Abstract
CCR7 chemokine-receptor expression on tumour cells of gastric carcinoma has been associated with lymph-node metastasis and is thought to play an important role in metastasis. However, so far it is unknown whether CCR7 is newly up-regulated on gastric carcinoma or already expressed in non-neoplastic gastric epithelium. Therefore, epithelial CCR7 expression was investigated in the process of gastric carcinogenesis: non-inflamed mucosa –Helicobacter pylori gastritis – intestinal metaplasia/dysplasia – gastric carcinoma. CCR7 was expressed by gastric epithelium in non-inflamed gastric mucosa (n = 5), H. pylori gastritis (n = 17), intestinal metaplasia (n = 10), dysplasia (n = 3) and on tumour cells in 20 of 24 patients with gastric carcinoma (13/14 intestinal-type; 7/10 diffuse-type) as tested by immunohistochemistry. As CCR7 expression by gastric epithelium was significantly stronger in H. pylori gastritis than in non-infected mucosa, the influence of H. pylori on CCR7 receptor expression of gastric epithelial cells was investigated by fluorescence activated cell sorter analysis. H. pylori strains up-regulated the CCR7 chemokine-receptor in CCR7-positive cell lines. No difference in CCR7 up-regulation between cag+ and cag–H. pylori strains was found. Epithelial CCR7 up-regulation by H. pylori may alter the metastatic fate of gastric carcinoma. Additionally, CCR7 expression not only on gastric carcinoma, but also on non-neoplastic gastric epithelium, suggests a novel biological function.
Keywords: CCR7, gastric carcinoma, Helicobacter pylori, Helicobacter pylori gastritis, intestinal metaplasia/dysplasia
Introduction
Chemokines are involved in acute and chronic inflammatory processes by attracting neutrophils, monocytes and T cells to the site of inflammation via the corresponding chemokine receptors [1]. Several chemokines have been found to be expressed constitutively in lymphoid tissue, suggesting that these chemokines might exhibit homeostatic functions by regulating lymphocyte trafficking to or within lymphoid organs [2–4].
The biology of chemokines and chemokine receptors has become more complex with the demonstration of functional chemokine receptors on epithelial cells, which make them capable of responding to chemokines. Recent studies on tumour cells of breast, lung and melanoma suggest that tumour cells expressing CCR7 might use chemokine-mediated mechanisms during the process of lymph node metastasis [5–7]. In this context, expression of CCR7 by gastric carcinoma was found recently to be associated with lymph node metastasis [8].
In the stomach, infection with the bacterium Helicobacter pylori causes inflammation of the underlying mucosa, which results in chronic active H. pylori gastritis. Because most gastric carcinomas develop against the background of chronic active H. pylori gastritis via the epithelial precursor lesions intestinal metaplasia and dysplasia, the bacterium H. pylori is defined as a definitive carcinogen by the World Health Organization (WHO) [9].
So far it is not known whether CCR7 is newly up-regulated in gastric carcinoma or already expressed in non-neoplastic gastric epithelium during carcinogenesis. Therefore, we investigated CCR7 expression in the sequence of gastric carcinogenesis: non-inflamed stomach–H. pylori gastritis–intestinal metaplasia/gastric dysplasia–gastric carcinoma.
As, in our study, CCR7 expression by gastric epithelium was stronger in H. pylori gastritis than in the non-infected mucosa and CCR7 is known to be up-regulated by bacterial products on dendritic cells [10–12], the influence of H. pylori on CCR7 expression was tested in functional studies on gastric epithelial cell lines.
Materials and methods
Patients and selection of gastric tissue
Surgical tissue specimens from gastric antrum and corpus mucosa of five patients without H. pylori gastritis (four with tumour of the pancreas, one with oesophageal carcinoma) and of 17 patients with chronic active H. pylori gastritis were investigated in this study. Ten samples of these patients with chronic active H. pylori gastritis showed areas with intestinal metaplasia and three with low-grade dysplasia. In the patients without H. pylori gastritis no inflammatory cells were detectable in antrum and corpus mucosa and H. pylori colonization was excluded by a modified Giemsa stain. Chronic active H. pylori gastritis showed a moderate to severe chronic inflammatory infiltrate of T and B lymphocytes, plasma cells and monocytes and a mild to severe activity with neutrophils in the lamina propria, within the gastric epithelium and in the foveolar lumen. H. pylori colonization was mild to severe, as determined by a modified Giemsa stain.
