Abstract
Excessive Th1 cell function is importantly involved in the pathogenesis of Behcet's disease (BD). We previously found that Txk, a member of the Tec family of tyrosine kinases, acts as a Th1 cell specific transcription factor. To investigate immune aberration in the pathogenesis of BD, we studied the expression of Txk and Th1 cytokines in peripheral blood lymphocytes (PBL) and skin lesions in patients with BD. Cytokine production by the lymphocytes was assessed using ELISA. PBL produced excessive Th1 associated cytokines including IFN-γ and IL-12 spontaneously and in response to exogenous HSP60-derived peptide stimulation, which was shown to induce proliferation of PBL, in patients with BD. Circulating CD4+ T cells expressed excessive Txk protein. A majority of cells infiltrating into skin lesions expressed IFN-γ in the BD specimens. IL-12 and IL-18 were also expressed in the mononuclear cell aggregates. Lymphocytes accumulating in the skin lesion expressed higher levels of Txk as compared with atopic dermatitis lesions, a typical Th2 disease. IFN-γ, IL-18 and Il-12 were detected in the BD skin lesions, which may induce preferential development of Th1 cells in patients with BD. The mononuclear cell aggregates contained Txk expressing cells in such skin lesions. Collectively, Txk expressing Th1 cells and the Th1 associated cytokines may play a critical role in the development of skin lesions in BD.
Keywords: Txk, tyrosine kinases, Th1 cytokine production, Behcet's disease, T lymphocytes
Introduction
Behcet's disease (BD) is a multi systemic inflammatory disease and is characterized by recurrent attacks of uveitis, oral aphtha, genital ulcers and erythema nodosum [1]. The aetiology and pathogenesis of this disease are unknown. While genetic factors are thought to play an important role in the pathogenesis of this disease [2–4], environmental factors are also involved [5,6]. Previous reports suggested that immunological disorders were importantly involved in its pathogenesis [1,2]. T lymphocytes from patients with BD had a distinctly lower threshold for interferon (IFN)-γ production than lymphocytes from patients with rheumatoid arthritis and from healthy control subjects when stimulated with a bacterial superantigen [7]. Serum IFN-γ was elevated in patients with BD [8]. Patients with active BD had significantly more IFN-γ producing CD4+ cells than did inactive case subjects and control subjects [9]. Serum IL-12 level and disease activity were correlated in the disease [10]. These results suggest that Th1 cells may play an important role in the immunopathogenesis of BD [11,12], although others have reported that the cytokine production profile has a mixed Th1/Th2 cell type in active BD [13]. More recently, BD was found to associate with an up-regulation of T-bet expression, a Th1 cell specific transcription factor in T cells [14]. We have reported that Txk, a member of Tec family nonreceptor tyrosine kinase is expressed on Th1/Th0 cells and Txk regulates specifically IFN-γ gene expression. Txk bound to the specific site of human IFN-γ gene promotor and acts as a Th1 cell specific transcription factor [15,16].
Human heat shock protein (HSP) is an unique antigen with a potent immunostimulatory property [17]. HSP is involved in the pathogenesis of autoimmune diseases [18]. It has been shown that human 60-kD HSP are involved in the development of BD [19–21]. Selected peptides derived from the sequences of human HSP60 induced significant proliferation of T cells in patients with BD. Especially the peptide (336–351) of human HSP60 induced proliferation of CD4+ T cells by an antigen-specific fashion [20]. The HSP peptide-stimulated PBMC expressed mRNA of proinflammatory cytokines in patients with BD [20].
To further clarify the involvement of cytokines and HSP60 in the Th1 cell dominant expansion in patients with BD, we investigated immunohistochemically whether Th1 cell associated cytokines were produced in skin lesions of patients with BD.
Patients and methods
Patients
We studied totally 35 patients with BD. The mean age (± SD) of these patients was 36·1 ± 9·4 years. (range 25–56 years). Patients fulfilled the diagnostic criteria proposed by both the BD Research Committee of Japan and the International Study Group of BD [22,23]. 30 healthy volunteer blood donors served as control subjects. Their mean age (± SD) was 37·2 ± 6· 5years (range 26–53 years). None of the patients have been treated with intermediate ∼ high dose corticosteroid therapy (more than 10 mg prednison/day). We also excluded those who had ciclosporin and other immunosuppressive agents from the patient group. Active phase of BD was defined as follows. One of the following symptoms was found including uveitis, subcutaneous venous thrombosis, skin lesion like erythema nodosum, genital ulcers, arthralgia, intestinal ulceration, progressive central nervous system lesions, progressive vasculitis and epididymitis. Inflammatory findings are also evident from clinical examination and/or clinical laboratory findings (serum CRP, ESR, findings in cerebral fluid, findings by colonic fiberscopy and others) [24]. Human studies’ committee's approval and individual informed consent from each patient were obtained before we conducted the present study. Specimens were obtained by skin biopsy of erythema nodosum (4 patients) and a genital ulcer (1 patient) in total 5 patients with BD. Because atopic dermatitis (AD) is a well known Th2 disease, skin biopsy specimens of patients with AD served as a control disease.
