Abstract
Adoptive antigen-specific immunotherapy is an attractive concept for the treatment of cancer because it does not require immunocompetence of patients, and the specificity of transferred lymphocytes can be targeted against tumour-associated antigens that are poorly immunogenic and thus fail to effectively trigger autologous T cell responses. As the isolation and in vitro expansion of antigen-specific lymphocytes is difficult, ‘conventional’ adoptive T cell therapy can only be carried out in specialized centres in small numbers of patients. However, T cell receptor (TCR) genes isolated from antigen-specific T cells can be exploited as generic therapeutic molecules for ‘unconventional’ antigen-specific immunotherapy. Retroviral TCR gene transfer into patient T cells can readily produce populations of antigen-specific lymphocytes after a single round of polyclonal T cell stimulation. TCR gene modified lymphocytes are functionally competent in vitro, and can have therapeutic efficacy in murine models in vivo. TCR gene expression is stable and modified lymphocytes can develop into memory T cells. Introduction of TCR genes into CD8+ and CD4+ lymphocytes provides an opportunity to use the same TCR specificity to produce antigen-specific killer and helper T lymphocytes. Thus, TCR gene therapy provides an attractive strategy to develop antigen-specific immunotherapy with autologous lymphocytes as a generic treatment option.
Keywords: cancer, CTL, immunotherapy, gene therapy, TCR
Therapeutic vaccination
Vaccination, the administration of antigen preparations in an immune-stimulating formulation, is an effective form of immune prevention with major health benefits for human populations worldwide. Vaccines have led to the eradication of some infectious diseases, such as smallpox, and to the control of spread of many other transmissible diseases. In general, effective vaccines are immunogenic and stimulate potent T cell and antibody responses, and they are usually administered prior to exposure to the disease-causing pathogen. In contrast, the therapeutic benefit of vaccines is much less impressive when given to patients with ongoing acute or chronic infections complications [1]. In this case, the disease-causing pathogen has already established its presence and, in so doing, suppressed or escaped protective immunity. This is similar to the situation in cancer patients, where malignant cells suppress or escape protective immune responses. Immune responses against cancer cells are dampened further by weak immunogenicity of tumour-associated antigens, which often fail to trigger strong immune responses as they are also expressed in normal tissues [2]. Expression in normal tissues can lead to clonal T and B cell deletion, development of anergy or the induction of antigen-specific CD4+ CD25+ immunosuppressive regulatory T cells. The weak immunogenicity of tumour antigens, the existence of tolerance mechanisms and the compromised immunocompetence of many cancer patients provide a strong incentive for adoptive immunotherapy approaches that do not rely on the patient's own immune responses.
Conventional adoptive immunotherapy
The targeting of tumour-associated antigens, such as CD20, Her2/Neu and CD33, with antigen-specific monoclonal antibodies has been tested in large numbers of patients and has shown clear clinical benefits [3]. The passively acquired antibodies can trigger apoptosis in tumour cells and activate complement-mediated or antibody-dependent cellular cytotoxicity in patients. In contrast to the widely documented clinical benefit of antibody treatment, the experience with adoptive transfer of antigen-specific T cell populations is much more limited. The most convincing demonstration of the clinical benefit of adoptive T cell transfer came from studies in immunosuppressed transplant patients who developed Epstein–Barr virus (EBV)-driven lymphoblastoid proliferation. Transfer of EBV-specific T cell populations into these immunosuppressed patients can be used therapeutically to clear lymphoblasts, or it can be used prophylactically to prevent disease development [4]. Similarly, the adoptive transfer of cytomegalovirus (CMV)-specific T cell populations can control CMV load in immunosuppressed post-transplant patients [5,6]. The antigen-specific T cell populations used for adoptive immunotherapy of EBV and CMV disease are derived from healthy donors who were exposed to the pathogen. Thus, the immune response was initiated in vivo in immunocompetent individuals, followed by in vitro expansion of antigen-specific T cells to obtain sufficient numbers for adoptive transfer.
