice-cold PBS and analysed. Apoptosis was assessed using the TACSTM annexin V-FITC apoptosis detection kit (R&D Systems, UK), as we have described previously [21], by annexin-V binding to phosphatidylserine expressed on the surface of apoptotic cells. Flow cytometric analysis was performed using a FACscan equipped with an argon ion laser (Becton Dickinson, CA, USA).
FasL mRNA quantification
Whole cell lysates from 2 × 106 Jurkat cells were prepared using 1× cell lysis diluent supplied with the Quantikine mRNA kit (R&D Systems) in a volume of 400 µl. Sample/probe hybridization and detection was carried out as per the manufacturer's instructions. Colorimetric detection was carried out by measuring the optical density at 490 nm using a microplate reader.
Measurement of NFκB activation
Nuclear extracts were prepared as follows: 1 × 107 Jurkat cells washed twice with 1 ml ice-cold PBS. The pellet was resuspended in 1 ml ice-cold hypotonic buffer (20 m m HEPES, pH 7·5, 0·1 m m EDTA, 10 µl protease inhibitor cocktail/5 ml). Cells were allowed to swell on ice for 15 min 50 µl 10% Igepal CA-630 was added to cell suspension and mixed gently. The homogenate was centrifuged for 30 s at 4°C in a microfuge. The nuclear pellet was resuspended in 50 µl complete lysis buffer [supplied in commercial NFκB kit (Active Motif, Belgium)] and incubated on ice for 30 min on a shaking platform. Nuclear extracts were centrifuged at 14 000 g for 10 min at 4°C. The protein concentrations of the extracts were determined using a Bradford-based assay. NFκB was measured as per the manufacturer's instructions using colourimetric detection. Colorimetric detection was carried out by measuring the optical density at 450 nm. Analysis of oligonucleotide binding specificity was also assessed via competitive binding experiments using wild-type and mutated NFκB consensus oligonucleotides supplied with the kit.
Blocking apoptosis with Fas : Fc inhibitor
Jurkat cells were treated with anti-CD3 MoAb, rhIL-2 and/or taurine as before in the presence of saturated levels of FasL inhibitor (Alexix Corporation, Nottingham, UK) (50 µg/ml), which was free in solution. At this concentration of inhibitor, FasL expression on stimulated cells cannot be detected via anti-FasL MoAb, indicating its blockade.
Statistical analysis
Results are expressed as mean ± s.e.m. of (n) independent experiments. Statistical significance was evaluated by means of one-way analysis of variance (anova) or t-test. A value of P < 0·05 was considered significant. Analysis was carried out using the statistical package for social science (SPSS Inc., Chicago, IL, USA).
Results
Taurine attenuates anti-CD3/IL-2-induced apoptosis of Jurkat cells and peripheral T cells sensitized to AICD
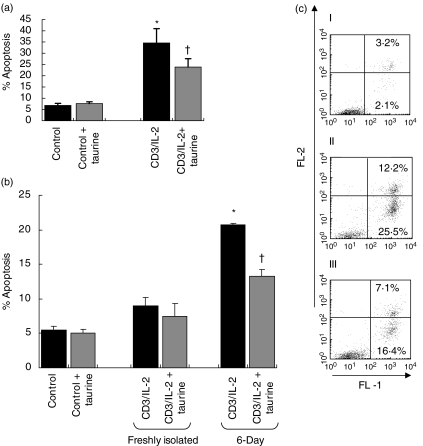
Jurkat cells are sensitive to Fas-mediated apoptosis, while freshly isolated T cells are resistant [22,23]. Prolonged activation of peripheral T cells results in sensitization of the cells to AICD [20,23]. We measured the ability of anti-CD3/IL-2 to induce apoptosis in Jurkat, freshly isolated peripheral T cells and 6-day T cells. In addition we examined the effect of taurine on CD3/IL-2-induced T cell apoptosis. Incubating the cells for 64 h with taurine allows the amino acid to enter the cells via passive diffusion. Control Jurkat cells and cells incubated in medium supplemented with taurine had similar levels of apoptosis. Stimulation of the cells with anti-CD3/IL-2 induced a significant level of apoptosis (P < 0·01), which was significantly lowered in taurine preloaded cells (P < 0·05) (Fig. 1a,c). We assessed whether our observations concerning the reduction in AICD by taurine held true in peripheral CD4+ T cells. As observed with Jurkat cells, taurine preloading significantly reduced CD3-IL-2-induced AICD in sensitized 6-day T cells (Fig. 1b).
