Abstract
Psoriasis is believed to be a T cell-mediated autoimmune disease, but also exhibits autoantibody production. Calpastatin is an endogenous inhibitor of calpain, a ubiquitous protease that regulates inflammatory processes. Anti-calpastatin autoantibody was first identified as an autoantibody specific to rheumatoid arthritis, but has been also detected in other autoimmune diseases. In this study, we examined the presence and levels of anti-calpastatin antibody in 77 psoriasis patients by enzyme-linked immunosorbent assay. Compared with normal controls, psoriasis patients exhibited significantly elevated IgG anti-calpastatin antibody levels that were similar to those found in rheumatoid arthritis patients. Remarkably, IgG anti-calpastatin autoantibody in sera from psoriasis patients inhibited calpastatin activity. Calpain II expression was up-regulated in psoriasis skin lesions compared with normal skin while calpastatin expression was normal. The results of this study reveal the presence of anti-calpastatin autoantibody in psoriasis.
Keywords: autoantibody, calpain, calpastatin, psoriasis
Introduction
Psoriasis is a chronic inflammatory disease of the skin and small joints that seriously impairs quality of life. Psoriasis is characterized by keratinocyte hyperproliferation, epidermal influx of neutrophils and infiltration of mononuclear cells, mainly activated memory T lymphocytes, in the papillary dermis and epidermis [1,2]. Although its pathogenesis is not fully clarified, psoriasis exhibited many features of a T cell-mediated autoimmune disease [2–4]. T cell-directed immunosuppressants are effective for controlling the disease activity of psoriasis [5]. Psoriasis lesions are fully induced when T cells from psoriasis patients are injected into symptomless psoriasis skin engrafted onto SCID mice [6]. In addition to the T cell-mediated autoimmunity, many reports have shown that psoriasis is associated with the production of various autoantibodies, including antistratum corneum antibody [7–9], antikeratin antibody [10] and antilipocortin-I antibody [11]. Thus, abnormal regulation of humoral immune response characterized by autoantibody production may also contribute to the development of inflammation associated with psoriasis.
Calpain (EC 3.4.22.17) is a calcium-dependent neutral protease involved in the regulation of various basic cellular functions, such as cell cycle, apoptosis and exocytosis [12,13]. Furthermore, calpain regulates the initiation and development of inflammation; for example, calpain-mediated degradation of IκB induces nuclear factor-κB (NF-κB) activation that leads to production of proinflammatory cytokines and expression of adhesion molecules [14,15]. The two isoforms of calpain are identified: calpain I (or µ-calpain) and calpain II (or m-calpain), which require low and high micromolar calcium for their activation, respectively [12]. Calpastatin is an endogenous, specific inhibitor of calpain and contains four repeating domains, each of which can inhibit calpain activity independently with domain I the highest activity [16]. Calpain I, calpain II and calpastatin are present in a variety of cell types, including keratinocytes [13,17].
Autoantibodies directed against calpastatin are recently identified autoantibodies that are detected initially in 10–57% of sera from patients with rheumatoid arthritis (RA) [18–21]. In RA, anti-calpastatin antibodies may contribute to the development of arthritis by enhancing the calpain activity as calpain functions as a proteoglycanase in cartilage destruction [22]. However, anti-calpastatin antibody is also detected in other various autoimmune disorders, including systemic sclerosis (38%), systemic lupus erythematosus (10–27%), polymyositis/dermatomyositis (24%), Sjögren's syndrome (19%) and overlap syndrome (29%) [18,20,21,23]. These findings suggest that anti-calpastatin antibody is not disease-specific, but contributes to inflammation associated with various autoimmune diseases. Therefore, in this study we examined the presence and levels of anti-calpastatin antibodies, their functional relevance and skin lesional expression of calpain and calpastatin in patients with psoriasis. The results of this study suggest that anti-calpastatin antibody contributes to the development of inflammation in psoriasis by augmenting the relative activity of calpain.