The production of T cell populations specific for tumour-associated antigens is more complicated. Tumour antigens are less immunogenic than viral antigens, and the immune response occurs in cancer patients who are often immunocompromised by the disease or by the treatment. Nevertheless, the expansion of T cell populations specific for tumour-associated antigens has been achieved in melanoma patients [2]. Recently, it was shown that the infusion of such T cell populations into melanoma patients conditioned by non-myeloablative chemotherapy resulted in substantial T cell expansion and in the reduction, even clearance, of tumour cells in patients [7,8]. To date, such impressive results are limited largely to melanoma. It is possible that melanoma cells are better antigen-presenting cells than other cancers, and that the melanoma-associated antigens, such as MelanA, tyrosinase and gp100, are more immunogenic than other tumour-associated antigens. Unlike melanoma antigens, other tumour-associated antigens are expressed more widely in normal tissues (e.g. p53; MDM2) or in cell types that are easily accessible to T cells, such as haematopoietic stem cells expressing the tumour-associated WT1 antigen [9,10]. As a consequence, tolerance mechanisms may purge high-avidity T cells with specificity for these tumour-associated antigens, while low-avidity T cells are retained in the autologous repertoire.
Because low-avidity cytotoxic T lymphocytes (CTL) were shown to be less effective in providing in vivo protection than high-avidity CTL [11,12], it is important to increase the avidity of CTL responses against tumour-associated antigens. This can be achieved by exploiting alloreactive CTL to circumvent partial or complete tolerance to tumour-associated antigens [13]. As tolerance is major histocompatibility complex (MHC)-restricted [14,15], it is possible to use allogeneic responder T cells to isolate high-avidity CTL specific tumour-associated antigens [16]. Furthermore, it is possible to select CTL populations that kill tumour cells efficiently but not normal cells expressing lower levels of the CTL-recognized target protein [16–18]. Although such CTL are specific for a self-antigen, they are functionally tumour-reactive and do not show any signs of normal tissue damage when transferred adoptively in murine model experiments [19].
The isolation of CTL specific for tumour-associated antigens is a time-consuming and labour-intensive process that fails on many occasions. Hence, it is hugely attractive to exploit the specificity of a well-characterized, tumour antigen-specific CTL line and use it for therapy in many cancer patients. In this strategy, therapy is no longer achieved by adoptive transfer of T cell populations, but by molecular transfer of T cell specificity. This strategy does not require histocompatibility between donor T cells and recipient patients, and provides an opportunity to introduce the specificity of allogeneic T cells into autologous T cells.
Post-conventional adoptive immunotherapy
CTL specificity is exclusively dictated by the T cell receptor (TCR), consisting of a heterodimeric alpha and beta chain. Thus, the transfer of TCR genes from donor to recipient T cells results in specificity transfer (Fig. 1). TCR gene transfer was first demonstrated in the melanoma system, although the efficiency was low in the initial studies [20]. More recently, vectors and gene transfer protocols have been improved substantially and it is now possible to achieve gene transfer routinely into 30–60% of human and murine T cell populations [21–29].
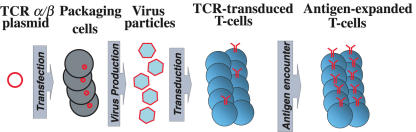
Fig. 1.
Retroviral TCR gene transfer. Retroviral DNA constructs are transfected into packaging cells to produce viral particles. Peripheral blood lymphocytes are polyclonally activated, using anti-CD3 antibodies or beads coated with anti-CD3/CD28 antibodies. Two days after activation lymphocytes are exposed to viral particles, and 5 days after activation TCR expression can be demonstrated by FACS analysis. Antigen-stimulation (in vitro or in vivo) leads to the expansion of cells expressing the introduced TCR.
All TCR gene transfer experiments to date were performed with retroviral vectors. The major advantage of retroviral vectors is that they have been studied extensively in experimental settings and there is substantial experience with these vectors in clinical trials [30]. This is an important aspect, as insertion of retroviral vectors into the host cell genome carries the risk of altering the expression pattern of genes flanking the insertion site. If such genes are involved in growth control, the altered expression profile may lead to uncontrolled growth and malignant transformation. For example, retroviral insertion into the LMO-2 locus in CD34+ haematopoietic stem cells is implicated in the development of leukaemia in immunodeficient children treated with retroviral vectors containing the gene for the common gamma chain of the IL-2 growth factor receptor, which is defective in these children. In a trial performed in France, leukaemia was observed in two of 12 treated children, suggesting a high risk of malignant transformation in this setting [31]. Fortunately, the risk of malignant transformation in mature T lymphocytes is substantially lower. For example, 31 patients were treated with more than 1011 lymphocytes infected with retroviral vectors encoding a truncated nerve growth factor receptor, and no adverse side effects due to retroviral insertions have been observed [30]. This indicates that retroviral gene transfer into mature lymphocytes is relatively safe. The relative safety in mature T cells is probably related to a lower risk of malignant transformation of fully differentiated end-stage cells compared to stem cells. In addition, it is likely that retroviral vectors insert at different sites in mature cells compared to stem cells, as insertion occurs preferentially near the promoter region of transcriptionally active genes.