Furthermore, surgical specimens from 24 gastric carcinomas were investigated. Gastric carcinomas were classified histopathologically according to Lauren [13] as either intestinal- (14/24) or diffuse-type (10/24).
This study, in which human tissue was collected from gastrectomy specimens used for routine histopathological diagnosis, was permitted by a local ethical commission.
Immunhistochemistry
The immunohistological staining procedure of formalin-fixed tissue sections was performed as described previously [14]. Anti-human CCR7 monoclonal antibody (BD Pharmingen, San Diego, CA, USA) was used as the first-step antibody.
As negative control the first monoclonal antibody was replaced by an isotype control antibody in the same dilution. Using this procedure no staining of the gastric tissue was detectable.
The intensity of CCR7 expression by epithelial cells was scored semiquantitatively by two independent researchers (B. S., M. E) as follows: none, weak, moderate, strong.
Cell culture and co-incubation with cagA-positive and cagA-negative H. pylori strains
Gastric carcinoma cell lines 3051, 23132/87, 4433 and 2957 (H. P. Vollmers, Institut für Pathologie, Universität Würzburg, Germany) and HM02 (gift from S. Suerbaum, Institut für Hygiene und Mikrobiologie, Medizinische Hochschule Hannover, Germany) were used for cell culture experiments.
H. pylori strain G27 is a clinical isolate which has been described previously [21]. G27/cag::kan is an isogenic derivative of H. pylori G27 in which a 1545 base pairs (bp) DNA fragment comprising part of the coding regions of hp0546 (cagC) and hp0547 (cagA) as well as the intergenic region between the two open reading frames (ORFs) has been replaced by a kanamycin resistance cassette via allelic exchange mutagenesis. When recovered from frozen stocks H. pylori strains were grown under microaerophilic conditions (Oxoid) on Columbia agar plates containing 5% horse blood, 0·2% cyclodextrin and Dent's or Skirrow's antibiotic supplement at 37°C for 3 days. After passaging on fresh plates, bacteria were cultured in a 5% CO2/95% air atmosphere for another 24 h at 37°C. Gastric epithelial cell lines were co-incubated with bacteria at a multiplicity of infection (MOI) of 50 bacteria per cell for 48 h at 37°C with 10% CO2. As negative control, in every experiment epithelial cells were incubated with RPMI-1640 medium without H. pylori under the same conditions.
Measurement of CCR7 expression on gastric epithelial cell lines by fluorescence activated cell sorter (FACS) analysis
Gastric epithelial cell lines were washed with phosphate buffered saline (PBS) containing 2% fetal calf serum (FCS) and reacted for 30 min with monoclonal mouse anti-CCR7 antibody (BD Pharmingen, San Diego, CA, USA) in an appropriate dilution. After washing, cells were reacted for 15 min with ptycoerythrin (PE)-conjugated donkey antimouse IgG Fab fragments (Jackson Immunoresearch Laboratories, West Grove, PA, USA). After staining cells were analysed on a FACScan flow cytometer (Becton Dickinson Biosciences, Mountain View, CA, USA) with appropriate gatings.
Results
Expression of CCR7 in non-inflamed gastric mucosa and in H. pylori gastritis
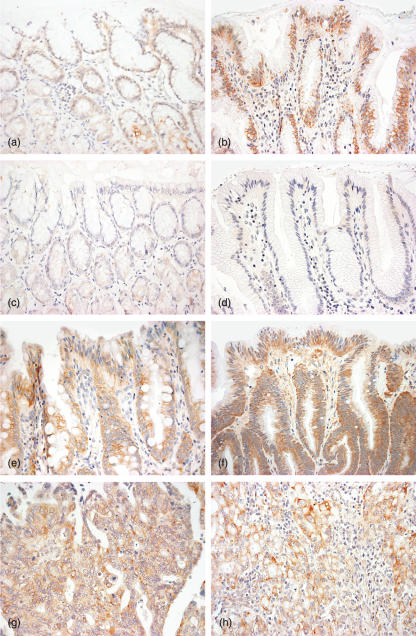
CCR7 was expressed by gastric epithelium in all patients with non-inflamed gastric mucosa (n = 5, Fig. 1a) and H. pylori gastritis (n = 17, Fig. 1b) as well in the antrum, as in the corpus. CCR7 expression by gastric epithelium was clearly stronger in H. pylori gastritis (Fig. 1b) than in the non-inflamed gastric mucosa (Fig. 1a). In the non-inflamed gastric mucosa CCR7 tended to be weak, whereas in H. pylori gastritis CCR7 expression was usually moderate to strong.