Cell separation and cell cultures
PBL were obtained by Ficoll-Hypaque centrifugation of heparinized blood from normal healthy donors and patients with BD [25,26]. Freshly isolated PBL were suspended in RPMI1640 medium containing 10% FCS, Penicillin and Streptomycin (Sigma–Aldrich, St. Louis, MO, USA). T lymphocytes were separated by rosette formation with neuraminidase treated SRBC, followed by Ficoll-Hypaque centrifugation. CD3+ T cells were further purified by using anti-CD16 mAb and autoMACS (Miltenyi Biotec GmbH, Gladbach, Germany). CD4+ T cells were similarly purified by using anti-CD8 and anti-CD16 mAbs.
PBL and T cell subpopulations were cultured with a synthetic peptide derived from sequence of human 60 kD HSP 336–351 (final concentration; 10 µg/ml) and a control peptide derived from the sequence of the Mycobacterium bovis 65-kD HSP 91–105 for 24 h. It has been reported that among a panel of the synthetic peptides derived from human HSP60 sequence, the peptide336–351 at this concentration induced potent proliferation of PBL in patients with BD in Japan [20]. The peptide91–105 did not provoke lymphocyte proliferation in normal individuals and in patients with BD in Japan [20], thus the peptide was used as a negative control peptide. The culture supernatants were recovered for estimation of cytokine production. Cytokine production by the lymphocytes was assayed by using ELISA kits. Human IFN-γ ELISA Kit (R & D Systems Inc., Minneapolis, MN, USA), Human IL-4 ELISA kit (R & D Systems Inc.), Human TNF-α ELISA Kit (R & D Systems Inc.), Human IL-10 ELISA kit (R & D Systems Inc.) and Human TGF-β1 ELISA kit (R & D Systems Inc.) were purchased.
SDS-PAGE and immunoblotting analysis
1 × 107 PBL were lysed in 100 µl Nonidet P40 lysis buffer containing protease inhibitors (1 mg/ml PMSF, 5 mm EDTA, 2 mg/ml aprotinin, and 2 mg/ml leupeptin) and protein concentration was measured [25,26]. Equal amounts of the cell lysates were electrophoresed on 4–20% SDS-PAGE gels. Proteins were electro-transferred to polyvinylidene difluoride membranes (Millipore, Beetford, MA, USA). The membrane was probed with anti-human Txk antibody, followed by incubation with biotin-labelled anti-goat IgG antibody and streptavidin-alkaline phosphatase. Visualization was carried out by chemiluminescence (Amersham, Tokyo, Japan). The intensity of the detected Txk bands was measured with gel plotting macros in NIH image 1·55 software, and was expressed as a relative intensity compared with that of actin bands (a control of the equal protein roading of different samples) of the same blot.
Immunohistochemical staining
Sections (5 µm) from frozen skin specimen were placed on poly L-lysine coated slides (Sigma Chemical Co., St. Louis, MO, USA) and fixed in cold acetone for 15 min at room temperature. Endogenous peroxidase was quenched in hydrogen peroxide. The tissue sections were incubated with BSA for blocking purposes. The first antibody and control mouse Ig were applied to the tissues and incubated over night at 4 °C. The tissue was washed three times with PBS. Visualization was carried out using a streptavidin-biotin complex immunoperoxidase system (DAKO Japan Co., Tokyo, Japan) according to the manufacturer's recommendation. 3-amino-9-ethylcarbazole was used as a chromogen and counterstained with haematoxylin.
Antibodies
Anti-TNF-α mAb, anti-IL-12 mAb and anti-IL-18 mAb were obtained from R & D systems (Minneapolis, MN, USA). Anti-IL-4 mAb and anti-IFN-γ mAb were obtained from Genzyme diagnostics (Cambridge, MA, USA). Anti-HSP60 mAb were obtained from Affinity BioReagents, Inc. (Golden, CO, USA). Goat anti-Txk Ab was from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Statistical analysis
Results of irregular distribution in the experiments were compared by Mann–Whitney U-test. A P-value less than 0·05 was judged to be statistically significant.