Fig. 1.
Taurine attenuates CD3/IL-2-induced T cell apoptosis. Jurkat cells were stimulated with anti-CD3 MoAb (5 µg/ml) and IL-2 (500 U/ml) for 18 h, and then re-stimulated with IL-2 (500 U/ml) only for an additional 18 h. CD4+ T cells isolated from peripheral blood of male volunteers were stimulated in culture with PMA (1 ng/ml) and PHA-P (0·5 µg/ml) for 3 days followed by IL-2 for a further 3 days (6-day). Freshly isolated and 6-day CD4+ T cells were then stimulated in same manner as the Jurkat cells. Apoptosis was measured by flow cytometry. The data are presented as the combination of the apoptotic (annexin-V positive) and late apoptotic/necrotic (annexin-V/propidium iodide positive). (a) Stimulation of Jurkat cells with CD3/IL-2 significantly increased levels of apoptosis compared to control (*P < 0·05 versus control). Preloading the cells with taurine (40 m m) for 64 h prior to CD3/IL-2 stimulation significantly reduced apoptosis induced by stimulation (†P < 0·05 versus control and CD3/IL-2). (b) Stimulation of 6-day T cells with CD3/IL-2 resulted in the induction of apoptosis (*P < 0·001 versus control). Preloading the 6-day T cells with taurine (40 m m for 64 h prior to CD3/IL-2 stimulation) significantly reduced CD3/IL-2-induced apoptosis (†P < 0·001 versus 6-day CD3/IL-2). (c) Representative flow cytometry diagrams of Jurkat apoptosis. The lower right quadrant represents annexin-V stained cells. The upper right quadrant represents annexin-V/propidium iodide stained cells. The lower left quadrant shows unstained, viable cells. (I) Control cell apoptosis; (II) CD3/IL-2-induced apoptosis; (III) apoptosis of CD3/IL-2-stimulated cells preloaded with 40 m m taurine for 64 h prior to stimulation. Data are expressed as the mean ± s.e.m. (n = 3). Statistical analysis was carried out by one-way anova with LSD post hoc correction.
Taurine does not modulate AICD through altered IL-2 bioavailability
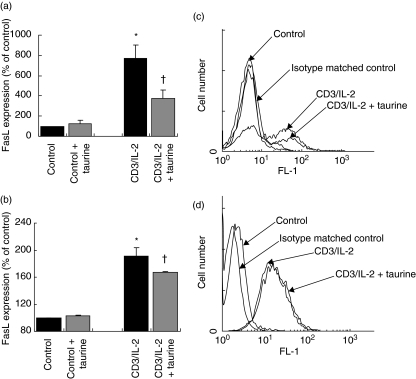
In order to rule out the possibility that the reduction in apoptosis was due to altered IL-2 availability to cells, we assessed the effect of taurine on IL-2r expression on Jurkat cells (Fig. 2d). We observed that while stimulation with anti-CD3/IL-2 resulted in a significant increase in IL-2r expression on cells, taurine preloading did not significantly alter IL-2r expression. Similar observations were made of freshly isolated peripheral CD4+ T cells also (data not shown).
Fig. 2.