Materials and methods
Patients and controls
Serum samples were obtained from 77 Japanese patients with psoriasis (49 males and 28 females, 47 ± 15 years). The clinical severity of psoriasis was evaluated by the Psoriasis Area and Severity Index (PASI) scores [24]. Psoriasis patients were classified into 55 patients with psoriasis vulgaris (37 males and 18 females, 49 ± 14 years, PASI scores 12·9 ± 9·5), 16 patients with psoriatic arthritis (11 males and five females, 43 ± 15 years, PASI scores 11·2 ± 7·8) and six patients with generalized pustular psoriasis (five males and one female, 35 ± 19 years, PASI scores 16·6 ± 3·2). The disease duration of patients with psoriasis vulgaris, generalized pustular psoriasis and psoriatic arthritis was 15 ± 10, 14 ± 7 and 14 ± 10 years, respectively. The serum and skin biopsy samples were obtained at the first visit to our clinic and patients received with treatment were excluded in this study. Therefore, none of the patients was treated with topical or oral steroids, oral retinoids, psoralen-ultraviolet A therapy or immunosuppressive drugs. The period from the last treatment to the first visit varied from 2 weeks to 1 month. Twenty-four Japanese patients with RA (three males and 21 females, 51 ± 11 years), who fulfilled the criteria for RA by the American College of Rheumatology [25], were also included in this study. Sixty-four age- and sex-matched Japanese healthy individuals (38 males and 26 females; age, 45 ± 11 years) were used as normal controls. Fresh venous blood samples were centrifuged shortly after clot formation. All samples were stored at −70°C prior to use. For analysis of immunohistochemistry and mRNA expression of calpastatin and calpain, five patients with psoriasis (four males and one female, 41 ± 9 years) and five normal individuals were evaluated. The disease duration of psoriasis patients was 13 ± 10 years. None of the patients for analysis of immunohistochemistry and mRNA expression was treated with topical or oral steroids, oral retinoids, psoralen-ultraviolet A therapy or immunosuppressive drugs. All investigations were performed after approval by the Kanazawa University Graduate School of Medical Science, and informed consent was obtained from all patients.
Enzyme-linked immunosorbent assay (ELISA) for anti-calpastatin antibody
ELISA was conducted as described previously [23]. Ninety-six well plates (EIA/RIA plate, Costar, Cambridge, MA, USA) were coated with human recombinant calpastatin domain I (5 µg/ml; Calbiochem-Novabiochem Corporation, La Jolla, CA, USA) in 0·1 m borate buffer, pH 8·4, at 4°C overnight. After washing, the wells were blocked with 2% bovine serum albumin and 1% gelatin for 1 h at 37°C. Serum samples diluted 1 : 200 were added to duplicate wells for 90 min at 20°C. After washing, the bound antibodies were detected with alkaline phosphatase-conjugated goat antihuman IgG or IgM antibodies (Cappel, Durham, NC, USA), using 10 mg/ml p-nitrophenyl phosphate (Sigma-Aldrich, St. Louis, MO, USA). The plates were read at 405 nm. Optical density (OD) greater than the mean ± 2 s.d. for the controls was considered positive. The sera were diluted at log intervals (1 : 10–1 : 105) and assessed for relative autoantibody levels as above, except that the results were plotted as OD versus dilution (log scale). The dilutions of sera giving half-maximal OD values were determined by linear regression analysis, thus generating arbitrary unit per millilitre values for comparison between sets of sera.
Calpastatin activity assay
IgG was purified from serum samples using magnetic beads coated with recombinant Protein G covalently coupled to the surface (Dynal Inc., Lake Success, NY, USA). The serum sample (10 µl) and Dynabeads (100 µl) were incubated for 40 min. The tube containing the mixture was placed in a magnetic column for 2 min and the magnetic column was then washed with phosphte buffered saline (PBS). Finally, the bound IgG was eluted off from the magnetic column with 0·1 m citrate (pH 2–3), and were then neutralized with 1 m Tris-HCl (pH 9·0). Final IgG concentration was measured by spectrophotometer (Gene Quant II, Amarsham Biosciences Inc., Piscataway, NJ, USA). The inhibitory activity of calpastatin for calpain was assessed by the capacity of calpain to proteolyse its substrate, casein [26]. Calpastatin (0·5 µg; Calbiochem-Novabiochem Corp.) was incubated with 50 µg of purified IgG or control blocking monoclonal antibody to calpastatin (Takara, Otsu, Japan) for 1 h at room temperature and then with 1 µg of calpain II (Calbiochem-Novabiochem Corp.) in 250 µl of reaction buffer (3 mg/ml of casein in 100 m m Tris-HCl, pH 7·5 containing 5 m m CaCl2 and 10 m m 2-mercaptoethanol) for 20 min at 30°C. The reaction was stopped by addition of a chilled solution (250 µl) of 10% trichloroacetic acid. After centrifugation (13 000 r.p.m) for 2 min, the absorbance at 280 nm was measured with a spectrophotometer (Gene Quant II, Amersham Biosciences Inc.).
