Abstract
Estrogen has extensive effects on the immune system. The aim of the present experiments was to compare the effects of 17β-estradiol (E2) and 4-estren-3α,17β-diol (estren) on T lymphopoiesis and T cell-dependent inflammation. In order to investigate the role of estrogen receptors (ER) in the effects of E2 and estren on the immune system, ER knock-out mice lacking both ERα and ERβ (DERKO) were used. T lymphopoiesis and T cell-dependent inflammation were studied by investigating thymus cellularity, the delayed-type hypersensitivity (DTH) reaction, CD4+ T cells in spleen and serum levels of interleukin (IL)-6. As expected, the presence of ERs was mandatory for all the effects of E2. In contrast, treatment with estren reduced thymus cellularity in ER knock-out mice, indicating an effect through ER-independent pathways. Interestingly, estren suppressed only DTH, the frequency of CD4+ T cells in spleen and serum levels of IL-6 in wild-type (WT) mice, but not in mice lacking ERs. Thus, our study is the first to show that estren inhibits T lymphopoiesis via ER-independent pathways, whereas its suppressive effects on inflammation are ER-dependent.
Keywords: 17β-estradiol, knock-out mice, estren, estrogen receptor, thymus
Introduction
The multiple effects of estrogens on the immune system in mice have been documented extensively. For example, B lymphopoiesis is suppressed during pregnancy [1] and elevated in estrogen-deficient mice [2], and treatment with estrogen induces thymus atrophy [3,4] and reduces the frequency of both T and B lymphopoietic cells [5,6]. Furthermore, estrogen treatment results in decreased delayed-type hypersensitivity (DTH) reaction [7,8], granulocyte-mediated inflammation [9] and levels of interleukin (IL)-6 in serum [10]. To date there are two known estrogen receptors (ERs), ERα and ERβ[11,12] and disruption of ERs have been reported to be associated with certain immune maladies at high ages [13,14]. In genomic signalling pathways of estrogen, the estrogen molecules bind to intracellular or membrane bound ERs, ultimately activating transcriptional activity in the target cell. However, reports have shown that a variety of cell types respond rapidly, within seconds/minutes, making the genomic signalling pathway unlikely and suggesting instead non-genomic signalling pathways.
Postmenopausal hormone replacement therapy (HRT) has beneficial effects on the skeleton, but is associated with well-known side effects. This has led to an increased focus on finding synthetic estrogen-like substances that reproduce only the beneficial effects of estrogen. 4-estren-3α,17β-diol (estren) is a synthetic compound with structural similarities to E2.
The aim of the present experiments was to compare the effects of E2 and estren on T lymphopoiesis and T cell-dependent inflammation. By the use of ER knock-out mice lacking both ERα and ERβ (DERKO) we were able to show that the presence of ERs was mandatory for all the effects of E2. In contrast, treatment with estren reduced thymus cellularity in ER knock-out mice but suppressed DTH, the frequency of CD4+ T cells in spleen and serum levels of IL-6 only in wild-type (WT) mice. These findings indicate that estren inhibits T lymphopoiesis via ER-independent pathways, whereas its suppression of peripheral immune functions is ER-dependent.
Materials and methods
This study was approved by the ethical committee for animal experiments at Göteborg University. Mice were kept in the animal facility at Göteborg University under standard conditions of temperature and light, and had free access to fresh water and soy-free food pellets R70 (Lactamin AB, Stockholm, Sweden).
Generation and identification of WT and DERKO mice
Male and female double heterozygous (ERα+/−β+/−) mice from a mixed C57Bl/6 J/129 background were mated, resulting in WT (ERα+/+β+/+) and DERKO (ERα–/–β–/–) offspring [15,16]. Genotyping of tail DNA was performed as described previously [17,18].
Ovariectomy and treatment
Eleven-month-old female WT and DERKO mice were ovariectomized under Ketalar® (Pfizer AB, Täby, Sweden)/Domitor® (Orion Pharma, Espoo, Finland) anaesthesia. Mice were given daily subcutaneous (s.c.) injections of 17β-estradiol-3-benzoate (E2) (Sigma, St Louis, MO, USA) (0·7 µg/mouse/day) or 4-estren-3α,17β-diol (estren) (Steraloids Inc., Newport, RI, USA) (75 µg/mouse/day) during 4 weeks (Fig. 1). Control mice received vehicle olive oil (Apoteksbolaget). Earlier studies have revealed that vehicle alone has no effect on the parameters studied [19]. Each group consisted of five to eight mice.
Fig. 1.