Results
Cytokine production of PBL in patients with BD
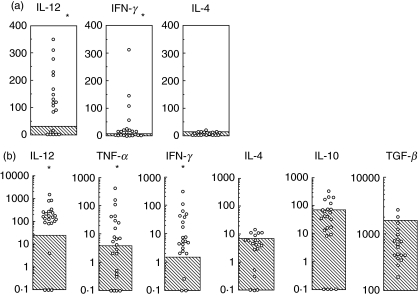
We first studied spontaneous cytokine production by freshly isolated PBL in patients with BD (Fig. 1a). We found that the PBL produced IFN-γ and IL-12 excessively in patients with BD. IL-4 production in patients with BD was almost comparable with that in normal individuals.
Fig. 1.
Cytokine production by PBL in patients with BD. (a) PBL recovered from patients with BD were cultured in culture media without mitogen for 24 h. Culture supernatants were used to measure the cytokine concentration (pg/ml) by using ELISA. Shaded area represents mean ± 3SD of 30 normal individuals. (b) PBL was stimulated with the HSP 336–351 peptide (10 µg/ml) and subsequent cytokine production (pg/ml) was assessed. Shaded area represents mean ± 3SD of 30 normal individuals. *P < 0·01 by Mann-Whitney U-test.
It has been shown that T cells from patients with BD responded to the selected peptides derived from human HSP [19]. Among the peptides studied so far, the peptide336–351 induced lymphocyte proliferation most potently in patients with BD [19–21] and administration of the peptide 336–351 induced uveitis in the rats [27]. We therefore studied the cytokine production by PBL stimulated with HSP derived peptide 336–351 (Fig. 1b). We found that the HSP peptide reactive PBL in patients with BD produced much more IFN-γ than normal PBL did. IL-4 production was almost comparable between patients with BD and normal individuals in response to the HSP peptide stimulation; less than 10–20 pg/ml. Control peptide stimulation induced only minimal cytokine production both in patients with BD and in normal individuals (data not shown). Furthermore, we did not find any significant association between disease activity and Th1 associated cytokine production in patients with BD. These results suggested that HSP reactive T cells predominantly produce IFN-γ and a majority of them can be categorized as Th1 cells in patients with BD.
Txk expression of peripheral blood T cells in patients with BD
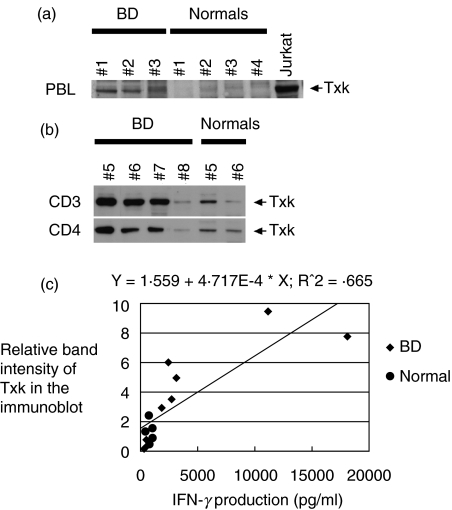
Txk is expressed in Th1 cells [15,28] and up regulates IFN-γ gene transcription [15,16]. We next studied the expression of Txk, a Th1 specific transcription factor. Txk expression of PBL was first assessed by immunoblotting method. We found that PBL from patients with BD expressed Txk much more than normal PBL (Fig. 2a). Some patients with BD and some normal individuals were selected for further study using purified CD3+ and CD4+ T cell subpopulations, because their PBL gave strong Txk band in the blot. CD3+ T cells and CD4+ T cells were separated from E-rosette positive cells of the selected patients and the control individuals by using autoMACS apparatus. Both CD3+ T cells and CD4+ T cells excessively expressed Txk protein in a majority of patients with BD (BD nos.5, 6 and 7) as compared with normal individuals (Normals nos. 5 and 6) (Fig. 2b).
Fig. 2.
Immunoblotting analysis of Txk expression in PBL in patients with BD. (a) PBL were separated from peripheral blood in patients with BD and were immediately lysed. Jurkat cells that had been transfected with a txk expression vector were included. The cell lysates were analysed by immunoblotting using affinity-purified anti-human Txk antibody. Arrowheads indicate 62 kD Txk. The results were representative of three independent experiments with similar results. (b) Some patients with BD and some normal individuals were selected for further study because their PBL gave a strong Txk band in the blot. CD3+ cells and CD4+ cells were separated and similarly analysed. PBL and T cell subpopulations from totally 6 normal donors were included. (c) A correlation of Txk expression in T cells with IFN-γ production in patients with BD (♦) and normal individuals (•). Relative band intensities of the Txk in association with the actin band intensities in the immunoblots were measured by using NIH image software as reported previously. In total, 8 patients and 5 normal individuals were measured, and the correlation coefficient was measured by using StatView J software. The P-value was less than 0·05 indicating the association of excessive Txk expression and enhanced IFN-γ production in patients with BD.