RNA isolation and real-time polymerase chain reaction (PCR)
Total cellular RNA was isolated from frozen tissue with Qiagen RNeasy spin columns (Qiagen Ltd, Crawley, UK). Total RNA from each sample was reverse transcribed into cDNA according to the protocol of the RNA PCR kit (Takara). Expression of calpain II and calpastatin was analysed using a real-time PCR quantification method according to the manufacturer's instructions (Applied Biosystems, Foster City, CA, USA). Sequence-specific primers and probes were designed by Applied Biosystems Assays-On-DemandsTM (Applied Biosystems). Real-time PCR (one cycle at 50°C for 2 min, at 95°C for 10 min; 40 cycles of at 92°C for 15 s, at 60°C for 60 s) was performed on an ABI Prism 7000 Sequence Detector (Applied Biosystems), on which fluorescent output was directly proportional to cDNA concentration. To ensure equality of loading, input cDNA concentration was normalized to housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) by use of photoelectron RNA control reagents (Applied Biosystems). To compare either calpain II or calpastatin and housekeeping (GAPDH) gene mRNA expression, relative expression of real-time PCR products was determined using the ΔΔCt method [27]. This method calculates relative expression using the equation: where Ct = the threshold cycle, i.e. the cycle number at which the sample's relative fluorescence rises about the background fluorescence and ΔΔCt = [Ct gene interest (unknown sample) – Ct GAPDH (unknown sample)]–[Ct gene interest (calibrator sample) – Ct GAPDH (calibrator sample)]. One of the control samples was chosen as a calibrator sample. Each sample was conducted in duplicate and the mean Ct was used in the equation.
Immunohistochemical analysis
Paraffin sections were deparaffinized and then incubated with 10% normal goat serum (10 min, 37°C) to block non-specific staining. Sections were then incubated (2 h, room temperature) with rabbit monoclonal antibody (MoAb) specific for calpain II (1 : 100 dilution, Chemicon, Temekula, CA, USA). Rabbit IgG (Southern Biotechnology associates Inc., Birmingham, AL, USA) was used as a control for non-specific staining. Sections were incubated sequentially (20 min, room temperature) with a biotinylated goat antirabbit IgG secondary antibody (Vectastain ABC method, Vector Laboratories, Burlingame, CA, USA), then horseradish peroxidase-conjugated avidin–biotin complexes (Vectastain ABC method, Vector Laboratories). Sections were developed with 3,3′-diaminobenzidine tetrahydrochloride and hydrogen peroxide, and then counterstained with methyl green. For immunohistochemical staining of Calpastatin, mouse anti-calpastatin MoAb (1 : 100 dilution, Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) was used.
Statistical analysis
Comparisons between two experimental groups of data were performed using a Mann–Whitney U-test. Comparisons among three or more experimental groups were performed using a one-way analysis of variance (anova), followed by a Bonferroni's test. Spearman's rank correlation coefficient was used to examine the relationship between two continuous variables. P-values less than 0·05 were considered statistically significant.
Results
Anti-calpastatin antibody levels in psoriasis
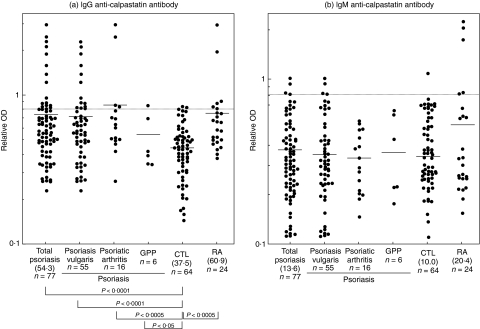
The presence and levels of anti-calpastatin autoantibodies in serum samples from patients with psoriasis, those with RA and normal controls were assessed by ELISA using human recombinant calpastatin domain I (Fig. 1). The dilution of sera giving half-maximal OD values in ELISAs was also determined to generate arbitrary units per millilitre that could be compared directly between patients and normal controls. Pooled sera from patients with RA had mean IgG anti-calpastatin antibody levels that were significantly 62% higher than those found in normal controls (P < 0·0005). Compared with normal controls, total 77 patients with psoriasis exhibited significantly elevated IgG anti-calpastatin antibody levels (45% increase, P < 0·0001) that were comparable with those found in patients with RA. Concerning subsets of psoriasis, IgG anti-calpastatin antibody levels were increased significantly in patients with psoriasis vulgaris (P < 0·0001), psoriatic arthritis (P < 0·0005) or generalized pustular psoriasis (P < 0·05) relative to normal controls. There was no significant difference in IgG anti-calpastatin antibody levels between patients with psoriasis vulgaris, those with psoriatic arthritis and those with generalized pustular psoriasis. By contrast, IgM anti-calpastatin antibody levels in total patients with psoriasis or patients with RA were normal. Anti-calpastatin antibody levels did not correlate with Psoriasis Area and Severity Index scores in patients with psoriasis (data not shown). Thus, psoriasis patients exhibited the elevation of IgG anti-calpastatin autoantibody to a similar level of patients with RA.