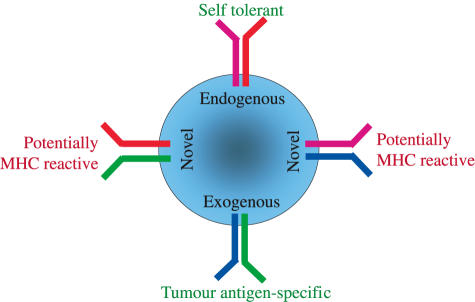
A concern of TCR gene transfer is the pairing of introduced TCR chains with endogenous chains (Fig. 2). Conceptually, it is possible that all transduced lymphocytes display four specificities (1): the specificity of the endogenous TCR α/β dimer; (2) the specificity of the introduced TCR α/β dimer; (3) the specificity of a TCR consisting of the endogenous α paired with introduced β chain; and (4) the specificity of a TCR consisting of endogenous β paired with introduced α chain. Specificities 3 and 4 are a safety risk as the newly assembled TCRs may be directed against patient MHC molecules. Theoretically, this risk would appear to be high, as studies of alloreactivity have shown that more than 1% of TCR heterodimers are MHC reactive [32]. Normally, MHC-reactive T cells are removed from the TCR repertoire during thymic T cell selection, a mechanism that will not apply to novel TCR specificities created by gene transfer into mature T cells. If each gene-transduced lymphocyte expresses two novel TCR specificities it would be expected that more than 2% of transduced cells display anti-MHC reactivity.
Fig. 2.
Pairing of TCR chains in TCR-transduced lymphocytes. In principle, each TCR-transduced T cell can express four TCR specificities, two of which are novel specificities with a risk of displaying reactivity towards patient MHC molecules. As discussed in the text, experimental observations suggest that most transduced T cells do not express two novel specificities.
Surprisingly, anti-MHC reactivity has not been observed to date in TCR gene transduced lymphocyte populations, suggesting that novel TCR pairs are not readily assembled and expressed. This could be due to preferential pairing of TCR α/β combinations that were naturally selected during thymic T cell selection. Because both the endogenous and introduced α/β dimers were naturally selected, they would be expected to be assembled most efficiently in lymphocytes expressing all TCR chains. Alternatively, it is possible that lymphocytes expressing multiple TCRs can display only one functional activity. This is unlikely to be the case, as recent experiments have shown that transfer of a TCR specific for HA1 into CTL expressing endogenous receptors for CMV produced CTL that recognized both HA1 and CMV epitopes [25].
The retroviral transfer of TCR genes into polyclonally activated lymphocytes can rapidly produce T cell populations of desired antigen-specificity. The original CTL and the TCR transduced T cell populations have the same fine specificity, as determined by the ability to recognize peptide variants, and peptide titration experiments showed that the avidity of the TCR transduced cells is similar to that of the original CTL [33]. TCR transduced human T cells show stable TCR expression and can be cultured in vitro by antigen-specific stimulation for several months without loss of function (unpublished). Furthermore, murine experiments demonstrated that TCR transduced T cells can be injected and protect mice against virus infection and against tumour challenge. The transfer of TCR transduced lymphocytes also established long-term immunological memory in recipient mice [26]. Together, the data to date show that TCR transduced human CTL display long-term antigen-specific activity in vitro, and murine TCR transduced CTL mediate disease protection in vivo.