Molecular structures of 17β-estradiol (E2) and 4-estren-3α,17β-diol (estren).
DTH reaction
Mice were sensitized by cutaneous application of 150 µl of 3% 4-ethoxymethylene-2-phenyloxazolone (OXA) (Sigma) dissolved in one-third acetone and two-thirds 99·5% ethanol, on the abdomen skin. Six days after sensitization, mice were challenged by application of 30 µl 1% OXA in olive oil on both sides of the right ear. The ear thickness was measured 24 h after challenge using an Oditest spring calliper (Kroeplin, Hessen, Germany). Results are presented as increase in ear thickness using the formula: ear thickness 24 h after challenge minus ear thickness of control ear (mm × 10−2).
Tissue collection and single-cell preparation
Mice were anaesthetized by Ketalar®/Domitor®, bled and killed by cervical dislocation. Thymi and spleens were removed and single-cell suspensions were prepared by mashing the organs through 70 µm cell strainers (Becton-Dickinson, Franklin Lakes, NJ, USA) (BD). After centrifugation at 515 g for 5 min, pelleted spleen cells were resuspended in Tris-buffered 0·83% NH4Cl solution (pH 7·29) for 5 min to lyse erythrocytes, and then washed in phosphate-buffered saline (PBS). The total number of leucocytes from the organs was calculated using an automated cell counter (Sysmex, Kobe, Japan). Thymus cells were resuspended in PBS, and spleen cells in complete medium (Iscove's medium (Sigma) enriched with 50 µg/ml gentamicin (Sigma), 4 mm l-glutamine (Sigma), 50 µm mercaptoethanol (Sigma) and 10% fetal calf serum (FCS) (Biological Ind., Beit Haemek, Israel) before use.
Flow cytometry
Spleen cells were subjected to fluorescence activated cell sorter (FACS) analysis. Cells were stained with phycoerythrin (PE) conjugated antibodies to CD4 (clone H129·19, BD PharMingen, Franklin Lakes, NJ, USA) and fluorescein isothiocyanate (FITC) labelled antibodies to CD8 (clone 53-6.7, BD PharMingen). Flow cytometry was performed on a FACSCalibur and analysed using Paint-A-Gate software (BD). The FACS data are presented as percentage positively stained spleen cells of all lymphocytes.
Serum E2 levels
Blood was collected at the termination of the experiment and sera were individually stored at −20°C until use. Serum levels of E2 were measured using an E2 radio immunoassay (RIA) scaled down 1 : 4 (Diagnostic Systems Laboratories Inc., Webster, TX, USA). The sensitivity of the assay was less than 1 pg/ml.
IL-6 bioassay
Levels of IL-6 in serum were measured by a bioassay, using cell line B13·29 subclone B9, which is dependent on IL-6 for growth. B9 cells were seeded into 96-well flat-bottomed plates (Nunc, Roskilde, Denmark) at a concentration of 5000 cells per well, and cultured in complete medium. Serum samples were diluted 1 : 50 and added in triplicate. Cultures were pulsed with 1 µCi [3H]-thymidine (Amersham Pharmacia Biotech, Uppsala, Sweden) after 68 h of culture, and the cells were harvested 4 h later. Recombinant mouse IL-6 (Nibsc, Hertfordshire, UK) was used as standard.
Statistical analysis
One-way anova followed by Fisher's exact test was used to compare data from control-, E2- or estren-treated mice. Results are presented as mean ± standard deviation. P < 0·05 was considered statistically significant.
Results
Treatment with estren does not influence serum levels of 17β-estradiol
WT and DERKO mice were treated with daily s.c. injections of 0·7 µg E2/mouse (Fig. 1) for 4 weeks, resulting in serum levels of 17β-estradiol similar to that found in normal female mice during the dioestrus phase [20]. In contrast, treatment with 75 µg estren/mouse/day (Fig. 1) resulted in serum levels of 17β-estradiol comparable to that of OVX vehicle-treated control mice.
Estren affects thymus cellularity via ER-independent pathways
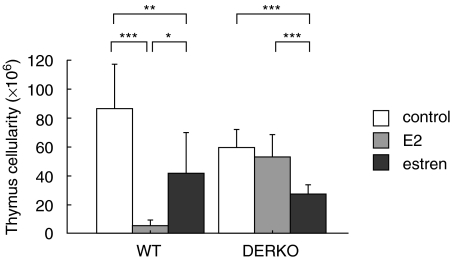
The effect of E2 and estren on thymus was investigated. We have shown previously that exposure to E2 of mice expressing ERα, but not ERβ, results in reduced thymus weight and cellularity [21]. In the present experiment we demonstrate the absence of E2-mediated reduction of thymus cellularity in mice lacking both ERα and ERβ (Fig. 2). In contrast, treatment with estren of WT and DERKO mice resulted in lower thymus cellularity in both genotypes (Fig. 2), indicating that estren affects the thymus by ER-independent pathways.