Fig. 1.
Anti-calpastatin antibody levels in patients with psoriasis. IgG or IgM anti-calpastatin antibody levels were determined by ELISA using human recombinant calpastatin domain I in patients with psoriasis vulgaris patients, those with psoriatic arthritis, those with generalized pustular psoriasis (GPP), those with RA and normal controls (CTL). The short bar indicates the mean value in each group. A broken line indicates the cut-off value (mean ± 2 s.d. of the control samples). Values in parenthesis represent the dilutions of pooled sera giving half-maximal OD values in ELISA, which were determined by linear regression analysis to generate arbitrary units per millilitre that could be directly compared between each group of patients and normal controls.
Frequency of anti-calpastatin antibody in psoriasis
Absorbance values higher than the mean ± 2 s.d. (0·802 for IgG anti-calpastatin antibody and 0·809 for IgM anti-calpastatin) of the control serum samples were considered positive in this study (Fig. 1). IgG or IgM anti-calpastatin antibodies were positive (i.e. above the mean ± 2 s.d. of normal control) in 26% of total patients with psoriasis, while they were positive in 38% of RA patients (Table 1). IgG isotype of this autoantibody (23%) was much more frequently positive than IgM isotype (4%) in total patients with psoriasis. By contrast, IgG or IgM anti-calpastatin antibody was positive in only 6% of healthy controls. Regarding the subsets of psoriasis, IgG or IgM anti-calpastatin antibody was positive in 27% of patients with psoriasis vulgaris, 25% of those with psoriatic arthritis and 17% of those with generalized pustular psoriasis. Thus, anti-calpastatin autoantibody was positive in ∼30% of patients with psoriasis.
Table 1.
Frequency of anti-calpastatin antibody in patients with psoriasis.a
| Anti-calpastatin antibody | |||
|---|---|---|---|
| IgGb | IgM | IgG or IgM | |
| Psoriasis (n = 77) | 18 (23) | 3 (4) | 20 (26) |
| ″Psoriasis vulgaris (n = 55) | 13 (24) | 3 (5) | 15 (27) |
| ″Psoriatic arthritis (n = 16) | 4 (25) | 0 | 4 (25) |
| Generalized pustular psoriasis (n = 6) | 1 (17) | 0 | 1 (17) |
| RA (n = 24) | 6 (25) | 5 (21) | 9 (38) |
| Normal (n = 64) | 3 (5) | 1 (2) | 4 (6) |
Values are the number (%) of patients with anti-calpastatin antibody that was determined by ELISA using human recombinant calpastatin domain I.
Isotypes (IgG or IgM) of anti-calpastatin antibody were determined using isotype-specific antihuman Ig antibodies.
Inhibition of calpastatin activity by anti-calpastatin antibody
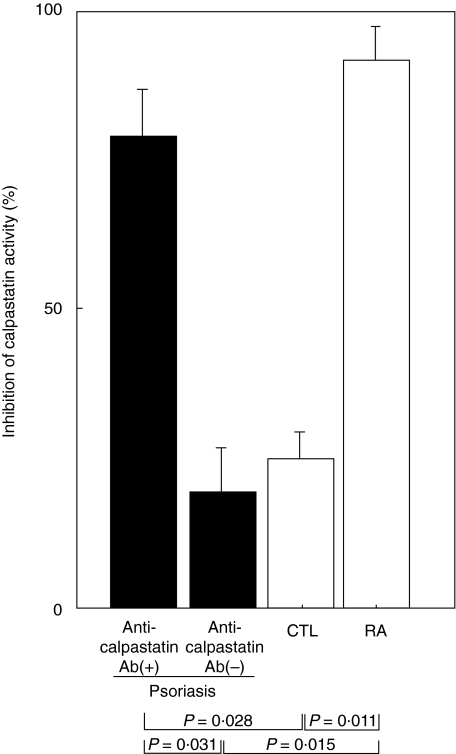
To determine the functional relevance of anti-calpastatin antibody in vivo, it was assessed whether anti-calpastatin autoantibody was able to inhibit enzymatic activity of calpastatin. The calpastatin activity was assessed by the inhibition of calpain activity that can proteolyse its substrate, casein. When compared with inhibition of calpastatin activity by control blocking anti-calpastatin monoclonal antibody that was defined as 100%, IgG isolated from serum samples of psoriasis patients positive for IgG anti-calpastatin antibody inhibited the calpastatin activity by 79% relative to psoriasis patients without IgG anti-calpastatin antibody (P = 0·031) and normal controls (P = 0·028). Similarly, the calpastatin activity was significantly blocked by IgG isolated from RA patients with IgG anti-calpastatin antibody compared with normal controls (P = 0·011); however, the extent of inhibition (92%) did not significantly differ from that by IgG from psoriasis patients with IgG anti-calpastatin antibody. Thus, IgG anti-calpastatin antibody from patients with psoriasis was able to block the inhibitory activity of calpastatin for calpain (Fig. 2).
