Abstract
Wiskott–Aldrich syndrome (WAS) is an X-linked immunodeficiency/platelet disease due to mutations of WASP, a cytoskeletal regulatory protein of blood cells. Patients exhibit a range of immune defects generally attributed to defective T-cell function, including poor response to immunization, skewed immunoglobulin isotypes, eczema, recurrent infections, autoimmune disease and increased frequency of malignancies. Here we show a deficit of total B-cells in WAS patients of various ages and identify phenotypic perturbations involving complement receptors and CD27. Whereas B-cells of normal healthy donors are overwhelmingly CD21/CD35-positive, B-cells expressing these receptors are significantly reduced in number in WAS patients, and their paucity may cause suboptimal antigen capture and presentation. The frequencies of IgD– and IgG+ patient B-cells were not different from healthy donors (although absolute numbers were decreased), indicating that isotype switching is occurring. In contrast, the frequency of cells positive for CD27, the marker of post germinal centre B-cells, was significantly decreased even among isotype-switched cells, and B-cells resembling germinal centre progenitors (CD10+CD27–CD38bright) were more frequent in adult patients, suggesting impaired germinal centre maturation/differentiation. The documentation of these phenotypic perturbations and deficit of total cells suggest that defects intrinsic to B-cells contribute to the impaired humoral immunity that characterizes this disease.
Keywords: immunodeficiency, B lymphocytes, complement receptors, post germinal centre B cells, isotype switching
Introduction
The Wiskott–Aldrich syndrome (WAS) is an X-linked immunodeficiency and platelet disease caused by mutations of the gene encoding for WASP (WAS protein), a cytoskeletal and signalling protein of blood cells. The disease was initially described by Wiskott in 1937 [1] and identified as X-linked by Aldrich et al. in 1954 [2]. It is characterized by thrombocytopenia with reduced platelet volume, eczema, recurrent bacterial and viral infections, a high rate of autoimmunity, and increased frequency of malignancies especially B-cell lymphoma. Serum of WAS patients has a skewed distribution of immunoglobulin isotypes with reduced levels of IgM, normal levels of IgG and elevated level of IgE and IgA. B-cell effector functions such as antibody responses to polysaccharide antigen and φx174 bacteriophage are defective (reviewed in [3–5]).
The affected protein WASP is the founding member of a protein family involved in transducing signals for rearrangement of the actin cytoskeleton. WASP is expressed in all nonerythroid blood lineages. Since the discovery of the underlying gene defect, the absence of WASP has been linked to in vitro functional defects in most blood cell lineages including macrophages, which show impaired chemotaxis and phagocytosis, and dendritic cells, which fail to polarize normally and have reduced translocational motility (reviewed in [6]). However, the major immune dysfunction in the patients is thought to result from WASP absence in T-cells. WASP-negative T cells have been extensively studied; they are deficient in proliferative response to anti-CD3 and actin polymerization at the contact site with antigen presenting cells and are impaired in capping of CD3 [7–9].
The role of WASP in B-cells has received less attention. Study of Epstein-Barr virus (EBV) transformed patient B-cell lines showed impaired intracellular calcium mobilization and tyrosine phosphorylation upon B cell antigen receptor cross-linking [10]. However, comparable studies for primary patient B-cells were inconsistent, one study showed decreased [10] and another showed normal calcium mobilization in response to B cell receptor (BCR) stimulation [11]. Several studies support a role for WASP in the cytoskeletal regulation of B-cells. Decreased F-actin content and diffused nonpolar α-actinin distribution were found in EBV transformed patient B-cell lines [12]. Although murine B-cells lacking WASP showed normal proliferative response upon stimulation, surface IgD capping was impaired [13]. Impaired cell polarization and spreading upon IL-4 and CD40 stimulation, and shorter microvilli at cell contact sites were also reported for WASP-null murine B cells [14].
Since the affected immune functions in the disease, e.g. response to immunization, require interactions of both T- and B-cells, it is likely that defects of both T- and B-cells contribute to the humoral immune deficiency seen in the patients. Indeed, a recent study of primary cells of WAS patients documented a selective deficit of T- and B- cells; the number of NK cells was normal [15]. The lower cell numbers were observed from infancy, suggesting that decreased production of T- and B-cells is a feature of WAS. We now report the results of immunophenotyping of primary B-cells of a cohort of WAS patients, aged several weeks to 54 years, in comparison to unaffected healthy control individuals of the same age range. The results show that a significant percent of patients’ B-cells lack complement receptors. Despite the skewed immunoglobulin isotype composition characteristic of patient plasma, the findings indicate normal frequency of isotype switching of patient B-cells. However, development of CD27+ post germinal centre B-cells is markedly deficient, and the deficit of CD27+ B-cells is associated with an increased frequency of CD10+ cells resembling germinal centre precursor B-cells.
Materials and methods
WAS patient and control subjects
Blood samples were collected with informed consent from diagnosed WAS patients and control individuals under protocols approved by the Institutional Review Boards of the CBR Institute for Biomedical Research, Boston, and the Research Institute for Paediatric Haematology, Moscow. The diagnosis of WAS was based on male sex, thrombocytopenia with small platelets, eczema, and immunodeficiency of variable severity; diagnosis was verified by identified WAS mutation [16] (M.I. Lutskiy et al. unpublished observation), and thus all patients met definitive international diagnostic criteria [17]. In addition, based on the presence or absence of eczema, recurrent infections, autoimmune disease and malignancy, the patients’ disease was graded as mild (thrombocytopenia with or without mild eczema), moderate (thrombocytopenia and severe eczema or frequent infections), or severe (thrombocytopenia, severe eczema and severe frequent infections and/or autoimmune or malignant disease). Data for a total of 30 patients who contributed blood samples for study are described in supplemental table S1. The number of patient samples included in each analysis is indicated in the text and/or figures and legends.