In the parallel experiments, we measured IFN-γ production by the lymphocyte subpopulations, and studied for the association of Txk protein expression of lymphocytes and their cytokine production. As shown in Fig. 2c, CD3+ T cells were simultaneously tested for their IFN-γ production by ELISA and Txk protein expressions by immunoblotting. Relative band intensities of the Txk in association with the actin band intensities in the immunoblots were measured by using NIH image soft ware. Totally 8 patients and 5 normal individuals were studied. The ‘r’ value was 0·815 and the P-value was less than 0·05 indicating that the extent of IFN-γ production by the lymphocytes correlated with the expression of Txk protein by the lymphocytes in the blot (Fig. 2c). Thus, it is possible that the excessive expression of Txk leads to the skewed Th1/Th2 balance and subsequent Th1 dominant immune responses in the pathogenesis of BD.
Th1 cytokine production in the skin lesions
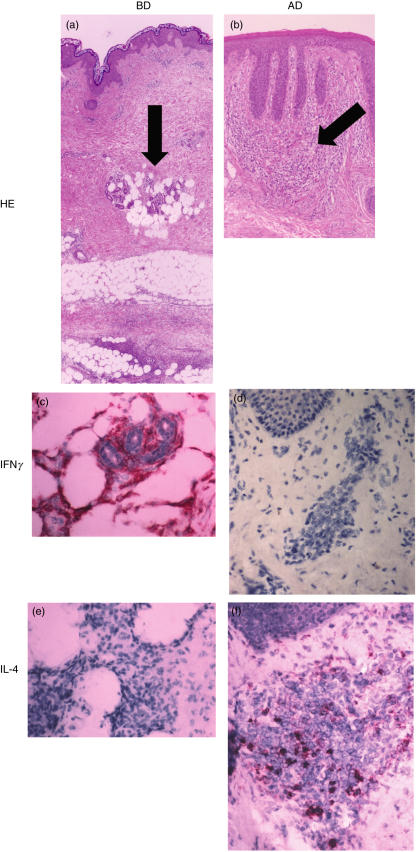
Th1 cells and Th2 cells show distinct patterns of cytokine secretion. Th1 cells typically produce IL-2, IFN-γ and TNF-β, and Th2 cells were characterized by their production of IL-4, IL-5, IL-6 and IL-13 [29,30]. To clarify whether infiltrating T cells in the skin lesion belonged to Th1 cells, we studied IL-4 and IFN-γ producing cells in the mononuclear cell aggregates of the skin lesions in patients with BD. As a disease control, eczematous lesions of atopic dermatitis, a typical Th2 disease, were included. IFN-γ producing cells were detected in mononuclear cell infiltrating area in the skin lesions in all patients with BD (Fig. 3e), while IL-4 producing cells were scarcely detected (Fig. 3g).
Fig. 3.
Expression of Th1/Th2 cytokines on skin lesions in patients with BD. (a) Biopsy specimen of the skin lesion (erythema nodosum) in patients with BD was stained with HE. (b) Eczematous lesion of atopic dermatitis (AD) was stained with HE. Arrows indicate the positions analysed in the immunochemical staining mentioned below. (c) IFN-γ expressing cells were detected in the lesion in patients with BD (an arrow in (a)). (d) IFN-γ producing cells were hardly detected in the lesion in patients with AD. (e) IL-4 positive mononuclear cells were not detected in the skin lesion. (f) Biopsy specimen of atopic dermatitis included IL-4 producing cells (an arrow in (b)). Magnification is × 25 (low) and ×100 (high). Control staining with nonimmune mouse IgG did not stain at all, thus was omitted. The result was a representative of total 5 experiments.
In contrast, IFN-γ producing cells were not detected in the lesions of atopic dermatitis (Fig. 3f). IL-4 producing cells were detected in the same skin lesions in all patients with atopic dermatitis (Fig. 3h). Thus, Th1 cells may predominantly infiltrate in the skin lesions in patients with BD.
Txk expression in the skin lesions in patients with BD
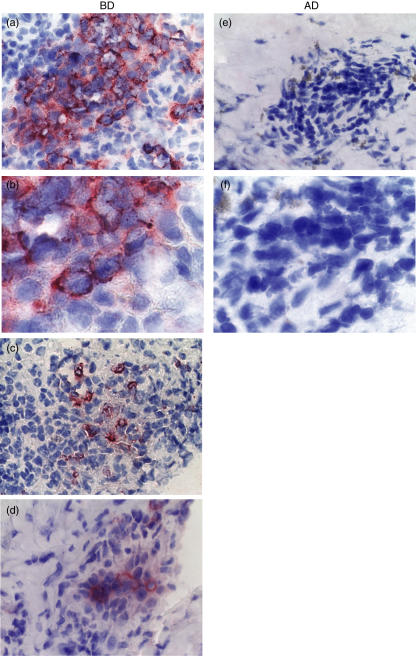
We next focused on studying whether lymphocytes infiltrating into the lesion express Th1 cell specific Txk protein. We found that Txk protein was expressed in the mononuclear cell aggregates of erythema nodosum in patients with BD (Fig. 4). None of the eczematous lesions of AD contained lymphocytes expressing Txk (Fig. 4). These finding confirm that Th1 cells predominantly infiltrate in the skin lesions in patients with BD.