Fig. 2.
Inhibition of calpastatin activity by IgG anti-calpastatin antibody from patients with psoriasis. IgG was purified from serum samples of psoriasis patients positive for IgG anti-calpastatin antibody by ELISA [anti-calpastatin antibody (+)], those negative for IgG anti-calpastatin antibody [anti-calpastatin antibody (–)], RA patients with IgG anti-calpastatin antibody and normal control (CTL). Purified IgG or control blocking monoclonal antibody to calpastatin was incubated with calpastatin and the calpastatin activity was assessed by the inhibition of calpain activity that can proteolyse its substrate, casein. Inhibition of calpastatin activity by isolated IgG is shown compared with that by control blocking anti-calpastatin monoclonal antibody that was defined as 100%. Each histogram shows the mean (± s.d.) results obtained for five patients in each group.
Quantitative real-time PCR analysis of calpain and calpastatin mRNA expression in psoriasis
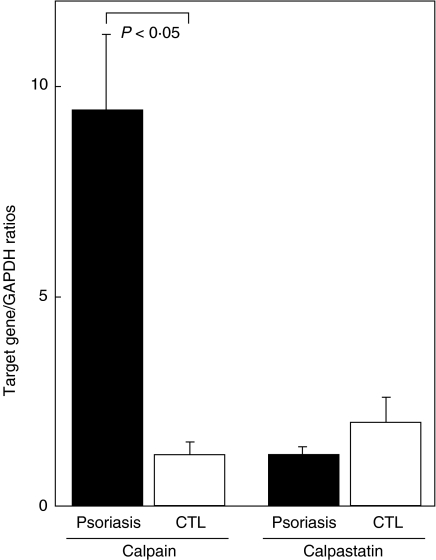
To assess the involvement of calpain and calpastatin in skin lesions of patients with psoriasis, mRNA expression of calpain II and calpastatin in the skin was quantified by real-time PCR. Calpain II mRNA levels were up-regulated by sixfold in the lesional skin from psoriasis patients compared with normal skin (P < 0·05; Fig. 3). Relative calpastatin mRNA expression in the psoriasis lesions did not differ significantly from that in normal skin. Thus, calpain II mRNA expression was augmented in the affected skin of psoriasis.
Fig. 3.
Expression of calpain II and calpastatin mRNA in skin lesions of psoriasis. Relative mRNA amount of calpain II and calpastatin was assessed by real-time PCR using the ΔΔCt method in lesion skin of psoriasis and normal skin (CTL). Total RNA was isolated from frozen skin tissues, reverse transcribed into cDNA, and then amplified using primers specific for calpain II and calpastatin. One of the control samples was chosen as a calibrator sample. Each histogram shows the mean (± s.d.) results obtained for five patients in each group.
Immunohistochemical analysis of calpain expression in psoriasis
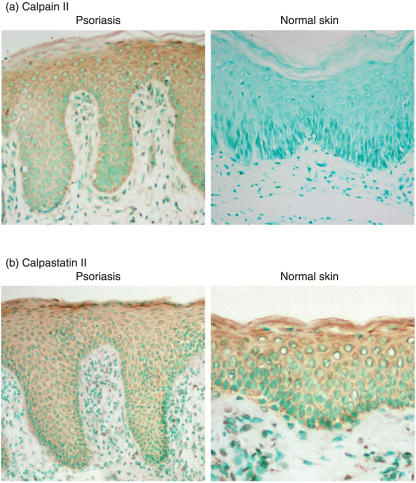
Calpain expression in the skin was further assessed immunohistochemically. In normal skin, calpain II expression was not detected (Fig. 4a). By contrast, calpain II was expressed in the epidermis of the psoriasis lesion with greater staining intensity in the upper to middle layers of the epidermis. Furthermore, calpain II was detected in vascular endothelial cells and infiltrating leucocytes of the psoriasis lesion. Calpastatin was also expressed throughout the epidermis: however, the staining intensity was similar between normal and psoriatic skin (Fig. 4b). We performed immunohistochemical staining in the three serial sections and similar results were obtained. Thus, calpain II expression was up-regulated in the psoriasis lesional skin.
Fig. 4.