The normal control population included healthy adults, primarily males in their third, or less frequently, fourth or fifth decade. Pediatric control blood samples consisted of ‘discarded materials’, i.e. portions of clinical samples remaining on completion of the assays for which they were drawn. These samples included blood samples submitted from children (WAS siblings and others) suspected to be WAS patients and subsequently diagnosed as ‘not suffering from WAS.’ Remainders of blood samples collected for clinical assays of immune markers at the Institute for Pediatric Hematology, Moscow, from normal children were also studied as paediatric control samples. Criteria for normal samples included the absence of known or suspected immune disease; criteria for all samples included absence of obvious current infection.
Monoclonal antibodies
Reagents used were: fluorescein-labelled monoclonal antibodies (MoAb) to CD5 (clone BL1a), CD23 (clone 9P25), CD21 (BL13), CD35 (J3D3), CD38 (T16), CD62L (DREG56), CD80 (MAB104) (Beckman Coulter, Brea, CA, USA) and CD72 (J4-117) (BD Biosciences, San Diego, CA, USA); phycoerythrin-labelled MoAb to CD10 (ALB1), CD19 (J4·119), CD22 (SJ10·1H11), CD40 (MAB89), CD95 (UB2) (Beckman Coulter), CD27 (M-T271) and CD138 (Mi15)(BD Biosciences); and phycoerythrin-cyanin 5 labelled MoAb to CD19, CD10, and CD45 (J33) (Beckman Coulter) and allophycocyanin labelled MoAb to CD45 and CD19 (Beckman Coulter). MoAb to IgG (clone G18-145) and IgD (IA6-2) (BD Biosciences) were used to stain isolated peripheral blood mononuclear cells (PBMCs).
Flow cytometric analysis
Whole blood for patient and control individuals was collected in acid-citrate-dextrose (NIH formula A) and processed immediately or after overnight shipment at ambient temperature. Comparable results were obtained when different FACS-Calibur instruments (Becton Dickinson, San Jose, CA, USA) were compared and when blood samples processed after overnight storage were compared to aliquots processed immediately after blood drawing. No significant differences were noted for blood samples drawn on multiple occasions for the same normal blood donors (n = 4) and patients (n = 3).
Blood cell subpopulations were quantified by differential analysis of whole blood using the Max-M (Coulter Corp, Hialeah, FL, USA). Leucocytes in whole blood were stained with three-colour antibody combinations by incubating 100 µl blood with saturating concentrations of MoAb for 15 min. All steps were done at approximately 22°C. To lyse erythrocytes, 900 µl of FACSTM Lysing Solution (BD Biosciences) was added, and the mixture was vortexed gently, and incubated for 15 min in the dark. The cells were diluted to 4 ml with phosphate-buffered saline (PBS) with 1% fetal calf serum (FCS), pelleted, and washed again with 4 ml PBS-FCS. The stained cells were suspended for analysis in 1·2% formaldehyde in PBS, stored at 4°C and analysed within 24 h.
PBMCs of patients and control individuals were surface stained for IgD, IgG, and CD27 as described above except for deletion of the erythrocyte lysis step. In some cases previously frozen patient and normal PBMCs were studied. No detectable difference was found for frozen and fresh cells from the same individual.
Stained cells were analysed using FACS Calibur and CellQuest software (Becton Dickinson). Data analysis was performed by gating on the lymphocyte population on a forward versus side scatter dot plot. The first gate was combined with a second gate around the CD19+ population. Differential values were used to calculate absolute cell concentration. All data are reported as mean ± SEM
Data analysis
Statistical differences were compared within age groups (infants, children, and adults); Student's t-test (unpaired) was used. For CD21– B-cell size analysis, forward light scatter intensity values for CD21– and CD21+ B-cells were compared (paired t-test). P-values less than 0·05 were considered to be statistically significant.
Results
Peripheral B cell deficit in WAS patients
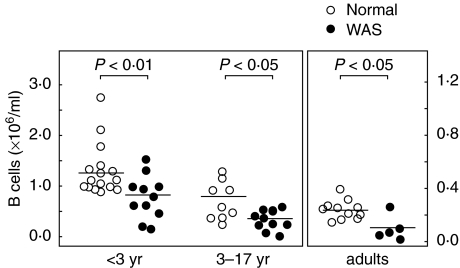
Immunophenotyping of peripheral blood cells of WAS patients (Table S1) showed a deficit of total B-cells (CD19+ cells) compared to normal healthy donors. Because B-cell counts change substantially during childhood [18], the comparisons were made in the context of age. The B-cell deficit was noted for patients within each age category (Fig. 1 and [15]). The number for normal healthy infants (< 36 months), 1·28 ± 0·12 × 106 B-cells/ml, was decreased to 0·78 ± 0·13 × 106/ml (P < 0·01) for infants with WAS. For children (3–17 years), the number for normals, 0·70 ± 0·13 × 106, was decreased to 0·33 ± 0·06 × 106 for patients (P < 0·05), and for adults, the normal number, 0·24 ± 0·02 × 106, was decreased to 0·09 ± 0·04 × 106/ml for patients (P < 0·05). Disparity of age distribution within categories was not a significant factor because age ranges were similar. Median ages for healthy versus patient groups, respectively, were 12 months (range 1–36 months) versus 10·5 months (range 1–25 months) for infants; 10 years (range 3–13) versus 12 years (range 4–16 years) for children, and 30 years (range 23–55 years) versus 41 years (range 21–54 years) for adults.