Fig. 4.
Txk expression on mononuclear cells in the skin lesions of patients with BD. Txk expression of the skin lesion in patients with BD was studied by using anti-Txk antibody. Control staining with nonimmune goat IgG did not stain at all, and thus was omitted. Significant numbers of mononuclear cells accumulating in the skin lesions expressed Txk in patients with BD (a), (c) and (d) corresponded to patients no.1, no.2 and no.3, respectively. The positions of each panel were almost equal with the arrow in Fig. 3a). Txk expression was not detected on the mononuclear cells collected in the biopsy specimen of an atopic dermatitis (AD) patient (the positions of each panel were almost equal with the arrow in Fig. 3b). (b) and (f) were higher magnifications of (a) and (e), respectively. Magnification is × 25 (low) and ×100 (high).
Production of cytokines that induce Th1 cell differentiation in the lesions
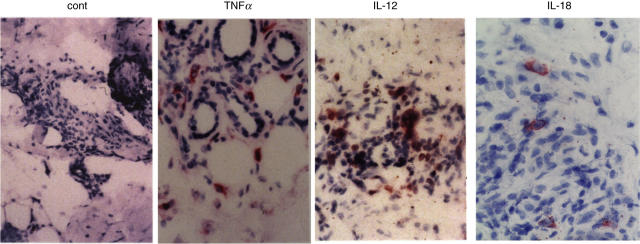
To determine the local cytokine production of cells present at the site of skin disease, we examined Th1 cell associated cytokine production in the lesions. We focused on TNF-α, IL-12, IL-18 and IFN-γ that may affect Th1 cell development. IL-12, IL-18 and IFN-γ have been shown to positively modulate Th1 cell induction [29]. We also found that pretreatment with IL-12, IL-18 and IFN-γ of normal T cells up-regulated Txk protein expression (NS et al. manuscript in preparation). Thus, if the Th1 associated cytokines are produced in situ, differentiation into Th1 cells of naive T cells may be enhanced in the lesions. Indeed, we found IL-12, IL-18 and IFN-γ producing cells in mononuclear cell infiltrating area in the skin lesions in all patients (Figs 3 and 5). Thus, Th1 associated cytokines may lead to preferential development of Th1 dominant responses in situ in BD. These data suggest that local production of the Th1 associated cytokines and accumulation of Txk expressing lymphocytes may be involved in pathogenesis of BD.
Fig. 5.
Th1 associated cytokine expression on mononuclear cells in the skin lesions of patients with BD. Th1 associated cytokine production in the skin lesion in patients with BD was studied by using anti-cytokine antibodies. Cells expressing TNF-α, IL-12 and IL-18 were accumulating in the lesion (erythema nodosum) in patients with BD. The positions of each panel were almost equal with the arrow in Fig. 3a. Magnification is × 100 (a–c) and ×200 (d). The result was a representative of total 5 experiments.
Discussion
In the pathogenesis of BD, a variety of immunological parameters can be demonstrated to be abnormal [1,31–35]. In this study, we examined spontaneous cytokine production and that in response to HSP derived peptide stimulation of PBL. In addition, expression of Txk, a Tec family tyrosine kinase that acts as a Th1 cell specific transcription factor was studied. After confirming Th1 dominant phenotype of this disease, production of cytokines with Th1 cell inducing activity in the PBL and in the skin lesions was also studied.
A possible polarization of T lymphocytes toward the Th1 type in BD has been suggested previously [9–13]. Plasma levels of IL-12, tumour necrosis factor receptor75, and soluble IL-2 receptor correlate with disease activity in BD [36]. Activated macrophages induce cellular immunity by activating Th1 cell responses and by suppressing Th2 cell responses [37]. IFN-γ represents the key cytokine produced by Th1 cells. IFN-γ is a potent immunoregulatory factor that plays an important role in the activation of macrophages by regulating antigen presentation. IL-4 drives the differentiation of naive CD4-positive cells in a Th2 direction [29,30]. IL-4 can alter the Th1/Th2 balance in the direction of Th2 cells. In the patients, normal levels of Th2 type cytokine production were recognized (Fig. 1b).