Fig. 2.
Estren affects thymus cellularity via ER-independent pathways. Thymus cellularity in 11-month-old OVX WT and DERKO mice is presented. The mice were given daily s.c. injections of 0·7 µg E2/mouse or 75 µg estren/mouse during 4 weeks. Control mice received vehicle olive oil. Treatment with E2 results in reduced thymus cellularity in WT mice but not in DERKO mice, while treatment with estren results in lower thymus cellularity also in DERKO mice. One-way anova followed by Fisher's exact test was used to compare data from control mice with E2 or estren-treated mice. *P < 0·05, **P < 0·01, ***P < 0·001. Results are presented as mean ± standard deviation. n = 5–8 mice in each group.
Estren inhibits T cell-dependent inflammation via ERs
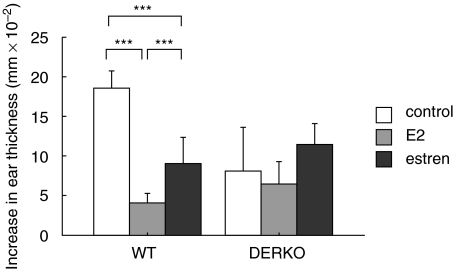
Seven days before termination of the experiment, the mice were sensitized by cutaneous application of 3% OXA. Six days later the mice were challenged by administration of 1% OXA on the right ear, and the DTH response was measured as swelling of the ear 24 h later. Results showed that both treatment with E2 and estren inhibited DTH responses in WT mice, while this could not be seen in DERKO mice (Fig. 3).
Fig. 3.
Estren inhibits the DTH response via ERs. DTH response of 11-month-old OVX WT and DERKO mice is presented. The mice were given daily s.c. injections of 0·7 µg E2/mouse or 75 µg estren/mouse during 4 weeks. Control mice received vehicle olive oil. Seven days before termination of the experiment, the mice were sensitized by administration of 3% OXA. Six days later the mice were challenged by administration of 1% OXA on the right ear, and swelling of the ear was measured 24 h later. Results are presented as thickness of challenged ear minus thickness of control ear (mm × 10−2). Treatment with both E2 and estren inhibited DTH responses in WT mice, while this could not be seen in DERKO mice. One-way anova followed by Fisher's exact test was used to compare data from control mice with E2- or estren-treated mice. ***P < 0·001. Results are presented as mean ± standard deviation. n = 5–8 mice in each group.
Estren-mediated inhibition of splenic CD4+ T cells is ER-dependent
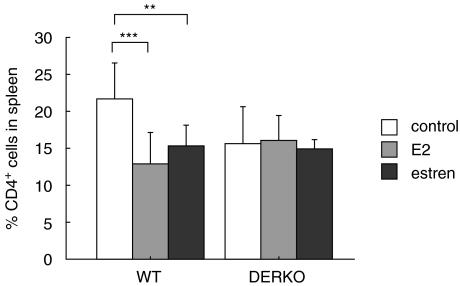
Flow cytometry analysis of spleen cells was performed. WT mice treated with E2 or estren displayed lower frequencies of CD4+ cells in the spleen compared to control mice, while this could not be seen in DERKO mice (Fig. 4). No differences could be detected in the frequency of CD8+ cells in the spleen (data not shown).
Fig. 4.
Estren inhibits the frequency of CD4+ T cells through ERs. Flow cytometry analysis of CD4+ cells in spleen of 11-month-old OVX WT and DERKO mice are presented. The mice were given daily s.c. injections of 0·7 µg E2/mouse or 75 µg estren/mouse during 4 weeks. Control mice received vehicle olive oil. Treatment with E2 or estren resulted in lower frequency of CD4+ cells in the spleen compared to control mice, while this could not be seen in DERKO mice. One-way anova followed by Fisher's exact test was used to compare data from control mice with E2- or estren-treated mice. **P < 0·01, ***P < 0·001. Results are presented as mean ± standard deviation. n = 5–8 mice in each group.