Fig. 1.
CCR7 is expressed on epithelium of non-inflamed gastric mucosa (a), H. pylori gastritis (b), intestinal metaplasia (e), dysplasia (f) and gastric carcinoma (g,h) as shown by immunohistochemistry. CCR7 control staining was negative, as shown clearly in non-inflamed gastric mucosa (c) and H. pylori gastritis (d). Epithelium of H. pylori gastritis, intestinal metaplasia, dysplasia and gastric carcinoma clearly expressed CCR7 stronger than the epithelium of non-inflamed gastric mucosa, suggesting that CCR7 is up-regulated during carcinogenesis. Original magnification: a–h ×400.
Additionally, CCR7 was detected in H. pylori gastritis on mononuclear cells (Fig. 1b), morphologically representing lymphocytes and dendritic cells. In the non-inflamed gastric mucosa CCR7 was not expressed in other cells than gastric epithelium (Fig. 1a).
Expression of CCR7 in gastric carcinoma and its precursor lesions, intestinal metaplasia and dysplasia
CCR7 showed a moderate to strong expression in the gastric mucosa of patients with H. pylori gastritis in all areas with intestinal metaplasia (n = 10, Fig. 1e) and dysplasia (n = 3, Fig. 1f). CCR7 was expressed on tumour cells in 13 of 14 patients with intestinal-type (Fig. 1g) and in seven of 10 patients with diffuse-type gastric carcinoma (Fig. 1h). In the CCR7-positive gastric carcinomas the number of CCR7-expressing tumour cells ranged from 5% to 80%, whereas in 15 of 20 tumours CCR7 expression was found in over 40% of the carcinoma cells.
CCR7 expression intensity of intestinal metaplasia/dysplasia and gastric cancer was similar to the gastric epithelium of H. pylori gastritis.
In most gastric carcinomas tumour infiltrating lymphocytes were positive for CCR7. In particular, all CCR7-negative gastric carcinomas (n = 4) showed CCR7 expressing tumour infiltrating lymphocytes as internal positive control.
CCR7 expression of gastric epithelial cell lines is up-regulated by H. pylori independent of the cag status
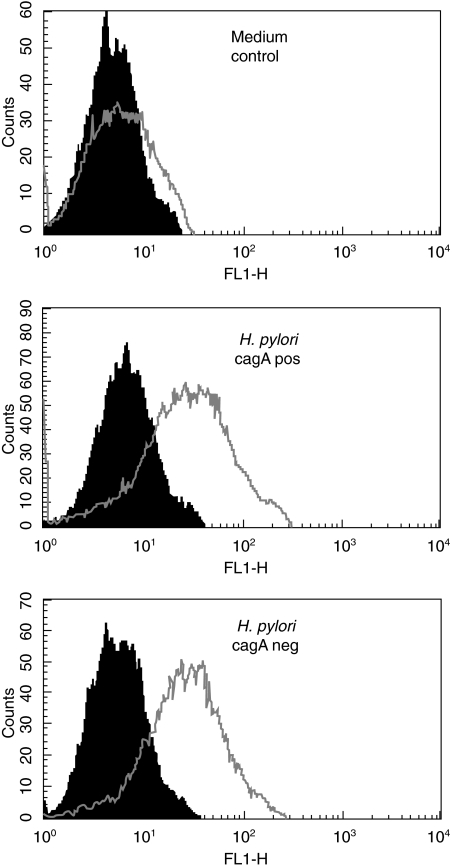
Because, in immunohistochemical staining, epithelial CCR7 expression was stronger H. pylori gastritis than in non-infected mucosa, the influence of H. pylori on CCR7 receptor expression was investigated in vitro using gastric epithelial cell lines. CCR7 was expressed in three of five gastric epithelial cell lines (3051, 2957, HM02) as determined by FACS analysis. Co-incubation with cag+ as cag–H. pylori strains also resulted in two of the CCR7-positive cell lines (3051, HMO2) in CCR7 up-regulation when compared with medium control (Fig. 2). There was no difference in CCR7 receptor up-regulation between cag+ and cag–H. pylori strains (Fig. 2), indicating that this process is independent of the cag status of H. pylori.
Fig. 2.
H. pylori up-regulates the CCR7 receptor on human gastric epithelial cell lines as determined by FACS analysis. Incubation of the cell line HM02 with a cag+ H. pylori strain as well as with the corresponding cag– deletion mutant resulted in up-regulation of CCR7 after 48 h when compared with medium control. The x-axis indicates fluorescence intensity measured on a log10 scale, and the y-axis indicates event counts per channel on a linear scale. Filled histograms represent the isotype-antibody control for each staining; open histograms represent the staining results with CCR7 antibody.