Fig. 1.
Peripheral blood B cell counts. Number of B cells (CD19+ cells) ( × 106/ml) in peripheral blood of normal healthy individuals (○) and WAS patients (•) grouped according to age: infants, 0–36 months; children, 3–17 years; and adults, 18–55 years. Bars indicate mean cell counts. Note that the scale for adults differs from that for infants and children. These data are a composite of previously published [15] and new values. The difference in B cell counts for patients versus normal was statistically significant (P < 0·05) within each age group (Student's unpaired t-test).
To test for phenotypic perturbations, we examined a broad panel of surface markers. For both patient and normal donors, the frequencies of B-cells expressing CD22, CD40, and CD72 were comparably high (greater than 98% for CD22 and CD40; greater than 85% for CD72; N.S) and did not show age dependent changes. The frequency of CD5 expressing B-cells showed a comparable age-dependent decrease for both patients and healthy donors (data not shown). The frequency of CD23+ B-cells was highly variable for both patients and healthy donors (N.S). The frequencies of B-cells expressing CD95 and CD80 were low and not significantly different for patients and healthy donors. In contrast, expression of CD21 and CD35 differed in patients and normal individuals.
Complement receptor positive B cells are reduced in patients
For CD21 (complement receptor 2, CR2), the overwhelming majority of healthy donor B-cells were positive, but a significant percent of patient B-cells were negative. The frequency of B-cells lacking CD21 was 7·3 ± 1·0% for healthy infants versus 19·8 ± 3·8% for WAS infants (P < 0·01); 7·2 ± 1·2% for healthy children versus 21·9 ± 4·2% for WAS children (P < 0·01); and 4·2 ± 0·6% CD21– for healthy adults versus 14·3 ± 3·0% for WAS adults (P < 0·05) (Fig. 2a). Despite the patients’ overall deficit of B-cells, their absolute number of CD21– B-cells was increased, although not significantly, compared to counterpart healthy donors in all three age categories (data not shown).
Fig. 2.
Increased frequency of complement receptor negative B cells in WAS patients. Frequency of CD21–cells (a) and CD35– cells (b) as a percent of total B cells (CD19 gated cells) for normal healthy individuals (○) and WAS patients (•). (c) Representative dot plots (from analysis of 11 patients) of CD35 and CD21 expression on B cells of a normal individual (an adult) and a WAS patient (12 years). (d) Correlation of CD21-negativity and CD35-negativity of B cells of 19 patients.
B-cells lacking CD35 (CR1) were also increased in patients (Fig. 2b). For healthy infants and infants with WAS, the frequency was 2·8 ± 0·7% versus 16·9 ± 5·8%, respectively (P < 0·05); 4·6 ± 0·6% versus 26 ± 4·4% for healthy children and WAS children, and 2·9 ± 0·6% versus 14·8 ± 0·6% for normal and patient adults, respectively. Analysis of 11 patients showed that the majority of B-cells lacking complement receptors were negative for both CD21 and CD35 (Fig. 2c). Data for 19 patient samples showed positive correlation (Pearson R = 0·77, P < 0·001) of CD21-negativity and CD35-negativity (Fig. 2d).
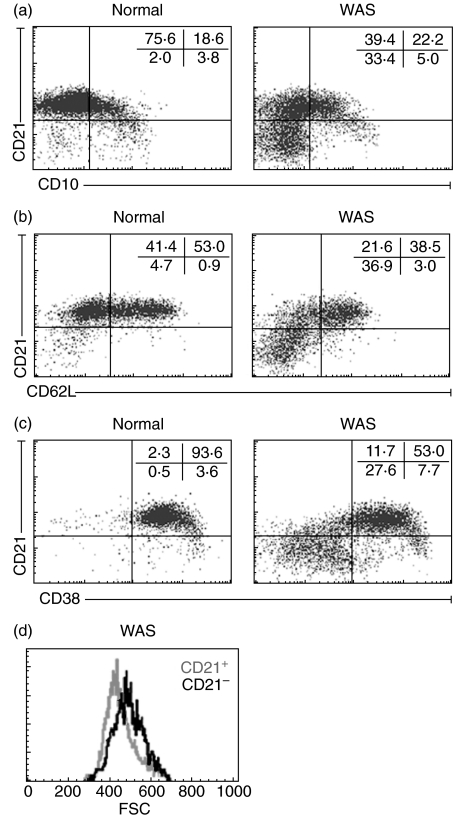
To characterize the CD21– patient B-cells, we measured CD10, a marker of immature B-cells primarily confined to bone marrow [19]. A small number of CD21–CD10bright cells were found in infant patients and were also present in normal infants (Fig. 3a, lower right quadrants). However, the vast majority of CD21– patient B cells are CD10– and hence unlikely to be immature B-cells (Fig. 3a). We also measured CD62L and CD38, markers on peripheral blood CD21– plasma cells [20]. Patient CD21– B-cells were CD62L– and CD38–, indicating that these are not peripheral plasma cells (Fig. 3b,c). Expression of CD138, another plasma cell marker, was also negligible (<1% of cells) (data not shown). Size analysis showed that CD21– patient B-cells are larger than CD21+ cells (Fig. 3d); mean forward light scatter intensity was 381 ± 21 for CD21– B-cells of 14 patients compared to 355 ± 21 for their CD21+ B cells (P < 0·01). The small number of CD21– B-cells in normal individuals are also larger than CD21+ B-cells (data not shown). Taken together, the phenotype of the CD21– B-cells in patients, CD35–CD10–CD62L–CD38–CD138– large cells, most closely resembles blast-like activated cells (CD21–CD35–CD62L– large cells) previously described for patients with HIV [21] (see Discussion).