Immunohistochemical expression of calpain II and calpastatin in skin lesions of psoriasis. Calpain II (a) and calpastatin (b) expression in normal skin and lesion skin of psoriasis was evaluated by immunohistochemistry using anti-calpain II and anti-calpastatin antibody. Sections were counterstained with methyl green. Magnification ×200.
Discussion
In the current study, anti-calpastatin antibody was positive in 26% of psoriasis with a similar frequency in psoriasis vulgaris, psoriatic arthritis and generalized pustular psoriasis. This prevalence of anti-calpastatin antibody was comparable with that in RA (38%) and also that in other systemic autoimmune diseases (10–38%) [18,20,21,23]. Remarkably, anti-calpastatin antibody in psoriasis patients was able to block the inhibitory activity of calpastatin for calpain protease activity to a similar level of RA patients. The current study is the first to reveal the presence of anti-calpastatin autoantibody in psoriasis that may be related to inflammation associated with psoriasis.
Calpain has been shown to play an important role in regulating inflammatory processes. Autodigestion of calpain exhibits a chemotactic activity for neutrophils [28], which may be related to neutrophil accumulation in the psoriatic epidermis. Furthermore, calpain activates neutrophils by promoting exocytosis of granules and superoxide anion production [29]. Calpain-mediated proteolytic processing of pre-interleukin (IL)-1α directly activates IL-1α[30]. IL-1α production by lesional psoriatic keratinocytes is augmented [31], which may be responsible for dermal inflammatory infiltration and epidermal hyperproliferation [32,33]. Expression of adhesion molecules, including intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) are also increased by calpain-mediated activation of NF-κB pathway [32]. ICAM-1 expression is up-regulated consistently in the epidermis and vascular endothelial cells of psoriasis lesions while VCAM-1 expression is increased in vascular endothelial cells [34]. These findings suggest that calpain activity contributes in part to the development of inflammation in psoriasis.
Calpain II expression was up-regulated in the skin lesions of psoriasis at both mRNA and protein levels compared with normal skin. In contrast, calpain I expression, which is detected in the middle to upper layers of the normal epidermis, is down-regulated in psoriatic epidermis [35]. This suggests that calpain II may play a more important role in inflammation of psoriasis than calpain I. Calpastatin was expressed at a similar level in normal and psoriatic skin at both mRNA and protein levels. Consistent with this, a previous study has shown that calpastatin is expressed in immortalized human HaCaT keratinocytes [17]. Thus, increased calpain II expression along with normal calpastatin expression in psoriasis suggests that augmented calpain II activity is involved in the development of psoriatic skin lesions.
Calpastatin acts intracellularly to inhibit calpain. However, extracellular location of calpain and calpastatin is demonstrated in the cell-free synovial fluid obtained from the knee joint of healthy adult humans as well as several patients with RA [36]. This may be released from the damaged synovial cells or inflammatory cells. The development of psoriasis was triggered frequently by viral or bacterial infection and drugs, some of which can induce the keratinocyte injury, including apoptosis [37]. This keratinocyte injury may release calpain and calpastatin extracellularly. Released calpain may further damage the keratinocyte and induce inflammation, which could be inhibited by similarly released calpastatin. Therefore, it is possible that anti-calpastatin antibody is formed by calpastatin released from damaged keratinocytes and then inhibits calpastatin activity.
Acknowledgments
We thank Ms M Matsubara and Y. Yamada for technical assistance.