Discussion
The biology of chemokines and chemokine receptors has become more complex with the demonstration of functional chemokine receptors on malignant tumours, which make them capable of responding to chemokines. In particular, the chemokine receptor CCR7, which is normally essential for migration of lymphocytes and dendritic cells to lymph nodes, is thought to play an important role in the metastatic destination of many carcinomas, e.g. carcinomas of the breast, lung and stomach [5,6,8].
Because most gastric carcinomas arise from the background of H. pylori gastritis via the epithelial precursor lesions, intestinal metaplasia and dysplasia, the question arises as to whether CCR7 is newly up-regulated in gastric carcinoma, perhaps by genetic or transcriptional changes, or already expressed during carcinogenesis in the non-neoplastic gastric epithelium.
In this study CCR7 was expressed by most gastric carcinomas, which is in line with previous data [8]; but for the first time CCR7 expression could also be demonstrated in the precancerous lesions, intestinal metaplasia and dysplasia, as in the non-neoplastic epithelium of H. pylori gastritis and of non-inflamed gastric mucosa.
Chemokine receptors expressed on non-neoplastic epithelial cells are suggested to play a role in epithelial physiology. For example, CXCR4 expressed on the colon epithelium may regulate epithelial maintenance and renewal [15,16]. In the human kidney constitutively expressed CCR7 is hypothezised to be involved in the process of tissue homeostasis and regeneration [17]. Therefore, expression of CCR7 on non-neoplastic epithelium of the gastric mucosa suggests a novel biological function in addition to metastasis in malignant epithelial cells.
In our study, in vivo epithelial CCR7 expression was significantly stronger in H. pylori gastritis than in non-inflamed gastric mucosa and CCR7 was up-regulated in vitro by H. pylori. Therefore, it may be concluded that CCR7 is expressed constitutively on non-neoplastic gastric epithelium, up-regulated during H. pylori infection and then preserved in the process of carcinogenesis. Interestingly, epithelial CCR7 up-regulation was independent of the virulence factor cytotoxin-associated antigen (CagA) of H. pylori, which is known to be associated with more severe gastritis, gastric carcinoma and MALT-type lymphoma [18,19].
CCR7 receptor regulation on epithelial cells is not without precedence and has been described recently in squamous cell carcinoma of the head and neck [20]. Furthermore, CCR7 regulation by H. pylori is in line with studies on immune cells, where CCR7 receptor expression is modulated by bacterial products such as lipopolysaccharide and flagellin [10–12].
In this study we have demonstrated that CCR7 is expressed not only on gastric carcinoma, but also on non-neoplastic gastric epithelium, and that CCR7 expression is regulated by H. pylori. Further studies are required to show the biological function of CCR7 expression on non-neoplastic epithelium and whether up-regulation of CCR7 is relevant to specifically alter the metastastic fate of gastric carcinoma.
Acknowledgments
This study was supported by the Deutsche Forschungsgemeinschaft grant EC 203/1 and EC 203/2. We thank E. Bachmann and E. Schmitt for excellent technical assistance.