Fig. 3.
Characterization of CD21– patient B cells. (a–c) Dot plots of gated B cells of a normal healthy individual (left, 7 months) and a WAS patient (right, 18 months) stained for CD21 and (a) CD10 (b) CD62L, and (c) CD38. The majority of patient CD21– B cells are negative for CD10, a marker of immature bone marrow B cells, and negative for CD62L and CD38, markers of blood plasma cells. (d) Representative histogram showing larger size of patient CD21– B cells compared to CD21+ B cells.
Immunoglobulin isotype switching occurs in patient B cells
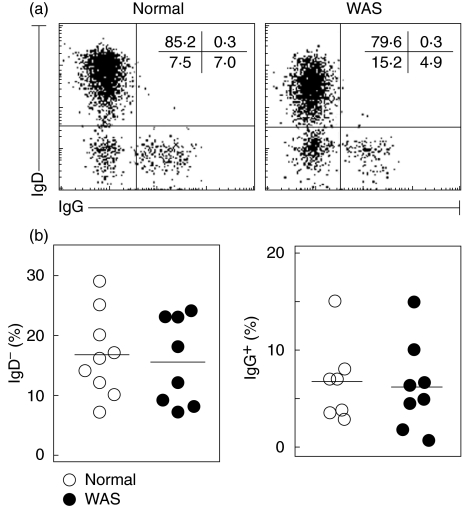
Since B-cell effector functions are defective in WAS patients, the frequency of immunoglobulin isotype switched B-cells was studied on PBMCs of patients 15 years of age and older. Surprisingly, the frequency of IgD– B-cells was not different for WAS patients (15·5 ± 2·5%) and healthy donors (16·7 ± 2·4%) (Fig. 4). The frequency of IgG+ B cells was also not different for WAS patients (6·2 ± 1·6%) and healthy donors (6·7 ± 1·6%) (Fig. 4). These findings strongly suggest that isotype switching occurs at normal frequency for WAS patients. However, due to the overall deficit of B-cells, the absolute number of isotype switched B-cells is decreased in patients.
Fig. 4.
Immunoglobulin isotype expression. Isolated PBMCs of normal healthy individuals and WAS patients, 15 years of age or older, were stained for IgD and IgG. (a) Representative dot plots gated for CD19+. (b) Frequencies of IgD– (left) and IgG+ (right) B cells.
Deficit of CD27+ B cells in patients
We also examined CD27, a marker of post germinal centre B-cells [22]. As anticipated, the frequency of CD27+ B-cells was low for infants – both for healthy infants and infant patients. For the healthy donor group, we noted the anticipated age-dependent increased frequency of CD27+ B-cells, from 7·0 ± 0·8% for infants to 23·6 ± 3·2% for adults (Fig. 5a). For patients, the frequency of CD27+ cells, which was 5·5 ± 1·1% for infants, virtually failed to increase in the older groups (Fig. 5a). As a result, the frequency of CD27+ B-cells, which was 20·0 ± 2·1% for healthy children, was 7·2 ± 1·2% for WAS children (P < 0·01). The difference was not due to age discrepancy since the median age for WAS children (12 years) was greater than that of healthy children (8·5 years). For adults, the frequency of CD27+ B-cells was 23·6 ± 3·2% for normal healthy donors and 6·8 ± 2·1% for adults with WAS (P < 0·01).
Fig. 5.
Deficit of CD27+ postgerminal centre B cells in WAS patients. (a) Frequency of CD27+ B cells of normal healthy individuals (○) and WAS patients (•). Whole blood staining results are shown. Note the age-dependent increase in CD27+ B cells for the normal healthy group but not for WAS patients. (b,c) Expression of CD27 as a function of surface IgD and IgG on PBMCs of normal healthy individuals and WAS patients 15 years and older. (b) Representative dot plots of CD19+ gated cells stained for CD27 and IgD. (c) Decreased frequencies of CD27+ B cells within IgD– and IgG+ B cell subsets. CD27+ B cells were also significantly decreased within the IgD+ population (15 ± 2·5% for normal donors versus 6·6 ± 2·3% for patients; data not shown).
The deficit of CD27+ postgerminal centre B-cells in patients was somewhat surprising in light of the normal frequency of IgG+ and IgD– cells. We further investigated the frequency of CD27+ B-cells within IgG+ and IgD– B cells. Isolated PBMC of patients 15 years and older were used. As previously reported [23], CD27 was expressed on the majority (80 ± 2·2%) of IgD– cells and the majority (74 ± 2·2%) of IgG+ B-cells of healthy donors (Fig. 5b,c). In contrast, patients showed a significantly (P < 0·01) decreased frequency of CD27+ within IgD– (50 ± 5·4%) and IgG+ (50 ± 5%) populations (Fig. 5b,c). Thus, the frequency of CD27+ post germinal centre B-cells was significantly decreased in WAS patients even within isotype-switched populations.
Increased frequency of CD10+ B cells in adult patients
Staining for CD10 revealed an increased frequency of CD10+ B-cells in adult patients, 33·5 ± 5·2%, compared to 11·6 ± 2·5% in healthy adults (P < 0·05) (Fig. 6a). Characterization of these cells for three adult patients showed that they are CD38bright, CD27– (Fig. 6b), IgD+, and CD21+ (not shown). The phenotype of CD10+ CD38bright CD27– B-cells was previously described for circulating germinal centre progenitor cells [24].
Fig. 6.