Taurine reduces CD3/IL-2-induced FasL expression on T cells. (a) Jurkat cells were stimulated with anti-CD3 MoAb (5 µg/ml) and IL-2 (500 U/ml) for 18 h, and then re-stimulated with IL-2 (500 U/ml) only for an additional 18 h. FasL expression was measured by flow cytometry. Stimulation of Jurkat cells with CD3/IL-2 up-regulated FasL expression relative to unstimulated control (*P < 0·05 versus control). Preloading with taurine (40 m m for 64 h prior to CD3/IL-2 stimulation) significantly reduced CD3/IL-2-induced FasL expression on Jurkat cells (†P < 0·01 versus CD3/IL-2). Data are expressed as the mean ± s.e.m. (n = 5). Statistical analysis was performed by one-way anova with LSD post hoc correction. (b) CD4+ T cells freshly isolated from peripheral blood of male volunteers were stimulated with anti-CD3 MoAb (5 µg/ml) and IL-2 (500 U/ml) for 18 h, and then re-stimulated with IL-2 (500 U/ml) only for an additional 18 h. FasL expression was measured by flow cytometry. Stimulation with CD3/IL-2 significantly up-regulated expression of FasL on freshly isolated CD4+ peripheral T cells compared to unstimulated control (*P < 0·05 versus control). Preloading the cells with taurine (40 m m for 64 h prior to CD3/IL-2 stimulation) significantly down-regulated CD3/IL-2-induced FasL expression on peripheral CD4+ lymphocytes (†P < 0·05 versus CD3/IL-2). Data are expressed as the mean ± s.e.m. (n = 3). Statistical analysis was carried out by one-way anova with LSD post hoc correction. (c) Flow cytometry histogram of Jurkat FasL expression. Binding of anti-FasL antibody is indicated by the arrows. There is no antibody binding to control cells as resting Jurkat cells do not express FasL. (d) Flow cytometry histogram of Jurkat IL-2r expression. Binding of anti-IL-2r antibody is indicated by the arrows. Statistical analysis was performed by one-way anova with LSD post hoc correction.
Taurine down-regulates anti-CD3 MoAb/IL-2-induced Jurkat and peripheral blood CD4+ T cell FasL expression
IL-2 enhances proliferation and up-regulates FasL expression on activated peripheral lymphocytes and T cell clones [22]. Stimulation with anti-CD3 and IL-2 resulted in a significant increase in expression of FasL on Jurkat T cells. It has been shown that FasL can be found in soluble form [24,25]. The soluble form of FasL is much less potent than the membrane-bound form [24,25], and it has been suggested that the cleavage of membrane-bound FasL by matrix metalloproteinases is a method of functional down-regulation of FasL [26,27]. Therefore in this study only the effect of taurine on the more potent form of FasL, the membrane-bound form, was determined. Taurine preloading had no effect on FasL expression in unstimulated Jurkat cells, but significantly down-regulated FasL expression induced by stimulation (Fig. 2a,c). We tested the effect of taurine on FasL protein expression on freshly isolated peripheral CD4+ T cells. As with Jurkat cells, treatment of CD4+ cells with anti-CD3/IL-2 significantly up-regulated FasL expression. Preloading the cells with taurine significantly reduced FasL expression on stimulated cells (Fig. 2b).
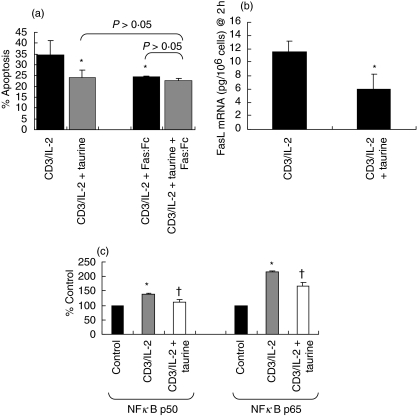
As we had observed that taurine resulted in a decrease in FasL expression, we endeavoured to determine whether the reduction in apoptosis due to taurine was indeed a direct result of decreased FasL expression. To accomplish this we stimulated Jurkat cells in the presence of an inhibitor of FasL. Blockade of FasL signalling with Fas : Fc, used at saturation, reduced apoptosis induced by anti-CD3/IL-2 to levels similar to those in the taurine preloaded group. This suggests that taurine reduces CD3/IL-2-induced T cell apoptosis via a FasL-dependent mechanism (Fig. 3a).