References
- 1.Nickoloff BJ. The immunologic and genetic basis of psoriasis. Arch Dermatol. 1999;135:1104–10. doi: 10.1001/archderm.135.9.1104. [DOI] [PubMed] [Google Scholar]
- 2.Bos JD, De Rie MA. The pathogenesis of psoriasis: immunological facts and speculations. Immunol Today. 1999;20:40–6. doi: 10.1016/s0167-5699(98)01381-4. [DOI] [PubMed] [Google Scholar]
- 3.Ghoreschi K, Thomas P, Breit S, et al. Interleukin-4 therapy of psoriasis induces Th2 responses and improves human autoimmune disease. Nat Med. 2003;9:40–6. doi: 10.1038/nm804. [DOI] [PubMed] [Google Scholar]
- 4.Barker JN. Psoriasis as a T cell-mediated autoimmune disease. Hosp Med. 1998;59:530–3. [PubMed] [Google Scholar]
- 5.Mueller W, Herrmann B. Cyclosporin A for psoriasis. N Engl J Med. 1979;301:555. doi: 10.1056/NEJM197909063011016. [DOI] [PubMed] [Google Scholar]
- 6.Nickoloff BJ, Wrone-Smith T. Injection of pre-psoriatic skin with CD4+ T cells induces psoriasis. Am J Pathol. 1999;155:145–58. doi: 10.1016/S0002-9440(10)65109-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jablonska S, Chorzelski TP, Beutner EH, Maciejowska E, Jarzabek C, Rzesa G. Autoimmunity in psoriasis. Relation of disease activity and forms of psoriasis to immunofluorescence findings. Arch Dermatol Res. 1978;261:135–46. doi: 10.1007/BF00447158. [DOI] [PubMed] [Google Scholar]
- 8.Qutaishat SS, Kumar V, Beutner EH, Jablonska S. A distinct stratum corneum antigen in psoriasis and its reactions with stratum corneum autoantibodies. APMIS. 1992;100:341–6. doi: 10.1111/j.1699-0463.1992.tb00881.x. [DOI] [PubMed] [Google Scholar]
- 9.Tagami H, Iwatsuki K, Yamada M. Profile of anti-stratum corneum autoantibodies in psoriatic patients. Arch Dermatol Res. 1983;275:71–5. doi: 10.1007/BF00412877. [DOI] [PubMed] [Google Scholar]
- 10.Aoki S, Yaoita H, Kitajima Y. An elevated level of autoantibodies against 48- to 50-kd keratins in the serum of patients with psoriasis. J Invest Dermatol. 1989;92:179–83. doi: 10.1111/1523-1747.ep12276700. [DOI] [PubMed] [Google Scholar]
- 11.Rivers JK, Podgorski MR, Goulding NJ, et al. The presence of autoantibody to recombinant lipocortin-I in patients with psoriasis and psoriatic arthritis. Br J Dermatol. 1990;123:569–72. doi: 10.1111/j.1365-2133.1990.tb01472.x. [DOI] [PubMed] [Google Scholar]
- 12.Croall DE, DeMartino GN. Calcium-activated neutral protease (calpain) systemml: structure, function, and regulation. Physiol Rev. 1991;71:813–47. doi: 10.1152/physrev.1991.71.3.813. [DOI] [PubMed] [Google Scholar]
- 13.Saido TC, Sorimachi H, Suzuki K. Calpain: new perspectives in molecular diversity and physiological–pathological involvement. FASEB J. 1994;8:814–22. [PubMed] [Google Scholar]
- 14.Wang KK, Yuen PW. Calpain inhibition: an overview of its therapeutic potential. Trends Pharmacol Sci. 1994;15:412–19. doi: 10.1016/0165-6147(94)90090-6. [DOI] [PubMed] [Google Scholar]
- 15.Cuzzocrea S, McDonald MC, Mazzon E, et al. Calpain inhibitor I reduces the development of acute and chronic inflammation. Am J Pathol. 2000;157:2065–79. doi: 10.1016/S0002-9440(10)64845-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Emori Y, Kawasaki H, Imajoh S, Minami Y, Suzuki K. All four repeating domains of the endogenous inhibitor for calcium-dependent protease independently retain inhibitory activity. Expression of the cDNA fragments in Escherichia coli. J Biol Chem. 1988;263:2364–70. [PubMed] [Google Scholar]
- 17.Garach-Jehoshua O, Ravid A, Liberman UA, Reichrath J, Glaser T, Koren R. Upregulation of the calcium-dependent protease, calpain, during keratinocyte differentiation. Br J Dermatol. 1998;139:950–7. doi: 10.1046/j.1365-2133.1998.02548.x. [DOI] [PubMed] [Google Scholar]
- 18.Mimori T, Suganuma K, Tanami Y, et al. Autoantibodies to calpastatin (an endogenous inhibitor for calcium-dependent neutral protease, calpain) in systemic rheumatic diseases. Proc Natl Acad Sci USA. 1995;92:7267–71. doi: 10.1073/pnas.92.16.7267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Despres N, Talbot G, Plouffe B, Boire G, Menard HA. Detection and expression of a cDNA clone that encodes a polypeptide containing two inhibitory domains of human calpastatin and its recognition by rheumatoid arthritis sera. J Clin Invest. 1995;95:1891–6. doi: 10.1172/JCI117870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saulot V, Vittecoq O, Salle V, et al. Autoantibodies directed against the amino-terminal domain I of human calpastatin (ACAST-DI Ab) in connective tissue diseases. High levels of ACAST-DI Ab are associated with vasculitis in lupus. J Autoimmun. 2002;19:55–61. doi: 10.1006/jaut.2002.0598. [DOI] [PubMed] [Google Scholar]