The current protocols of T cell transduction involve in vitro activation to achieve T cell proliferation that is required for effective retroviral infection. It is currently not known to what extent the in vitro activation conditions affect the in vivo performance of transduced T cell populations. It is likely that polyclonal activation with lectins (e.g. ConA) or antibodies (e.g. anti-CD3, anti-CD28 antibodies) affects the expression pattern of adhesion molecules, chemokines and chemokine receptors of T cell populations. This, in turn, may affect the in vivo migration and homing. Equally, it is likely that the cytokine milieu used forin vitro activation will impact on the cytokine production profile of TCR-transduced T cell populations. T cell activation in the presence of interleukin (IL)-4 and IL-10 would be expected to produce TH2-type T cell populations, while activation in the presence of interferon (IFN)-γ and IL-12 would be expected to produce primarily TH1-type cells. Unfortunately, retroviral gene transfer cannot be achieved in resting lymphocytes. This may be achievable with lentiviral vectors, which can infect non-dividing lymphocytes exposed to low doses of cytokines such as IL-2 and IL-7. Adoptive transfer of such minimally stimulated cells will reveal whether in vivo transfer of TCR transduced resting T cells provides more effective protection from disease and better memory development than transfer of fully activated T cells.
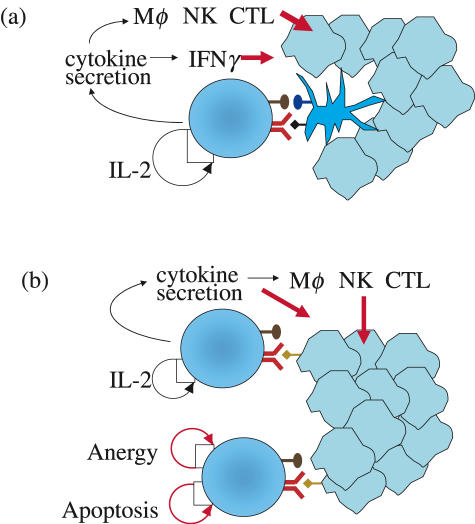
TCR gene transfer can also be used to produce antigen-specific CD4+ helper T cells. One possibility is to transfer the TCR genes of donor CD4+ helper cells to recipient T cells, thus producing MHC class II-restricted lymphocytes [23]. The presentation of antigen of MHC class II-negative tumours, in this situation, is mediated by professional antigen-presenting cells (APCs) that pick up tumour antigens and present epitopes in the context of MHC class II molecules (Fig. 3a). Alternatively, it is possible to use TCR gene transfer to produce MHC class I-restricted helper T cells. TCRs isolated from CD8-independent CTL would be expected to function in CD4+ helper T cells, enabling them to recognize directly epitopes presented by MHC class I molecules on tumour cells (Fig. 3b). It is also possible to exploit the TCR of CD8-dependent CTL for production of class I-restricted helper T cells. In this scenario, CD8 genes are introduced alongside TCR genes into CD4+ T cells producing double-positive CD4+ CD8+ lymphocytes with a class I restricted antigen receptor. CD8α homodimers and CD8 α/β heterodimers may have distinct effects on the cytokine production and proliferation potential of transduced CD4+ helper T cells.
Fig. 3.
The function of CD4+ helper T cells in tumour immunity. (a) Conventional CD4+ T cells recognize tumour antigens presented by MHC class II molecules on the surface of DCs. TCR engagement and signals derived from the interaction of co-stimulatory molecules such as B7·1, B7·2 and CD40 trigger the production of cytokines, including IL-2, which is required for proliferation. IFN-γ can have direct effects by inhibiting cells of the tumour stroma, or it can activate antitumour effector function of macrophages, NK cells and CTL. (b) CD4+ T cells expressing an MHC class I-restricted TCR can interact directly with tumour cells. In this case, the interaction is dominated by the TCR and is not assisted by co-stimulatory signals. It is currently not known if this will trigger full effector function and T cell expansion, or if TCR signal without co-stimulation may lead to lack of IL-2 production, the development of anergy or even the triggering of apoptosis.
The in vivo interaction of conventional CD4+ T cells is restricted largely to MHC class II-positive professional antigen-presenting cells. The interaction of CD4+ T cells and dendritic cells (DCs) is particularly important because CD40L/CD40 binding can deliver a ‘competence’ signal required for DC to acquire the ability to activate CD8+ T cells. It is likely that class I-restricted CD4+ T cells can also provide ‘competence’ signals to DCs. In this case TCR recognition of MHC class I-presented epitopes will trigger CD40L expression, thus leading to CD40 ligation on the surface of DC. Recognition of DC presented antigen triggers the production of cytokines by conventional CD4+ T cells. IL-2 has a direct effect on the CD4+ T cells and triggers proliferation and can prevent anergy induction. Other cytokines, such as IFN-γ, can activate macrophages and natural killer (NK) cells and enhance the functional activity of CTL (Fig. 3a). In addition, IFN-γ can have antitumour effects by inhibiting the formation of new blood vessels in the tumour stroma [34].