Fig. 3.
(a) Taurine reduces CD3/IL-2-induced Jurkat cell apoptosis via a FasL-dependent mechanism. Jurkat cells were stimulated with anti-CD3 MoAb (5 µg/ml) and IL-2 (500 U/ml) for 18 h, and then re-stimulated with IL-2 (500 U/ml) only for an additional 18 h. Apoptosis was measured by flow cytometry. The data are presented as the combination of the apoptotic (annexin-V positive) and late apoptotic/necrotic (annexin-V/PI positive) cell populations. In the presence or absence of excess levels of Fas : Fc (50 µg/ml), an inhibitor of FasL. FasL inhibitor reduced CD3/IL-2-induced apoptosis to a level similar to that observed when the cells were preloaded with taurine (40 m m for 64 h prior to CD3/IL-2 stimulation) (*P < 0·05 versus CD3/IL-2). Fas : Fc and taurine in combination reduced of CD3/IL-2 stimulated Jurkat cell apoptosis to similar levels obtained with Fas : Fc (P > 0·05) or taurine (P > 0·05) alone. Data are expressed as mean ± s.e.m. (n = 3). Statistical analysis was carried out by one-way anova with LSD post hoc correction. (b) Taurine decreases CD3/IL-2-induced FasL mRNA expression in Jurkat cells. Jurkat cells were stimulated with anti-CD3 MoAb (5 µg/ml) and IL-2 (500 U/ml) for 2 h in the presence or absence of 40 m m taurine (preloaded for 64 h prior to CD3/IL-2 stimulation). FasL mRNA in whole cell lysates was measured by quantitative enzyme-linked immunosorbent assay (ELISA). Stimulation of Jurkat cells with CD3/IL-2 in the presence of taurine significantly reduced FasL mRNA expression relative to that observed in the absence of taurine (*P < 0·05 versus CD3/IL-2). Data are expressed as mean ± s.e.m. (n = 3). Statistical analysis was performed by Student's t-test. (c) Taurine decreases CD3/IL-2-induced NFκB p50 and p65 activation in Jurkat cells. Jurkat cells were stimulated with anti-CD3 MoAb (5 µg/ml) and IL-2 (500 U/ml) for 90 min. Following preparation of nuclear extracts, NFκB p50 and p65 subunit expression were measured by ELISA using NFκB oligonucleotide probes, antip50 antobody and antip65 antibody. Stimulation with CD3/IL-2 increased NFκB p50 and p65 expression in the nucleus relative to unstimulated control (*P < 0·05 versus control). Preloading the cells with taurine (40 m m for 64 h prior to CD3/IL-2 stimulation) significantly reduced both NFκB p50 and p65 expression induced by CD3/IL-2 stimulation alone (†P < 0·05 versus CD3/IL-2). Data are expressed as mean ± s.e.m. (n = 3). Statistical analysis was performed by one-way anova with LSD post hoc correction.
As taurine decreases expression of membrane FasL expression we determined its effect on FasL gene transcription in Jurkat cells. Expression levels of FasL mRNA were found to peak 2 h following stimulation after which time levels begin to decline (data not shown). Resting Jurkat cells did not express detectable levels of FasL mRNA. The effect of taurine on FasL mRNA expression was measured 2 h following stimulation. Figure 3b shows that taurine significantly reduces levels of FasL mRNA expression in Jurkat cells.