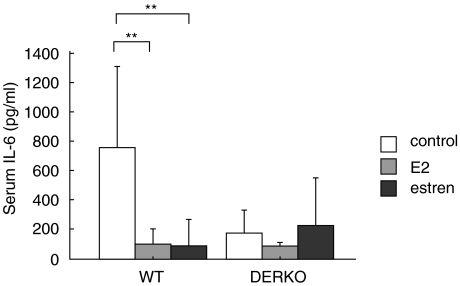
Estren suppresses serum levels of IL-6 via ER-dependent pathways
Serum samples taken at termination of the experiment were analysed for the presence of IL-6. Treatment with E2 or estren resulted in inhibited serum levels of IL-6 in WT mice but not in DERKO mice (Fig. 5).
Fig. 5.
Estren inhibits the level of IL-6 in serum through ERs. The levels of IL-6 in serum (pg/ml) of 11-month-old OVX WT and DERKO mice are presented. The mice were given daily s.c. injections of 0·7 µg E2/mouse or 75 µg estren/mouse during 4 weeks. Control mice received vehicle olive oil. Treatment with E2 or estren resulted in inhibited serum levels of IL-6 in WT mice but not in DERKO mice. One-way anova followed by Fisher's exact test was used to compare data from control mice with E2- or estren-treated mice. **P < 0·01. Results are presented as mean ± standard deviation. n = 5–8 mice in each group.
Discussion
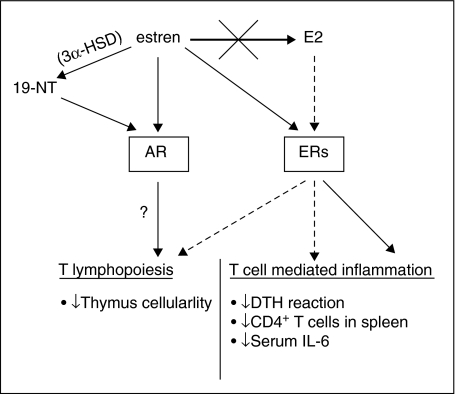
Postmenopausal hormone replacement therapy (HRT) has beneficial effects on the skeleton but is associated with well-known side effects. This has led to an increased focus on finding synthetic estrogen-like substances that reproduce only the beneficial effects of estrogen. The aims of the present study were to compare the effects of E2 and estren on thymus and T cell-dependent inflammation. WT and DERKO mice were used in order to investigate the ER dependence of E2 and estren-mediated effects on these parameters. Our study is the first to show that estren inhibits T lymphopoiesis via ER-independent pathways, whereas its suppressive effects on peripheral immune functions are ER-dependent. A schematic summary of possible pathways for these effects is shown in Fig. 6.
Fig. 6.
Schematic summary of the effects on immune responses and signalling pathways of E2 and estren. Treatment with estren (black arrows) inhibits T lymphopoiesis via ER-independent pathways (see also Fig. 2). Because AR stimulation is known to down-regulate both T and B lymphopoiesis [28–30], the non-ER-mediated effects of estren on thymus could result from activation of AR. One possibility is direct binding of estren to AR, or via 3α-HSD-mediated conversion of estren into 19–NT [25]. In contrast, the suppressive effects of estren on peripheral immune functions are ER-dependent (black arrow) (see also Figs 3–5). These effects are not due to conversion of estren into E2, as treatment with estren did not result in increased serum levels of 17β-estradiol (bold black arrow). As expected, the presence of ERs was mandatory for all the effects of E2 (hatched arrows) (see also Figs 2–5).
In previously published papers, Kousteni et al. proposed that sex steroids affect reproductive tissues by classical genomic signalling, while the bone sparing effect of sex steroids is mediated through a non-genomic pathway. It was also suggested that ERα, ERβ or the androgen receptor (AR) can transmit the non-genomic signalling pathway irrespective of whether the ligand is an estrogen or an androgen. Furthermore, Kousteni et al. showed that treatment with estren increases bone mass in OVX mice without affecting the reproductive organs, suggesting that estren is a mechanism-specific compound that reproduces only the non-genomic signalling of estrogen and thus can also affect target cells through the AR [22–24]. In a recently published paper, Centrella et al. found limited ER-dependent effects by estren in osteoblast cultures, and also showed that it binds poorly to both ER and AR in vitro. However, estren potently regulated AR-dependent effects on gene expression in osteoblasts. Interestingly, they also showed that estren can be metabolized into 19-NT by action of 3α–hydroxysteroid dehydrogenase (3α-HSD) (Fig. 6), and that 19-NT binds to AR with an affinity that was approximately 40% of that of dihydrotestosterone. Furthermore, estren activates the AR indirectly to regulate both androgen- and estrogen-like transcriptional responses by osteoblasts. In conclusion, these in vitro results support the hypothesis that the ER-independent effects of estren may be mediated through the AR [25].