Frequency and characteristics of CD10+ B cells in adult WAS patients. (a) Frequency of CD10+ B cells of normal individuals (○) and WAS patients (•). The frequency of these cells is significantly increased in adult patients. (b) Representative dot plots of B cells of a normal healthy adult (left, 40 years old) and an adult patient (right, 40 years old) stained for CD27, CD38, and CD10. CD10− cells are shown as red dot events. CD10+ cells, shown as blue dot events, are seen as CD27– and CD38bright. The near absence of CD27+ B cells is conspicuous in the patient. Similar results were obtained for two additional comparisons of adult patients and normal healthy adults.
Discussion
The findings of this cross-sectional study reveal previously unappreciated phenotypic perturbations of B cells in patients with the Wiskott–Aldrich syndrome. The findings are representative of the disease since the patient blood specimens that were studied were randomly selected (all samples received over a 3-years period) and the affected boys and men have 25 different WASP mutations (Table S1). Representation of mild and severe phenotype patients was approximately equal. The finding that total B-cell number is decreased even in infant patients suggests that export from the bone marrow and/or peripheral survival of B-cells is decreased. The additional decreased frequency of B-cells expressing complement receptors CD21 and CD35 may lead to impaired B-cell capacity for capture and presentation of antigens. In contrast to age-matched healthy children and adults, patients with WAS showed significant deficit of CD27+ postgerminal centre B-cells, consistent with impaired differentiation into post germinal centre cells. Despite the deficit of CD27+ B-cells, the frequency (although not the number) of isotype switched cells was normal.
CD21– B-cells, a small population in healthy donors, were increased as a percent of B-cells in patients, confirming an earlier report [25]. The increased frequency of CD21– B-cells was noted in all age categories including infants, indicating that this perturbation is not secondary to maturation/ageing process. The vast majority of patient CD21– B-cells were CD10– and thus unlikely to be immature bone marrow B-cells and also unlikely to be transitional B-cells, which in humans are positive for both CD62L and CD21 [26]. CD21– B-cells from both patients and normal healthy donors are larger than CD21+ cells, suggesting that CD21– B-cells in patients are a normal cell population at abnormally increased frequency. Patient CD21– B-cells were negative for plasma cell markers CD62L, CD38 and CD138. B-cells with a similar phenotype, CD21–CD35– CD62L– large blast-like ‘plasmacytoid’ cells, were described in HIV infection, and when isolated from HIV infected patients, produced increased amounts of IgG [21,27]. Hence, CD21– B-cells of WAS patients, if they share this higher capacity for IgG synthesis, might explain the patients’ normal serum IgG levels despite their deficit of B-cells.
The frequency of CD35– B-cells was also increased in patients, and the majority of complement receptor negative B cells were negative for both CD21 and CD35. These two receptors have important functional roles in humoral immunity. They have been shown to augment signal transduction by BCR [28–30]. CD21 serves as complement binding subunit of the CD19/CD21 signalling complex [31]. In mice, CD21 and CD35, which are alternatively spliced products of a single gene Cr2 [32], are essential for T-dependent antibody response to low dose antigenic challenge [33] and for survival of germinal centre B-cells [34]. The decreased density of CD21/CD35 expressing B-cells resulting from decreased frequency and deficit of total B-cells suggests a suboptimal B cell capacity to capture and present opsonized antigens, particularly when antigen is limiting. Of note, both WAS patients and individuals with complement deficiencies share defective antibody responses to φx174 [35,36]. Furthermore, the deficit of CD21/35-expressing B-cells may contribute to the failure of WAS patients to generate CD27+ B-cells.
The deficit of CD21/CD35 expressing B-cells may also predispose the patients to autoimmunity, a major clinical problem in the WAS. As many as 40% of WAS patients develop autoimmune disorders including haemolytic anaemia, arthritis, and renal disease [37]. Currently, several lines of evidence suggest that the down regulation of complement receptors plays a role in the breakdown of tolerance and the development of autoimmunity. Patients with systemic lupus erythematosus have decreased expression of CD21 and CD35 on B-cells [38,39], and a deficit of B-cells expressing CD35 has been reported for patients with arthritis [40]. In murine lupus models, decreased CR2 expression was observed before the onset of nephritis [41], and the lack of Cr2 led to an exacerbated lupus activity [42]. Importantly, self-reactive murine B-cells deficient in CD21/CD35 were not anergized by self antigen [42], suggesting a role for complement in B-cell tolerance.
We also investigated two parameters of B-cells differentiation: immunoglobulin isotype switching, which occurred at normal frequency for patient B-cells, and expression of CD27, which was substantially decreased. CD27 expression has been defined as a marker of post germinal centre B-cells with somatic hypermutation [22,43] and thus the deficit of CD27+ B-cells in patients suggests a deficit of somatically mutated post germinal centre cells. The deficit of CD27+ B-cells is consistent with the deficient response of patients to immunization [3,4]. Although not directly measured in this study, the overall deficit of CD27+ B-cells strongly suggest that the patients will be deficient in IgM+/CD27+ memory B-cells which are thought to be important for protection against encapsulated bacteria [44]. A deficit of IgM+/CD27+ memory B cells in WAS patients would be in accordance with the deficient response to polysaccharide antigens characteristic of the disease (reviewed in [3,5]). The discordance in the patients between normal frequency of isotype switching and decreased CD27+ expression suggests that (some) isotype switched B-cells develop in WAS patients independent of germinal centre maturation.