Unlike conventional CD4+ T cells, MHC class I-restricted helper cells can recognize peptide epitopes presented by MHC class II-negative non-professional APCs, such as tumour cells. Thus, it will be important to determine if this interaction leads to anergy of helper T cells, as suggested by some studies, or, as suggested by others, to T cell activation (Fig. 3b) [35–37]. This is a critical issue as anergic class I-restricted helper T cells may inhibit ongoing antitumour immune responses and thus promote tumour growth, while class I-restricted helper cells activated upon peptide recognition on the surface of tumour cells would enhance antitumour immunity. At present, it is difficult to predict if unconventional class I-restricted CD4+ T cells will, like conventional helper cells, increase long-term survival and memory development of CD8+ CTL, or whether the interaction of class I-restricted helper cells with non-professional APCs will be detrimental for their helper function.
Conclusion
The isolation and expansion of monoclonal T cells of defined antigen specificity is technically difficult. Once achieved, it is appealing to exploit monoclonal TCRs as generic reagents similar to the way monoclonal antibodies have been exploited. TCRs are most effective on the surface of CTL and T helper cells, where they can trigger a wide spectrum of effector functions including cytotoxicity and cytokine production. In addition, the injection of TCR expressing lymphocytes can have long-lasting therapeutic effects, due to the ability of lymphocytes to develop into memory cells. The introduction of unmodified TCR genes into lymphocytes is particularly attractive, because modified TCR chains may provoke immune responses and trigger rejection of injected lymphocytes, thus preventing memory development. Although far behind antibody-based therapies in many aspects, the TCR approach is one step ahead in terms of immunogenicity. High-avidity TCRs are isolated readily from human T cells and can be introduced reliably into patient lymphocytes. There is no requirement for histocompatibility between donor T cells and patients, and cloned TCR genes become generic molecules for therapy of all patients with a malignancy that expresses the TCR-recognized antigen and HLA allele required for antigen presentation.
Acknowledgments
This work was supported by the Leukaemia Research Fund, The Dinwoodie Trust and the Medical Research Council.
References
- 1.Hilleman MR. Strategies and mechanisms for host and pathogen survival in acute and persistent viral infections. Proc Natl Acad Sci USA. 2004;101:4560–6. doi: 10.1073/pnas.0404758101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenberg SA. Progress in human tumour immunology and immunotherapy. Nature. 2001;411:380–4. doi: 10.1038/35077246. [DOI] [PubMed] [Google Scholar]
- 3.Glennie MJ, van de Winkel JG. Renaissance of cancer therapeutic antibodies. Drug Discov Today. 2003;8:503–10. doi: 10.1016/s1359-6446(03)02714-4. [DOI] [PubMed] [Google Scholar]
- 4.Rooney CM, Smith CA, Ng CY, et al. Infusion of cytotoxic T cells for the prevention and treatment of Epstein–Barr virus-induced lymphoma in allogeneic transplant recipients. Blood. 1998;92:1549–55. [PubMed] [Google Scholar]
- 5.Walter EA, Greenberg PD, Gilbert MJ, et al. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N Engl J Med. 1995;333:1038–44. doi: 10.1056/NEJM199510193331603. [DOI] [PubMed] [Google Scholar]
- 6.Peggs KS, Verfuerth S, Pizzey A, et al. Adoptive cellular therapy for early cytomegalovirus infection after allogeneic stem-cell transplantation with virus-specific T-cell lines. Lancet. 2003;362:1375–7. doi: 10.1016/S0140-6736(03)14634-X. [DOI] [PubMed] [Google Scholar]