- 21.Vittecoq O, Salle V, Jouen-Beades F, et al. Autoantibodies to the 27 C-terminal amino acids of calpastatin are detected in a restricted set of connective tissue diseases and may be useful for diagnosis of rheumatoid arthritis in community cases of very early arthritis. Rheumatology. 2001;40:1126–34. doi: 10.1093/rheumatology/40.10.1126. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki K, Shimizu K, Hamamoto T, Nakagawa Y, Murachi T, Yamamuro T. Characterization of proteoglycan degradation by calpain. Biochem J. 1992;285:857–62. doi: 10.1042/bj2850857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sato S, Hasegawa M, Nagaoka T, et al. Autoantibodies against calpastatin in sera from patients with systemic sclerosis. J Rheumatol. 1998;25:2135–9. [PubMed] [Google Scholar]
- 24.Fredriksson T, Pettersson U. Severe psoriasis − oral therapy with a new retinoid. Dermatologica. 1978;157:238–44. doi: 10.1159/000250839. [DOI] [PubMed] [Google Scholar]
- 25.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 26.Imajoh S, Kawasaki H, Suzuki K. Limited autolysis of calcium-activated neutral protease (CANP): reduction of the Ca2+-requirement is due to the NH2-terminal processing of the large subunit. J Biochem (Tokyo) 1986;100:633–42. doi: 10.1093/oxfordjournals.jbchem.a121755. [DOI] [PubMed] [Google Scholar]
- 27.Meijerink J, Mandigers C, van de Locht L, Tonnissen E, Goodsaid F, Raemaekers J. A novel method to compensate for different amplification efficiencies between patient DNA samples in quantitative real-time PCR. J Mol Diag. 2001;3:55–61. doi: 10.1016/S1525-1578(10)60652-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kunimatsu MH, Igashiyama S, Sato K, Ohkubo I, Sasaki M. Calcium dependent cysteine proteinase is a precursor of a chemotactic factor for neutrophils. Biochem Biophys Res Commun. 1989;164:875–82. doi: 10.1016/0006-291x(89)91540-4. [DOI] [PubMed] [Google Scholar]
- 29.Pontremoli S, Melloni E, Damiani G, et al. Effects of a monoclonal anti-calpain antibody on responses of stimulated human neutrophils. Evidence for a role for proteolytically modified protein kinase C. J Biol Chem. 1988;263:1915–19. [PubMed] [Google Scholar]
- 30.Kobayashi Y, Yamamoto K, Saido T, Kawasaki H, Oppenheim JJ, Matsushima K. Identification of calcium-activated neutral protease as a processing enzyme of human interleukin 1α. Proc Natl Acad Sci USA. 1990;87:5548–52. doi: 10.1073/pnas.87.14.5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Debets R, Hegmans JP, Troost RJ, Benner R, Prens EP. Enhanced production of biologically active interleukin-1α and interleukin-1β by psoriatic epidermal cells ex vivo: evidence of increased cytosolic interleukin-1β levels and facilitated interleukin-1 release. Eur J Immunol. 1995;25:1624–30. doi: 10.1002/eji.1830250623. [DOI] [PubMed] [Google Scholar]
- 32.Groves RW, Mizutani H, Kieffer JD, Kupper TS. Inflammatory skin disease in transgenic mice that express high levels of interleukin 1α in basal epidermis. Proc Natl Acad Sci USA. 1995;92:11874–8. doi: 10.1073/pnas.92.25.11874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grossman RM, Krueger J, Yourish D, et al. Interleukin 6 is expressed in high levels in psoriatic skin and stimulates proliferation of cultured human keratinocytes. Proc Natl Acad Sci USA. 1989;86:6367–71. doi: 10.1073/pnas.86.16.6367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Boer OJ, Wakelkamp IM, Pals ST, Claessen N, Bos JD, Das PK. Increased expression of adhesion receptors in both lesional and non-lesional psoriatic skin. Arch Dermatol Res. 1994;286:304–11. doi: 10.1007/BF00402220. [DOI] [PubMed] [Google Scholar]
- 35.Miyachi Y, Yoshimura N, Suzuki S, et al. Biochemical demonstration and immunohistochemical localization of calpain in human skin. J Invest Dermatol. 1986;86:346–9. doi: 10.1111/1523-1747.ep12285556. [DOI] [PubMed] [Google Scholar]
- 36.Fukui I, Tanaka K, Murachi T. Extracellular appearance of calpain and calpastatin in the synovial fluid of the knee joint. Biochem Biophys Res Commun. 1989;162:559–66. doi: 10.1016/0006-291x(89)92347-4. [DOI] [PubMed] [Google Scholar]
- 37.Ortonne JP. Aetiology and pathogenesis of psoriasis. Br J Dermatol. 1996;135(Suppl. 49):1–5. doi: 10.1111/j.1365-2133.1996.tb15660.x. [DOI] [PubMed] [Google Scholar]