We found that MNC infiltrating in the skin lesions of patients with BD consisted of mainly IFN-γ producing cells. Gul et al. reported the immunohistology of skin pathergy reaction in BD [38]. In pathergy reaction, they found that MNC infiltrate was predominantly composed of CD4+ T cells [38]. Charteris et al. [39] reported that lymphocytes in retinal perivasculitis in patients with BD were activated CD4+ T cell. Our results suggest that Th1 cells expressing Txk may be involved in the pathogenesis of skin lesions in patients with BD. Reduced Txk expression was recently reported in patients with atopic dermatitis [40]. Th1 cells are critical to cell-mediated immunity required to eliminate some bacterial pathogens and protozoan parasites. Th1 cells in the lesions may result in the induction of inflammatory process in the skin lesions of BD. Ulcerated lesion of scrotal skin of the patient was similarly studied. Haematoxilin-Eosin staining revealed the nonspecifically inflamed skin tissue with ulcerated lesion containing many neurtophils. After recovering mRNA of the ulcerative lesion and subsequent RT-PCR, we found that txk mRNA was expressed in the lesion. This finding was consistent with the results of immunohistochemical staining of the erythema nodosum in patients with Behcet's disease.
In preliminary experiments, we have studied cytokine expression of other types of erythema nodosum. We found the findings were almost the same with those of patients with Behcet's disease. We also studied Th1 and Th2 associated chemokine receptor expression of lymphocytes in patients with BD. We found that CCR5 and CXCR3 receptors were expressed preferentially on the lymphocytes, further suggesting Th1 dominant immune aberration in this disease (manuscript submitted for publication).
Finally, we studied production of cytokines that preferentially induce Th1 cells in the lesion. We found excessive production of IFN-γ, IL-12 and IL-18 in the skin lesions. Hamzaoui et al. reported that IL-18 was produced in bronchoalveolar lavage from BD and recombinant IL-18 induced IFN-γ production in the BAL fluid from BD [41]. These cytokines may act in a synergistic fashion to induce preferential induction of Th1 cells in the lesion. In addition, IFN-γ mediated autocrine augmentation of Th1 cell induction may have happened in the lesion.
In conclusion, we suggested that excessive HSP60 expression in the skin lesions and accumulation of Th1 cells expressing Txk protein may play a role in the pathogenesis of BD. Th1 type cytokines may contribute to the immunopathogenesis of BD.
References
- 1.Sakane T, Takeno M, Suzuki N, Inaba G. Behcet's disease. N Engl J Med. 1999;341:1284–91. doi: 10.1056/NEJM199910213411707. [DOI] [PubMed] [Google Scholar]
- 2.Lee EB, Kim JY, Lee YJ, Park MH, Song YW. TNF and TNF receptor polymorphisms in Korean Behcet's disease patients. Hum Immunol. 2003;64:614–20. doi: 10.1016/s0198-8859(03)00057-0. [DOI] [PubMed] [Google Scholar]
- 3.Gul A, Hajeer AH, Worthington J, Ollier WE, Silman AJ. Linkage mapping of a novel susceptibility locus for Behcet's disease to chromosome 6p22–23. Arthritis Rheum. 2001;44:2693–6. doi: 10.1002/1529-0131(200111)44:11<2693::aid-art449>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 4.Kotter I, Gunaydin I, Stubiger N, et al. Comparative analysis of the association of HLA-B*51 suballeles with Behcet's disease in patients of German and Turkish origin. Tissue Antigens. 2001;58:166–70. doi: 10.1034/j.1399-0039.2001.580304.x. [DOI] [PubMed] [Google Scholar]
- 5.Lee S, Bang D, Cho YH, Lee ES, Sohn S. Polymerase chain reaction reveals herpes simplex virus DNA in saliva of patients with Behcet's disease. Arch Dermatol Res. 1996;288:179–83. doi: 10.1007/BF02505221. [DOI] [PubMed] [Google Scholar]
- 6.Yokota K, Hayashi S, Araki Y, et al. Characterization of Streptococcus sanguis isolated from patients with Behcet's disease. Microbiol Immunol. 1995;39:729–32. doi: 10.1111/j.1348-0421.1995.tb03249.x. [DOI] [PubMed] [Google Scholar]