- 7.Dudley ME, Wunderlich JR, Robbins PF, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–4. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenberg SA, Dudley ME. Cancer regression in patients with metastatic melanoma after the transfer of autologous antitumor lymphocytes. Proc Natl Acad Sci USA. 2004;101:14639–45. doi: 10.1073/pnas.0405730101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ellisen LW, Carlesso N, Cheng T, Scadden DT, Haber DA. The Wilms tumor suppressor WT1 directs stage-specific quiescence and differentiation of human hematopoietic progenitor cells. EMBO J. 2001;20:1897–909. doi: 10.1093/emboj/20.8.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baird PN, Simmons PJ. Expression of the Wilms’ tumor gene (WT1) in normal hemopoiesis. Exp Hematol. 1997;25:312–20. [PubMed] [Google Scholar]
- 11.Zeh HJ, III, Perry-Lalley D, Dudley ME, Rosenberg SA, Yang JC. High avidity CTLs for two self-antigens demonstrate superior in vitro and in vivo antitumor efficacy. J Immunol. 1999;162:989–94. [PubMed] [Google Scholar]
- 12.Derby M, Alexander-Miller M, Tse R, Berzofsky J. High-avidity CTL exploit two complementary mechanisms to provide better protection against viral infection than low-avidity CTL. J Immunol. 2001;166:1690–7. doi: 10.4049/jimmunol.166.3.1690. [DOI] [PubMed] [Google Scholar]
- 13.Stauss HJ. Immunotherapy with CTLs restricted by nonself MHC. Immunol Today. 1999;20:180–3. doi: 10.1016/s0167-5699(99)01443-7. [DOI] [PubMed] [Google Scholar]
- 14.Rammensee HG, Bevan MJ. Evidence from in vitro studies that tolerance to self antigens is MHC-restricted. Nature. 1984;308:741–4. doi: 10.1038/308741a0. [DOI] [PubMed] [Google Scholar]
- 15.Matzinger P, Zamoyska R, Waldmann H. Self tolerance is H-2-restricted. Nature. 1984;308:738–41. doi: 10.1038/308738a0. [DOI] [PubMed] [Google Scholar]
- 16.Sadovnikova E, Stauss HJ. Peptide-specific cytotoxic T lymphocytes restricted by nonself major histocompatibility complex class I molecules: reagents for tumor immunotherapy. Proc Natl Acad Sci USA. 1996;93:13114–18. doi: 10.1073/pnas.93.23.13114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sadovnikova E, Jopling LA, Soo KS, Stauss HJ. Generation of human tumor-reactive cytotoxic T cells against peptides presented by non-self HLA class I molecules. Eur J Immunol. 1998;28:193–200. doi: 10.1002/(SICI)1521-4141(199801)28:01<193::AID-IMMU193>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 18.Gao L, Bellantuono I, Elsasser A, et al. Selective elimination of leukemic CD34(+) progenitor cells by cytotoxic T lymphocytes specific for WT1. Blood. 2000;95:2198–203. [PubMed] [Google Scholar]
- 19.Goa L, Yang TH, Sadovnikova E, Hasserjian R, Stauss HJ. Allo-MHC-restricted CTL engraft in bone marrow transplanted recipients without causing graft versus host disease. Blood. 1999;94:2999–3006. [PubMed] [Google Scholar]
- 20.Clay TM, Custer MC, Sachs J, Hwu P, Rosenberg SA, Nishimura MI. Efficient transfer of a tumor antigen-reactive TCR to human peripheral blood lymphocytes confers anti-tumor reactivity. J Immunol. 1999;163:507–13. [PubMed] [Google Scholar]
- 21.Cooper LJ, Kalos M, Lewinsohn DA, Riddell SR, Greenberg PD. Transfer of specificity for human immunodeficiency virus type 1 into primary human T lymphocytes by introduction of T-cell receptor genes. J Virol. 2000;74:8207–12. doi: 10.1128/jvi.74.17.8207-8212.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chamoto K, Tsuji T, Funamoto H, et al. Potentiation of tumor eradication by adoptive immunotherapy with T-cell receptor gene-transduced T-helper type 1 cells. Cancer Res. 2004;64:386–90. doi: 10.1158/0008-5472.can-03-2596. [DOI] [PubMed] [Google Scholar]