References
- 1.Luster AD. Chemokines – chemotactic cytokines that mediate inflammation. N Engl J Med. 1998;338:436–45. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- 2.Campbell JJ, Butcher EC. Chemokines in tissue-specific and microenvironment-specific lymphocyte homing. Curr Opin Immunol. 2000;2:336–41. doi: 10.1016/s0952-7915(00)00096-0. [DOI] [PubMed] [Google Scholar]
- 3.Moser B, Loetscher P. Lymphocyte traffic control by chemokines. Nat Immunol. 2001;2:123–8. doi: 10.1038/84219. [DOI] [PubMed] [Google Scholar]
- 4.Muller G, Hopken UE, Lipp M. The impact of CCR7 and CXCR5 on lymphoid organ development and systemic immunity. Immunol Rev. 2003;195:117–35. doi: 10.1034/j.1600-065x.2003.00073.x. [DOI] [PubMed] [Google Scholar]
- 5.Muller A, Homey B, Soto H, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–6. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 6.Takanami I. Overexpression of CCR7 mRNA in nonsmall cell lung cancer: correlation with lymph node metastasis. Int J Cancer. 2003;105:186–9. doi: 10.1002/ijc.11063. [DOI] [PubMed] [Google Scholar]
- 7.Payne AS, Cornelius LA. The role of chemokines in melanoma tumor growth and metastasis. J Invest Dermatol. 2002;118:915–22. doi: 10.1046/j.1523-1747.2002.01725.x. [DOI] [PubMed] [Google Scholar]
- 8.Mashino K, Sadanaga N, Yamaguchi H, et al. Expression of chemokine receptor CCR7 is associated with lymph node metastasis of gastric carcinoma. Cancer Res. 2002;62:2937–41. [PubMed] [Google Scholar]
- 9.IARC. Schistosomes, liver flukes, and Helicobacter pylori: views and expert opinions of an IARC Working Group on the evaluation of carcinogenic risks to humans. Lyon: IARC; 1994. Working Group on the Evaluation of Carcinogenic Risks to Humans: Helicobacter pylori; pp. 177–240. [PMC free article] [PubMed] [Google Scholar]
- 10.Sallusto F, Schaerli P, Loetscher P, et al. A rapid and coordinated switch in chemokine receptor expression during dendritic cell maturation. Eur J Immunol. 1998;28:2760–9. doi: 10.1002/(SICI)1521-4141(199809)28:09<2760::AID-IMMU2760>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 11.Jarrossay D, Napolitani G, Colonna M, et al. Specialization and complementarity in microbial molecule recognition by human myeloid and plasmacytoid dendritic cells. Eur J Immunol. 2001;31:3388–93. doi: 10.1002/1521-4141(200111)31:11<3388::aid-immu3388>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 12.Means TK, Hayashi F, Smith KD, et al. The Toll-like receptor 5 stimulus bacterial flagellin induces maturation and chemokine production in human dendritic cells. J Immunol. 2003;170:5165–75. doi: 10.4049/jimmunol.170.10.5165. [DOI] [PubMed] [Google Scholar]
- 13.Eck M, Schmausser B, Scheller K, et al. Pleiotropic effects of CXC chemokines in gastric carcinoma: differences in CXCL8 and CXCL1 expression between diffuse and intestinal types of gastric carcinoma. Clin Exp Immunol. 2003;134:508–15. doi: 10.1111/j.1365-2249.2003.02305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmausser B, Andrulis M, Endrich S, et al. Expression and subcellular distribution of toll-like receptors TLR4, TLR5 and TLR9 on the gastric epithelium in Helicobacter pylori infection. Clin Exp Immunol. 2004;136:521–6. doi: 10.1111/j.1365-2249.2004.02464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murdoch C, Monk PN, Finn A. Functional expression of chemokine receptor CXCR4 on human epithelial cells. Immunology. 1999;98:36–41. doi: 10.1046/j.1365-2567.1999.00848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jordan NJ, Kolios G, Abbot SE, et al. Expression of functional CXCR4 chemokine receptors on human colonic epithelial cells. J Clin Invest. 1999;104:1061–9. doi: 10.1172/JCI6685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Banas B, Wornle M, Berger T, et al. Roles of SLC/CCL21 and CCR7 in human kidney for mesangial proliferation, migration, apoptosis, and tissue homeostasis. J Immunol. 2002;168:4301–7. doi: 10.4049/jimmunol.168.9.4301. [DOI] [PubMed] [Google Scholar]
- 18.Blaser MJ, Perez-Perez GI, Kleanthous H, et al. Infection with Helicobacter pylori strains possessing cag A is associated with an increased of developing adenocarcinoma of the stomach. Cancer Res. 1995;55:2111–15. [PubMed] [Google Scholar]
- 19.Eck M, Schmaußer B, Haas R, et al. MALT-type lymphoma of the stomach is associated with Helicobacter pylori strains expressing CagA protein. Gastroenterology. 1997;112:1482–6. doi: 10.1016/s0016-5085(97)70028-3. [DOI] [PubMed] [Google Scholar]
- 20.Wang J, Xi L, Hunt JL, et al. Expression pattern of chemokine receptor 6 (CCR6) and CCR7 in squamous cell carcinoma of the head and neck identifies a novel metastatic phenotype. Cancer Res. 2004;64:1861–6. doi: 10.1158/0008-5472.can-03-2968. [DOI] [PubMed] [Google Scholar]
- 21.Xiang Z, Censini SPF, Bayeli JL, et al. Analysis of expression of CagA and VacA virulence factors in 43 strains of Helicobacter pylori reveals that clinical isolates can be divided into two major types and that CagA is not necessary for expression of the vacuolating cytotoxin. Infect Immun. 1995;63:94–8. doi: 10.1128/iai.63.1.94-98.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]