Taurine decreases activation of the FasL transcription factor NFκB in Jurkat cells
A number of studies have shown that inhibition of NFκB activity results in decreased apoptosis and activation-induced FasL expression [28]. It has also been shown that chemical antioxidants inhibit AICD by blocking NFκB activation [29]. We sought to determine whether taurine-mediated down-regulation of FasL mRNA was associated with decreased NFκB activation. To examine this, Jurkat cells were stimulated, the proteins extracted and analysed subsequently for NFκB p50, p52, p65/RelA, RelB and c-Rel subunit content. An initial time-course of Jurkat p50 subunit activation over a period of 90 min revealed that nuclear levels of activated NFκB continue to increase steadily following CD3/IL-2 stimulation (data not shown). In subsequent experiments nuclear NFκB expression was measured 90 min following stimulation. CD3/IL-2 stimulation resulted in the activation of the p50 and p65/RelA subunits (Fig. 3c). The p50 and p65/RelA subunits are the predominant subunits of NFκB involved in FasL mRNA transcription [30]. Stimulation did not result in the activation of the p52, RelB or c-Rel subunits (data not shown). Preloading the cells with taurine significantly reduced levels of activated p50 and p65/RelA, but had no effect on p52, RelB or c-Rel.
Discussion
Use of the cytokine IL-2 in immunotherapy is hindered not only by its detrimental effect on the vascular system, but also by the induction of lymphopenia. This loss of lymphocytes may result ultimately from IL-2 stimulation in the absence of antigen resulting in the deletion of T cells by AICD under the rules of self-tolerance. While IL-2 therapy remains the most successful treatment for renal cell carcinoma and malignant melanoma, the effectiveness of treatment is hampered by its dose-limiting toxicity. Toxicity to IL-2 develops rapidly after administration and results in vascular leak syndrome [18]. Given the severity of the side effects, patients who might otherwise benefit from a complete term of treatment must discontinue therapy. Therefore it is desirable to persist with IL-2 treatment by decreasing the associated side effects.
In the present study we used a Jurkat T cell model of AICD, which involves activation through the TCR and stimulation with IL-2, and we explored the effect of taurine in this system. Taurine preloading was found to significantly reduce CD3/IL-2-induced apoptosis of both Jurkat cells and CD4+ peripheral T cells sensitized to AICD. To ensure differences in levels of apoptosis between stimulated and control cells were not due solely to differences in cell numbers due to proliferation, the cell populations were counted by haemocytometry before analysis of apoptosis. There was no apparent difference between the numbers of cells in controls compared to stimulated cells. The inclusion of taurine did not alter cell numbers in either group. Nor was the reduction in apoptosis due to a reduction in IL-2 bioavailability, as taurine did not alter IL-2r expression. These data support our previous findings that taurine attenuates apoptosis of other cell types, such as neutrophils [16], endothelial cells [18] and hepatocytes [17].
AICD of T cells is mediated by a number of receptor/ligand pathways such as FasL, TNF and TRAIL [5]. Given that FasL is considered to be the major executioner of T cell apoptosis we measured the effect of stimulation and taurine on the expression of FasL. Stimulation of peripheral T cells and Jurkat cells significantly increased protein and mRNA levels of FasL. Taurine was found to significantly reduce expression of FasL in both types of T cell. The FasL inhibitor reduced apoptosis to levels similar to that observed with taurine, indicating that the reduction in apoptosis due to taurine was dependent on FasL and reduced expression of the ligand. This was confirmed further by treating the cells in the presence of both Fas : Fc inhibitor and taurine. We have confirmed that the remaining apoptotic death in CD3/IL-2-stimulated T cells is due to the cellular release of TRAIL, which contributes to AICD in this model, but is unaffected by taurine (unpublished data).