- 7.Hirohata S, Hashimoto T. Abnormal T cell responses to bacterial superantigens in Behcet's disease (BD) Clin Exp Immunol. 1998;112:317–24. doi: 10.1046/j.1365-2249.1998.00572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamzaoui K, Ayed K, Slim A, Hamza M, Touraine J. Natural killer cell activity, interferon-gamma and antibodies to herpes viruses in patients with Behcet's disease. Clin Exp Immunol. 1990;79:28–34. doi: 10.1111/j.1365-2249.1990.tb05122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rajendram R, Rao NA. Molecular mechanisms in Behcet's disease. Br J Ophthalmol. 2003;87:1199–200. doi: 10.1136/bjo.87.10.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frassanito MA, Dammacco R, Cafforio P, Dammacco F. Th1 polarization of the immune response in Behcet's disease. a putative pathogenetic role of interleukin-12. Arthritis Rheum. 1999;42:1967–74. doi: 10.1002/1529-0131(199909)42:9<1967::AID-ANR24>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 11.Sugi-Ikai N, Nakazawa M, Nakamura S, Ohno S, Minami M. Increased frequencies of interleukin-2- and interferon-gamma-producing T cells in patients with active Behcet's disease. Invest Ophthalmol Vis Sci. 1998;39:996–1004. [PubMed] [Google Scholar]
- 12.Hamzaoui K, Hamzaoui A, Guemira F, Bessioud M, Hamza M, Ayed K. Cytokine profile in Behcet's disease patients. Relationship with disease activity. Scand J Rheumatol. 2002;31:205–10. doi: 10.1080/030097402320318387. [DOI] [PubMed] [Google Scholar]
- 13.Raziuddin S, al-Dalaan A, Bahabri S, Siraj AK, al-Sedairy S. Divergent cytokine production profile in Behcet's disease. Altered Th1/Th2 cell cytokine pattern. J Rheumatol. 1998;25:329–33. [PubMed] [Google Scholar]
- 14.Li B, Yang P, Zhou H, Zhang Z, Xie C, Lin X, et al. T-bet expression is upregulated in active Behcet's disease. Br J Ophthalmol. 2003;87:1264–7. doi: 10.1136/bjo.87.10.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kashiwakura J, Suzuki N, Nagafuchi H, et al. Txk, a nonreceptor tyrosine kinase of the Tec family, is expressed in T helper type 1 cells and regulates interferon gamma production in human T lymphocytes. J Exp Med. 1999;190:1147–54. doi: 10.1084/jem.190.8.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takeba Y, Nagafuchi H, Takeno M, Kashiwakura J, Suzuki N. Txk, a member of nonreceptor tyrosine kinase of Tec family, acts as a Th1 cell-specific transcription factor and regulates IFN-gamma gene transcription. J Immunol. 2002;168:2365–70. doi: 10.4049/jimmunol.168.5.2365. [DOI] [PubMed] [Google Scholar]
- 17.Lehner T, Lavery E, Smith R, van der Zee R, Mizushima Y, Shinnick T. Association between the 65-kilodalton heat shock protein, Streptococcus sanguis, and the corresponding antibodies in Behcet's syndrome. Infect Immun. 1991;59:1434–41. doi: 10.1128/iai.59.4.1434-1441.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rich T, Gruneberg U, Trowsdale J. Heat shock proteins, HLA-DR and rheumatoid arthritis. Nat Med. 1998;4:1210–1. doi: 10.1038/3172. [DOI] [PubMed] [Google Scholar]
- 19.Pervin K, Childrstone A, Shinnick T, et al. T cell epitope expression of mycobacterial and homologous human 65-kilodalton heat shock protein peptides in short term cell lines from patients with Behcet's disease. J Immunol. 1993;151:2273–82. [PubMed] [Google Scholar]
- 20.Kaneko S, Suzuki N, Yamashita N, et al. Characterization of T cells specific for an epitope of human 60-kirodalton heat shock protein in patients with Behcet's disease. Clin Exp Immunol. 1997;108:204–12. doi: 10.1046/j.1365-2249.1997.3611265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Direskeneli H, Eksioglu-Demiralp E, et al. T cell responses to 60/65 kDa heat shock protein derived peptides in Turkish patients with Behcet's disease. J Rheumatol. 2000;27:708–13. [PubMed] [Google Scholar]
- 22.Behcet's Disease Research Committee of Japan. Criteria for Diagnosis of Behcet's Disease. In: Mizushima Y, editor. Annual Report of Behcet's Disease Research Committee of Japan. Tokyo: Ministry of Health and Welfare of Japan; 1986. [Google Scholar]
- 23.International Study Group for Behcet's Disease. Criteria for diagnosis of Behcet's disease. Lancet. 1990;335:1078–80. [PubMed] [Google Scholar]