The deficit of CD27+ B-cells is consistent also with histomorphometric findings for patient secondary lymphoid organs. Cellular architectural defects identified in germinal centres ranged from a lack of defined structure [45] to a gross morphological defect characterized as ‘burnt out’ morphology [46]. The reduction of marginal zone thickness was also reported in spleen sections [46]. Since marginal zone B-cells not only include cells responsible for antibody responses, but also somatically mutated CD27+ B-cells [47], the histological studies are in agreement with the deficit of CD27+ B-cells in patient blood. The increased frequency in adult patients of circulating CD10+ CD38bright CD27– B-cells that resemble germinal centre progenitor B-cells [24] may reflect the failure of germinal centre reactions or aberrant migration of patient B-cells due to underlying cytoskeletal defect.
It is unlikely that the aberrant phenotypes reported here are the result of splenectomy. Two non-WAS splenectomized individuals did not show the aberrant phenotypes reported for WAS patients (data not shown). In addition, 10 of the 13 infants and many of the children were eusplenic at the time of specimen collection (Table S1). We also analysed frozen PBMCs of an adult patient prepared before and four years after splenectomy, and aberrant phenotypes were detected in both specimens. Although the number of patient samples analysed (30) was large for this relatively rare disease, attempts to correlate B cell findings with clinical phenotype of the patients did not reveal significant differences. This lack of correlation may simply reflect the small number of patients per group resulting from the need to consider patient data within subcategories based on age. It is thus possible that some of the B-cell perturbations reported here are more extreme in severe phenotype patients.
The present findings contribute by revealing consequences of WASP absence/defects in B-cells of patients, but they also suggest a need for further studies. It would be important, e.g. to more precisely define the identity and function of the perturbed B-cell populations and the mechanism of their generation. Such studies may be possible using the WASP gene deletion mouse models [9,13] assuming that comparable phenotypic perturbations will be found in this species.
Overall, this cross-sectional study demonstrates that decreased numbers of circulating B-cells is a feature of the WAS found already in infant patients. In addition, patients have phenotypic B-cell perturbations that involve complement receptors and CD27 as a marker. The severe deficit of CD27+ post germinal centre cells is consistent with the deficient antibody responses of patients to immunization. These cumulative perturbations strongly suggest that defects of B cells contribute (along with defects of T cells) to the impaired humoral immunity that characterizes this disease.
Acknowledgments
This work was supported by grants from the National Institutes of Health (HL59561, AI39574), and the Jeffrey Modell Foundation. We thank Mikayla Kob, F. Morgan Smyrl and Tatiana Radigina for flow cytometric measurements, Dr Dianne Kenney for encouragement in initiating the study, and Drs Klaus Rajewsky and Robert Barrington for critical manuscript review. We are grateful to the patients and families and control blood donors for their cooperation.
References
- 1.Wiskott A. Familiarer, angeobren Morbus Werlhofi? Monatschrift Kinderheil. 1937;68:212–6. [Google Scholar]
- 2.Aldrich R, Steinberg A, Campbell D. Pedigree demonstrating a sex-linked recessive condition characterized by draining ears, eczematoid dermatitis and bloody diarrhea. Pediatrics. 1954;13:133–8. [PubMed] [Google Scholar]
- 3.Sullivan KE. Recent advances in our understanding of Wiskott–Aldrich syndrome. Curr Opin Hematol. 1999;6:8–14. doi: 10.1097/00062752-199901000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Remold-O’Donnell E, Rosen FS, Kenney DM. Defects in Wiskott–Aldrich syndrome blood cells. Blood. 1996;87:2621–31. [PubMed] [Google Scholar]
- 5.Ochs HD, Rosen FS. Primary Immunodeficiency Diseases. New York: Oxford University Press; 1999. [Google Scholar]
- 6.Thrasher AJ. WASp in immune-system organization and function. Nat Rev Immunol. 2002;2:635–46. doi: 10.1038/nri884. [DOI] [PubMed] [Google Scholar]
- 7.Molina IJ, Sancho J, Terhorst C, Rosen FS, Remold-O’Donnell E. T cells of patients with the Wiskott–Aldrich syndrome have a restricted defect in proliferative responses. J Immunol. 1993;151:4383–90. [PubMed] [Google Scholar]
- 8.Badour K, Zhang J, Shi F, McGavin MK, Rampersad V, Hardy LA, Field D, Siminovitch KA. The Wiskott–Aldrich syndrome protein acts downstream of CD2 and the CD2AP and PSTPIP1 adaptors to promote formation of the immunological synapse. Immunity. 2003;18:141–54. doi: 10.1016/s1074-7613(02)00516-2. [DOI] [PubMed] [Google Scholar]
- 9.Snapper SB, Rosen FS, Mizoguchi E, et al. Wiskott–Aldrich syndrome protein × deficient mice reveal a role for WASP in T but not B cell activation. Immunity. 1998;9:81–91. doi: 10.1016/s1074-7613(00)80590-7. [DOI] [PubMed] [Google Scholar]