The transcription factor NFκB has been shown to be necessary for the expression of FasL mRNA in Jurkat cells and PBLs [28,30]. One study has shown that combination of niacin and taurine blocks lung injury and fibrosis by down-regulating bleomycin-induced activation of NFκB in mice [31]. Taurine preloading decreased the activation of the p50 and p65 subunits of NFκB induced by CD3/IL-2 stimulation of Jurkat cells. Given this, taurine is likely to reduce FasL expression, and indeed apoptosis, via a reduction in NFκB activation.
NFκB family members are involved in tumour growth and metastasis. Several Hodgkin's lymphoma cell lines contain constitutively nuclear NFκB complexes, and inhibition of NFκB activation by overexpression of a non-degradable IκB molecule inhibits proliferation and tumourigenicity of these cells [32]. High levels of activated NFκB are also associated with progression of breast cancer cells to oestrogen-independent growth [33] and human thyroid carcinoma cells require nuclear NFκB activity for proliferation and malignant transformation in vitro[34]. In prostate cancer NFκB has been shown to be a pivotal transcription factor in the metastasis of prostate cancer to bone [35]. It is possible that in vivo taurine has a direct antineoplastic effect by reducing constitutively activated NFκB levels in tumour cells, thereby reducing tumour growth and/or metastasis. This direct effect of taurine on tumour cell NFκB may explain how taurine has a beneficial effect on tumour growth and metastasis [13], while simultaneously reducing immune function.
In this study we have established that taurine attenuates CD3/IL-2-induced Jurkat and CD4+ peripheral T cell apoptosis. In Jurkat cells this is mediated through the modulation of FasL expression and NFκB activation. Our data suggest that the AICD mechanism may underlie the lymphopenia ultimately induced in IL-2 immunotherapy. From previous studies it is known that administration of taurine is not associated with the development of autoimmunity and can reduce vascular leak syndrome. Hence, we are continuing to investigate whether administration of taurine to patients may reduce the deleterious side effects of IL-2 treatment, such as vascular leak and lymphopenia, allowing for dose-escalation and extended treatment regimens to maximize the therapeutic benefit of IL-2 immunotherapy.
Acknowledgments
This study was supported by a grant from the Jervis Street Charitable Infirmary Trust.
References
- 1.Waldmann TA. Immunotherapy: past, present and future. Nat Med. 2003;9:269–77. doi: 10.1038/nm0303-269. [DOI] [PubMed] [Google Scholar]
- 2.Lenardo MJ. Fas and the art of lymphocyte maintenance. J Exp Med. 1996;183:721–4. doi: 10.1084/jem.183.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chambers CA, Kuhns MS, Egen JG, Allison JP. CTLA-4 mediated inhibition in regulation of T cell responses: mechanisms and manipulation in tumour immunotherapy. Ann Rev Immunol. 2001;19:565–94. doi: 10.1146/annurev.immunol.19.1.565. [DOI] [PubMed] [Google Scholar]
- 4.Nagata S. Apoptosis by death factor. Cell. 1997;88:355–65. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- 5.Maher S, Toomey D, Condron C, Bouchier-Hayes D. Activation-induced cell death. The controversial role of Fas and Fas ligand in immune privilege and tumour counterattack. Immunol Cell Biol. 2002;80:131–7. doi: 10.1046/j.1440-1711.2002.01068.x. [DOI] [PubMed] [Google Scholar]