- 24.Behcet's Disease Research Committee of Japan. Criteria for Activity of Behcet's Disease. In: Sakane T, editor. Annual Report of Behcet's Disease Research Committee of Japan. Tokyo: Ministry of Health and Welfare of Japan; 1994. [Google Scholar]
- 25.Wakisaka S, Takeba Y, Mihara S, et al. Aberrant Fas ligand expression in lymphocytes in patients with Behcet's disease. Int Arch Allergy Immunol. 2002;129:175–80. doi: 10.1159/000065878. [DOI] [PubMed] [Google Scholar]
- 26.Suzuki N, Kaneko S, Ichino M, Mihara S, Wakisaka S, Sakane T. In vivo mechanisms for the inhibition of T lymphocyte activation by long-term therapy with tacrolimus (FK-506): experience in patients with Behcet's disease. Arthritis Rheum. 1997;40:1157–67. doi: 10.1002/art.1780400622. [DOI] [PubMed] [Google Scholar]
- 27.Hu W, Hasan A, Wilson A, et al. Experimental mucosal induction of uveitis with the 60-kDa heat shock protein-derived peptide 336–351. Eur J Immunol. 1998;28:2444–55. doi: 10.1002/(SICI)1521-4141(199808)28:08<2444::AID-IMMU2444>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 28.Hu Q, Davidson D, Schwartzberg PL, et al. Identification of Rlk, a novel protein tyrosine kinase with predominant expression in the T cell lineage. J Biol Chem. 1995;270:1928–34. doi: 10.1074/jbc.270.4.1928. [DOI] [PubMed] [Google Scholar]
- 29.Szabo SJ, Sullivan BM, Peng SL, Glimcher LH. Molecular mechanisms regulating Th1 immune responses. Annu Rev Immunol. 2003;21:713–58. doi: 10.1146/annurev.immunol.21.120601.140942. [DOI] [PubMed] [Google Scholar]
- 30.Murphy KM, Ouyang W, Farrar JD, et al. Signaling and transcription in T helper development. Annu Rev Immunol. 2000;18:451–94. doi: 10.1146/annurev.immunol.18.1.451. [DOI] [PubMed] [Google Scholar]
- 31.Yurdakul S, Hamuryudan V, Yazici H. Behcet syndrome. Curr Opin Rheumatol. 2004;16:38–42. doi: 10.1097/00002281-200401000-00008. [DOI] [PubMed] [Google Scholar]
- 32.Zierhut M, Mizuki N, Ohno S, et al. Immunology and functional genomics of Behcet's disease. Cell Mol Life Sci. 2003;60:1903–22. doi: 10.1007/s00018-003-2333-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verity DH, Wallace GR, Vaughan RW, Stanford MR. Behcet's disease: from Hippocrates to the third millennium. Br J Ophthalmol. 2003;87:1175–83. doi: 10.1136/bjo.87.9.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zouboulis CC, May T. Pathogenesis of Adamantiades-Behcet's disease. Med Microbiol Immunol (Berl) 2003;192:149–55. doi: 10.1007/s00430-002-0167-5. [DOI] [PubMed] [Google Scholar]
- 35.Direskeneli H. Behcet's disease. infectious aetiology, new autoantigens, and HLA-B51. Ann Rheum Dis. 2001;60:996–1002. doi: 10.1136/ard.60.11.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Turan B, Gallati H, Erdi H, Gurler A, Michel BA, Villiger PM. Systemic levels of the T cell regulatory cytokines IL-10 and IL-12 in BD; soluble TNFR-75 as a biological marker of disease activity. J Rheumatol. 1997;24:128–32. [PubMed] [Google Scholar]
- 37.Desmedt M, Rottiers P, Dooms H, Fiers W, Grooten J. Macrophages induce cellular immunity by activating Th1 cell responses and suppressing Th2 cell responses. J Immunol. 1998;160:5300–8. [PubMed] [Google Scholar]
- 38.Gul A, Esin A, Dilsen N, Konice M, Wigzell H, Biberfeld P. Immunohistology of skin pathergy reaction in Behcet's disease. Br J Derm. 1995;132:901–7. doi: 10.1111/j.1365-2133.1995.tb16946.x. [DOI] [PubMed] [Google Scholar]
- 39.Charteris DG, Champ C, Rosenthal AR, Lightman SL. Behcet's disease: activated T lymphocytes in retinal perivasculitis. Br J Opth. 1992;76:499–501. doi: 10.1136/bjo.76.8.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arakawa S, Hatano Y, Katagiri K. Differential expression of mRNA for Th1 and Th2 cytokine-associated transcription factors and suppressors of cytokine signalling in peripheral blood mononuclear cells of patients with atopic dermatitis. Clin Exp Immunol. 2004;135:505–10. doi: 10.1111/j.1365-2249.2004.02405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hamzaoui A, Ghrairi H, Ammar J, Zekri S, Guemira F, Hamzaoui K. IL-18 mRNA expression and IFN-gamma induction in bronchoalveolar lavage from Behcet's disease. Clin Exp Rheumatol. 2003;21:S8–14. [PubMed] [Google Scholar]