- 10.Simon HU, Mills GB, Hashimoto S, Siminovitch KA. Evidence for defective transmembrane signaling in B cells from patients with Wiskott–Aldrich syndrome. J Clin Invest. 1992;90:1396–405. doi: 10.1172/JCI116006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henriquez NV, Rijkers GT, Zegers BJ. Antigen receptor-mediated transmembrane signaling in Wiskott–Aldrich syndrome. J Immunol. 1994;153:395–9. [PubMed] [Google Scholar]
- 12.Facchetti F, Blanzuoli L, Vermi W, et al. Defective actin polymerization in EBV-transformed B-cell lines from patients with the Wiskott–Aldrich syndrome. J Pathol. 1998;185:99–107. doi: 10.1002/(SICI)1096-9896(199805)185:1<99::AID-PATH48>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 13.Zhang J, Shehabeldin A, da Cruz LA, et al. Antigen receptor-induced activation and cytoskeletal rearrangement are impaired in Wiskott–Aldrich syndrome protein × deficient lymphocytes. J Exp Med. 1999;190:1329–42. doi: 10.1084/jem.190.9.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Westerberg L, Greicius G, Snapper SB, Aspenstrom P, Severinson E. Cdc42, Rac1, and the Wiskott–Aldrich syndrome protein are involved in the cytoskeletal regulation of B lymphocytes. Blood. 2001;98:1086–94. doi: 10.1182/blood.v98.4.1086. [DOI] [PubMed] [Google Scholar]
- 15.Park J, Kob M, Prodeus AP, Rosen FS, Shcherbina A, Remold-O’Donnell E. Early deficit of lymphocytes in Wiskott–Aldrich syndrome: Possible role of WASP in human lymphocyte maturation. Clin Exp Immunol. 2004;136:104–10. doi: 10.1111/j.1365-2249.2004.02409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shcherbina A, Rosen FS, Remold-O’Donnell E. WASP levels in platelets and lymphocytes of wiskott–aldrich syndrome patients correlate with cell dysfunction. J Immunol. 1999;163:6314–20. [PubMed] [Google Scholar]
- 17.Conley ME, Notarangelo LD, Etzioni A. Diagnostic criteria for primary immunodeficiencies. Representing PAGID (Pan-American Group for Immunodeficiency) and ESID (European Society for Immunodeficiencies) Clin Immunol. 1999;93:190–7. doi: 10.1006/clim.1999.4799. [DOI] [PubMed] [Google Scholar]
- 18.Comans-Bitter WM, de Groot R, van den Beemd R, Neijens HJ, Hop WC, Groeneveld K, Hooijkaas H, van Dongen JJ. Immunophenotyping of blood lymphocytes in childhood. Reference values for lymphocyte subpopulations. J Pediatr. 1997;130:388–93. doi: 10.1016/s0022-3476(97)70200-2. [DOI] [PubMed] [Google Scholar]
- 19.Uckun FM, Kersey JH, Vallera DA, Ledbetter JA, Weisdorf D, Myers DE, Haake R, Ramsay NK. Autologous bone marrow transplantation in high-risk remission T-lineage acute lymphoblastic leukemia using immunotoxins plus 4-hydroperoxycyclophosphamide for marrow purging. Blood. 1990;76:1723–33. [PubMed] [Google Scholar]
- 20.Medina F, Segundo C, Campos-Caro A, Gonzalez-Garcia I, Brieva JA. The heterogeneity shown by human plasma cells from tonsil, blood, and bone marrow reveals graded stages of increasing maturity, but local profiles of adhesion molecule expression. Blood. 2002;99:2154–61. doi: 10.1182/blood.v99.6.2154. [DOI] [PubMed] [Google Scholar]
- 21.Moir S, Malaspina A, Ogwaro KM, et al. HIV-1 induces phenotypic and functional perturbations of B cells in chronically infected individuals. Proc Natl Acad Sci USA. 2001;98:10362–7. doi: 10.1073/pnas.181347898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klein U, Rajewsky K, Kuppers R. Human immunoglobulin (Ig) M+IgD+ peripheral blood B cells expressing the CD27 cell surface antigen carry somatically mutated variable region genes: CD27 as a general marker for somatically mutated (memory) B cells. J Exp Med. 1998;188:1679–89. doi: 10.1084/jem.188.9.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agematsu K, Nagumo H, Yang FC, et al. B cell subpopulations separated by CD27 and crucial collaboration of CD27+ B cells and helper T cells in immunoglobulin production. Eur J Immunol. 1997;27:2073–9. doi: 10.1002/eji.1830270835. [DOI] [PubMed] [Google Scholar]
- 24.Bohnhorst JO, Bjorgan MB, Thoen JE, Natvig JB, Thompson KM. Bm1-Bm5 classification of peripheral blood B cells reveals circulating germinal center founder cells in healthy individuals and disturbance in the B cell subpopulations in patients with primary Sjogren's syndrome. J Immunol. 2001;167:3610–8. doi: 10.4049/jimmunol.167.7.3610. [DOI] [PubMed] [Google Scholar]
- 25.Morio T, Takase K, Okawa H, et al. The increase of non-MHC-restricted cytotoxic cells (gamma/delta-TCR-bearing T cells or NK cells) and the abnormal differentiation of B cells in Wiskott–Aldrich syndrome. Clin Immunol Immunopathol. 1989;52:279–90. doi: 10.1016/0090-1229(89)90179-7. [DOI] [PubMed] [Google Scholar]
- 26.Carsetti R, Rosado MM, Wardmann H. Peripheral development of B cells in mouse and man. Immunol Rev. 2004;197:179–91. doi: 10.1111/j.0105-2896.2004.0109.x. [DOI] [PubMed] [Google Scholar]
- 27.Rodriguez C, Thomas JK, O'Rourke S, Stiehm ER, Plaeger S. HIV disease in children is associated with a selective decrease in CD23+ and CD62L+ B cells. Clin Immunol Immunopathol. 1996;81:191–9. doi: 10.1006/clin.1996.0176. [DOI] [PubMed] [Google Scholar]