- 6.Lenardo MJ. Interleukin-2 programs mouse αβ T lymphocytes for apoptosis. Nature. 1991;353:858–61. doi: 10.1038/353858a0. [DOI] [PubMed] [Google Scholar]
- 7.Lotze MT, Matory YL, Ettinghausen SE, et al. In vivo administration of purified human interleukin 2, II. Half life, immunologic effects, and expansion of peripheral lymphoid cells in vivo with recombinant IL 2. J Immunol. 1985;135:2865–75. [PubMed] [Google Scholar]
- 8.Whitehead RP, Freidman KD, Clark DA, Pagini K, Rapp L. Phase I trial of simultaneous administration of interleukin 2 and interleukin 4 subcutaneously. Clin Cancer Res. 1995;1:1145–52. [PubMed] [Google Scholar]
- 9.Rosenberg SA, Lotze MT, Yang JC, et al. Experience with the use of high-dose interleukin-2 in the treatment of 652 cancer patients. Ann Surg. 1989;210:474–85. doi: 10.1097/00000658-198910000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finnegan NM, Redmond HP, Bouchier-Hayes DJ. Taurine attenuates recombinant interleukin-2-activated, lymphocyte-mediated endothelial cell injury. Cancer. 1998;82:186–99. doi: 10.1002/(sici)1097-0142(19980101)82:1<186::aid-cncr24>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 11.Itoh K, Balch CM, Murray JL, et al. Immunological properties of melanoma tumor-infiltrating lymphocytes before and after IL-2-based biotherapies. In Vivo. 1991;5:647–54. [PubMed] [Google Scholar]
- 12.Son YI, Dallal RM, Lotze MT. Combined treatment with interleukin-18 and low-dose interleukin-2 induced regression of a murine sarcoma and memory response. J Immunother. 2003;26:234–40. doi: 10.1097/00002371-200305000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Finnegan N, Toomey D, Condron C, Redmond HP, Da Costa M, Bouchier-Hayes D. Potentiation of the therapeutic index of IL-2 immunotherapy by combination with taurine in a syngeneic murine tumour model. Irish J Med Sci. 2002;170:223–7. doi: 10.1007/BF03168959. [DOI] [PubMed] [Google Scholar]
- 14.Stapleton PP, O'Flaherty L, Redmond HP, Bouchier-Hayes DJ. Host defence − a role for the amino acid taurine. JPEN Parenteral and Enteral Nutrition. 1998;22:42–8. doi: 10.1177/014860719802200142. [DOI] [PubMed] [Google Scholar]
- 15.Timbrell JA, Seabra V, Waterfield CJ. The in vivo and in vitro protective properties of taurine. Gen Pharmacol. 1995;26:453–62. doi: 10.1016/0306-3623(94)00203-y. [DOI] [PubMed] [Google Scholar]
- 16.Watson RW, Redmond HP, Wang JH, Bouchier-Hayes DJ. Mechanisms involved in sodium arsenite-induced apoptosis of human neutrophils. J Leukoc Biol. 1996;60:625–32. doi: 10.1002/jlb.60.5.625. [DOI] [PubMed] [Google Scholar]
- 17.Redmond HP, Wang JH, Bouchier-Hayes D. Taurine attenuates nitric oxide- and reactive oxygen intermediate-dependent hepatocyte injury. Arch Surg. 1996;131:1280–7. doi: 10.1001/archsurg.1996.01430240034004. [DOI] [PubMed] [Google Scholar]
- 18.Wang JH, Redmond HP, Watson RW, Condron C, Bouchier-Hayes DJ. The beneficial effect of taurine on the prevention of human endothelial cell death. Shock. 1996;6:331–8. doi: 10.1097/00024382-199611000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Lang F, Madlung J, Siemen D, Ellory C, Lepple-Wienhues A, Gulbins E. The involvement of caspases in the CD95 (Fas/Apo-1)-but not swelling-induced cellular taurine release from Jurkat T-lymphocytes. Pflugers Arch. 2000;440:93–9. doi: 10.1007/s004240000247. [DOI] [PubMed] [Google Scholar]