- 23.Fujio K, Misaki Y, Setoguchi K, et al. Functional reconstitution of class II MHC-restricted T cell immunity mediated by retroviral transfer of the alpha beta TCR complex. J Immunol. 2000;165:528–32. doi: 10.4049/jimmunol.165.1.528. [DOI] [PubMed] [Google Scholar]
- 24.Heemskerk MH, Hoogeboom M, de Paus RA, et al. Redirection of antileukemic reactivity of peripheral T lymphocytes using gene transfer of minor histocompatibility antigen HA-2-specific T-cell receptor complexes expressing a conserved alpha joining region. Blood. 2003;102:3530–40. doi: 10.1182/blood-2003-05-1524. [DOI] [PubMed] [Google Scholar]
- 25.Heemskerk MH, Hoogeboom M, Hagedoorn R, Kester MG, Willemze R, Falkenburg JH. Reprogramming of virus-specific T cells into leukemia-reactive T cells using T cell receptor gene transfer. J Exp Med. 2004;199:885–94. doi: 10.1084/jem.20031110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kessels HW, Wolkers MC, van den Boom MD, van der Valk MA, Schumacher TN. Immunotherapy through TCR gene transfer. Nat Immunol. 2001;2:957–61. doi: 10.1038/ni1001-957. [DOI] [PubMed] [Google Scholar]
- 27.Orentas RJ, Bircher LA, Roskopf S. Retroviral transfer of T-cell receptor genes produces cells with a broad range of lytic activity. Scand J Immunol. 2003;58:33–42. doi: 10.1046/j.1365-3083.2003.01277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stanislawski T, Voss RH, Lotz C, et al. Circumventing tolerance to a human MDM2-derived tumor antigen by TCR gene transfer. Nat Immunol. 2001;2:962–70. doi: 10.1038/ni1001-962. [DOI] [PubMed] [Google Scholar]
- 29.Tahara H, Fujio K, Araki Y, et al. Reconstitution of CD8+ T cells by retroviral transfer of the TCR alpha beta-chain genes isolated from a clonally expanded P815-infiltrating lymphocyte. J Immunol. 2003;171:2154–60. doi: 10.4049/jimmunol.171.4.2154. [DOI] [PubMed] [Google Scholar]
- 30.Bonini C, Grez M, Traversari C, et al. Safety of retroviral gene marking with a truncated NGF receptor. Nat Med. 2003;9:367–9. doi: 10.1038/nm0403-367. [DOI] [PubMed] [Google Scholar]
- 31.Hacein-Bey-Abina S, von Kalle C, Schmidt M, et al. A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency. N Engl J Med. 2003;348:255–6. doi: 10.1056/NEJM200301163480314. [DOI] [PubMed] [Google Scholar]
- 32.Zerrahn J, Held W, Raulet DH. The MHC reactivity of the T cell repertoire prior to positive and negative selection. Cell. 1997;88:627–36. doi: 10.1016/s0092-8674(00)81905-4. [DOI] [PubMed] [Google Scholar]
- 33.Schaft N, Willemsen RA, de Vries J, et al. Peptide fine specificity of anti-glycoprotein 100 CTL is preserved following transfer of engineered TCR alpha beta genes into primary human T lymphocytes. J Immunol. 2003;170:2186–94. doi: 10.4049/jimmunol.170.4.2186. [DOI] [PubMed] [Google Scholar]
- 34.Blankenstein T, Qin Z. The role of IFN-gamma in tumor transplantation immunity and inhibition of chemical carcinogenesis. Curr Opin Immunol. 2003;15:148–54. doi: 10.1016/s0952-7915(03)00007-4. [DOI] [PubMed] [Google Scholar]
- 35.Choi J, Enis DR, Koh KP, Shiao SL, Pober JS. T lymphocyte–endothelial cell interactions. Annu Rev Immunol. 2004;22:683–709. doi: 10.1146/annurev.immunol.22.012703.104639. [DOI] [PubMed] [Google Scholar]
- 36.Lawson C, McCormack AM, Moyes D, et al. An epithelial cell line that can stimulate alloproliferation of resting CD4+ T cells, but not after IFN-gamma stimulation. J Immunol. 2000;165:734–42. doi: 10.4049/jimmunol.165.2.734. [DOI] [PubMed] [Google Scholar]
- 37.Marelli-Berg FM, Lechler RI. Antigen presentation by parenchymal cells: a route to peripheral tolerance? Immunol Rev. 1999;172:297–314. doi: 10.1111/j.1600-065x.1999.tb01374.x. [DOI] [PubMed] [Google Scholar]