- 28.Boackle SA, Morris MA, Holers VM, Karp DR. Complement opsonization is required for presentation of immune complexes by resting peripheral blood B cells. J Immunol. 1998;161:6537–43. [PubMed] [Google Scholar]
- 29.Arvieux J, Yssel H, Colomb MG. Antigen-bound C3b and C4b enhance antigen-presenting cell function in activation of human T-cell clones. Immunology. 1988;65:229–35. [PMC free article] [PubMed] [Google Scholar]
- 30.Weiss L, Delfraissy JF, Vazquez A, Wallon C, Galanaud P, Kazatchkine MD. Monoclonal antibodies to the human C3b/C4b receptor (CR1) enhance specific B cell differentiation. J Immunol. 1987;138:2988–93. [PubMed] [Google Scholar]
- 31.Fearon DT, Carroll MC. Regulation of B lymphocyte responses to foreign and self-antigens by the CD19/CD21 complex. Annu Rev Immunol. 2000;18:393–422. doi: 10.1146/annurev.immunol.18.1.393. [DOI] [PubMed] [Google Scholar]
- 32.Kurtz CB, O'Toole E, Christensen SM, Weis JH. The murine complement receptor gene family. IV. Alternative splicing of Cr2 gene transcripts predicts two distinct gene products that share homologous domains with both human CR2 and CR1. J Immunol. 1990;144:3581–91. [PubMed] [Google Scholar]
- 33.Ahearn JM, Fischer MB, Croix D, et al. Disruption of the Cr2 locus results in a reduction in B-1a cells and in an impaired B cell response to T-dependent antigen. Immunity. 1996;4:251–62. doi: 10.1016/s1074-7613(00)80433-1. [DOI] [PubMed] [Google Scholar]
- 34.Fischer MB, Ma M, Goerg S, et al. Regulation of the B cell response to T-dependent antigens by classical pathway complement. J Immunol. 1996;157:549–56. [PubMed] [Google Scholar]
- 35.Ochs HD, Slichter SJ, Harker LA, Von Behrens WE, Clark RA, Wedgwood RJ. The Wiskott–Aldrich syndrome: studies of lymphocytes, granulocytes, and platelets. Blood. 1980;55:243–52. [PubMed] [Google Scholar]
- 36.Jackson CG, Ochs HD, Wedgwood RJ. Immune response of a patient with deficiency of the fourth component of complement and systemic lupus erythematosus. N Engl J Med. 1979;300:1124–9. doi: 10.1056/NEJM197905173002002. [DOI] [PubMed] [Google Scholar]
- 37.Schurman SH, Candotti F. Autoimmunity in Wiskott–Aldrich syndrome. Curr Opin Rheumatol. 2003;15:446–53. doi: 10.1097/00002281-200307000-00012. [DOI] [PubMed] [Google Scholar]
- 38.Marquart HV, Svendsen A, Rasmussen JM, Nielsen CH, Junker P, Svehag SE, Leslie RG. Complement receptor expression and activation of the complement cascade on B lymphocytes from patients with systemic lupus erythematosus (SLE) Clin Exp Immunol. 1995;101:60–5. doi: 10.1111/j.1365-2249.1995.tb02277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilson JG, Ratnoff WD, Schur PH, Fearon DT. Decreased expression of the C3b/C4b receptor (CR1) and the C3d receptor (CR2) on B lymphocytes and of CR1 on neutrophils of patients with systemic lupus erythematosus. Arthritis Rheum. 1986;29:739–47. doi: 10.1002/art.1780290606. [DOI] [PubMed] [Google Scholar]
- 40.Munson LG, Scott ME, Landay AL, Spear GT. Decreased levels of complement receptor 1 (CD35) on B lymphocytes in persons with HIV infection. Clin Immunol Immunopathol. 1995;75:20–5. doi: 10.1006/clin.1995.1047. [DOI] [PubMed] [Google Scholar]
- 41.Takahashi K, Kozono Y, Waldschmidt TJ, Berthiaume D, Quigg RJ, Baron A, Holers VM. Mouse complement receptors type 1 (CR1; CD35) and type 2 (CR2; CD21): expression on normal B cell subpopulations and decreased levels during the development of autoimmunity in MRL/lpr mice. J Immunol. 1997;159:1557–69. [PubMed] [Google Scholar]
- 42.Prodeus AP, Goerg S, Shen LM, Pozdnyakova OO, Chu L, Alicot EM, Goodnow CC, Carroll MC. A critical role for complement in maintenance of self-tolerance. Immunity. 1998;9:721–31. doi: 10.1016/s1074-7613(00)80669-x. [DOI] [PubMed] [Google Scholar]
- 43.Agematsu K, Hokibara S, Nagumo H, Komiyama A. CD27: a memory B-cell marker. Immunol Today. 2000;21:204–6. doi: 10.1016/s0167-5699(00)01605-4. [DOI] [PubMed] [Google Scholar]
- 44.Kruetzmann S, Rosado MM, Weber H, et al. Human immunoglobulin M memory B cells controlling Streptococcus pneumoniae infections are generated in the spleen. J Exp Med. 2003;197:939–45. doi: 10.1084/jem.20022020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gerwin N, Friedrich C, Perez-Atayde A, Rosen FS, Gutierrez-Ramos JC. Multiple antigens are altered on T and B lymphocytes from peripheral blood and spleen of patients with Wiskott–Aldrich syndrome. Clin Exp Immunol. 1996;106:208–17. doi: 10.1046/j.1365-2249.1996.d01-853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vermi W, Blanzuoli L, Kraus MD, et al. The spleen in the Wiskott–Aldrich syndrome: histopathologic abnormalities of the white pulp correlate with the clinical phenotype of the disease. Am J Surg Pathol. 1999;23:182–91. doi: 10.1097/00000478-199902000-00007. [DOI] [PubMed] [Google Scholar]
- 47.Spencer J, Perry ME, Dunn-Walters DK. Human marginal-zone B cells. Immunol Today. 1998;19:421–6. doi: 10.1016/s0167-5699(98)01308-5. [DOI] [PubMed] [Google Scholar]