- 20.Wesselborg S, Jannsen O, Kabelitz D. Induction of activation-driven death (apoptosis) in activated but not resting peripheral blood T cells. J Immunol. 1993;150:4338. [PubMed] [Google Scholar]
- 21.Fanning NF, Porter J, Shorten GD, et al. Inhibition of neutrophil apoptosis after elective surgery. Surgery. 1999;126:527–34. [PubMed] [Google Scholar]
- 22.Esser MT, Dinglasan RD, Krishnamurthy B, Gullo CA, Graham MB, Braciale VL. IL-2 induces FasL/Fas (CD95L/CD95) cytotoxicity in CD8+ and CD4+ T lymphocyte clones. J Immunol. 1997;158:5612–8. [PubMed] [Google Scholar]
- 23.Klas C, Debatin KM, Jonker RR, Krammer PH. Activation interferes with the APO-1 pathway in mature human T cells. Int Immunol. 1993;5:625–30. doi: 10.1093/intimm/5.6.625. [DOI] [PubMed] [Google Scholar]
- 24.Cheng J, Zhou T, Liu C, et al. Protection from Fas-mediated apoptosis by a soluble form of the Fas molecule. Science. 1994;263:1759–62. doi: 10.1126/science.7510905. [DOI] [PubMed] [Google Scholar]
- 25.Tanaka M, Suda T, Takahashi T, Nagata S. Expression of the functional soluble form of human Fas ligand in activated lymphocytes. EMBO J. 1995;14:1129–35. doi: 10.1002/j.1460-2075.1995.tb07096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tanaka M, Itai T, Adachi M, Nagata S. Downregulation of Fas ligand by shedding. Nat Med. 1998;4:31–6. doi: 10.1038/nm0198-031. [DOI] [PubMed] [Google Scholar]
- 27.Martinez-Lorenzo MJ, Anel A, Gamen S, et al. Activated human T cells release bioactive Fas ligand and APO2 ligand in microvesicles. J Immunol. 1995;163:1274–81. [PubMed] [Google Scholar]
- 28.Kasibhatla S, Genestier L, Green DR. Regulation of Fas-ligand expression during activation-induced cell death in T lymphocytes via nuclear factor κB. J Biol Chem. 1999;274:987–92. doi: 10.1074/jbc.274.2.987. [DOI] [PubMed] [Google Scholar]
- 29.Mercurio F, Manning AM. NFκB as a primary regulator of the stress response. Oncogene. 1999;18:6163–71. doi: 10.1038/sj.onc.1203174. [DOI] [PubMed] [Google Scholar]
- 30.Hsu SC, Gavrilin MA, Lee HH, Wu CC, Han SH, Lai MZ. NF-kappa B-dependent Fas ligand expression. Eur J Immunol. 1999;29:2948–56. doi: 10.1002/(SICI)1521-4141(199909)29:09<2948::AID-IMMU2948>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 31.Gurujeyalakshmi G, Wang Y, Giri SN. Suppression of bleomycin-induced nitric oxide production in mice by taurine and niacin. Nitric Oxide. 2000;4:399–411. doi: 10.1006/niox.2000.0297. [DOI] [PubMed] [Google Scholar]
- 32.Sovak MA, Bellas RE, Kim DW, et al. Aberrant nuclear factor-kappa B/Rel expression and the pathogenesis of breast cancer. J Clin Invest. 1997;100:2952–60. doi: 10.1172/JCI119848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bours V, Dejardin E, Goujon-Latawe F, Merville MP, Castronovo V. The NF-kappa B transcription factor and cancer: high expression of NF-kappa B- and I-kappa B-related proteins in tumour cell lines. Biochem Pharmacol. 1994;47:145–9. doi: 10.1016/0006-2952(94)90448-0. [DOI] [PubMed] [Google Scholar]
- 34.Hammarskjold ML, Simurda MC. Epstein–Barr virus latent membrane protein transactivates the human immunodeficiency virus type-1 long terminal repeat through induction of NF-kappa B activity. J Virol. 1992;66:6496–501. doi: 10.1128/jvi.66.11.6496-6501.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Andela VB, Gordon AH, Zotalis G, et al. NFkappaB: a pivotal transcription factor in prostate cancer metastasis to bone. Clin Orthop. 2003;415:S75–85. doi: 10.1097/01.blo.0000093048.96273.aa. [DOI] [PubMed] [Google Scholar]