However, we have demonstrated recently that the trabecular bone sparing effect of 17β–estradiol (E2) in vivo is mediated only via ERs and not via the AR. Furthermore, using ERα and ERβ expressing reporter cell lines, we showed that estren has the capacity to exert genomic effects via both ERα and ERβ. We showed that estren increases uterus weight and trabecular bone mineral density, and that these effects were mediated only via ERs and not via the AR. In conclusion, results from that study demonstrate that estren has the capacity to affect both bone and reproductive organs through classical genomic signalling via ERs [26].
In the present report we have studied the effects of estren and E2 on the immune system of aged ER-targeted mice. In previous studies we have demonstrated that estrogens affect the early development of the immune system in mice. The expression of ERα is mandatory for the full development of thymus and spleen [21]. Furthermore, ERα is required for the age-related increased frequency of IgM spot-forming cells in the bone marrow as well as for the increased production of IL-10 from cultured splenocytes in 18-month-old female mice [18]. We have also shown previously that young male DERKO mice display significantly lower serum levels of IL-6 compared to WT mice [27]. Thus, signalling through ERs is necessary for the development of an intact immune system.
As depicted schematically in Fig. 6, our study shows that estren inhibits T lymphopoiesis via ER-independent pathways. Because AR stimulation is also known to down-regulate both T and B cell development [28–30], the non-ER-mediated effects of estren on thymus may result from activation of AR. One possibility is direct binding of estren to AR, or via 3α-HSD-mediated conversion of estren into 19-NT [25]. Interestingly, our study showed clearly that the suppressive effects of estren on inflammation are ER-dependent. These effects were not due to conversion of estren into E2, because treatment with estren did not result in increased serum levels of 17β-estradiol. To investigate further how estren modulates the development of the immune system, we plan to use AR- and ER-blocking molecules.
Previous studies have shown that exposure to estrogen can ameliorate not only experimental autoimmune rheumatic diseases such as collagen type II induced arthritis [31], but can also be bone-protective and anti-arthritic in postmenopausal rheumatoid arthritis [32]. A limitation for the clinical use of estrogen is its well-recognized side effects, thus there is an increasing demand for new synthetic steroids with selective beneficial effects on bone and inflammation.
Acknowledgments
We thank Maud Petersson for excellent technical assistance. This study was supported by grants from the Börje Dahlin foundation, the Göteborg Medical Society, Association against Rheumatism, the King Gustav V's 80-year foundation, Reumaforskningsfond Margareta, the Medical Faculty of Göteborg University (LUA), the Novo Nordiska foundation, the Swedish Medical Research Council, KaroBio AB and the Swedish Cancer Fund.
References
- 1.Medina KL, Smithson G, Kincade PW. Suppression of B lymphopoiesis during normal pregnancy. J Exp Med. 1993;178:1507–15. doi: 10.1084/jem.178.5.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smithson G, Beamer WG, Shultz KL, Christianson SW, Shultz LD, Kincade PW. Increased B lymphopoiesis in genetically sex steroid-deficient hypogonadal (hpg) mice. J Exp Med. 1994;180:717–20. doi: 10.1084/jem.180.2.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marotti T, Sirotkovic M, Pavelic J, Gabrilovac J, Pavelic K. In vivo effect of progesterone and estrogen on thymus mass and T-cell functions in female mice. Horm Metab Res. 1984;16:201–3. doi: 10.1055/s-2007-1014742. [DOI] [PubMed] [Google Scholar]
- 4.Bodey B, Bodey B, Jr, Siegel SE, Kaiser HE. Involution of the mammalian thymus, one of the leading regulators of aging. In Vivo. 1997;11:421–40. [PubMed] [Google Scholar]
- 5.Medina KL, Kincade PW. Pregnancy-related steroids are potential negative regulators of B lymphopoiesis. Proc Natl Acad Sci USA. 1994;91:5382–6. doi: 10.1073/pnas.91.12.5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rijhsinghani AG, Thompson K, Bhatia SK, Waldschmidt TJ. Estrogen blocks early T cell development in the thymus. Am J Reprod Immunol. 1996;36:269–77. doi: 10.1111/j.1600-0897.1996.tb00176.x. [DOI] [PubMed] [Google Scholar]
- 7.Carlsten H, Holmdahl R, Tarkowski A, Nilsson LA. Estradiol- and testosterone-mediated effects on the immune system in normal and autoimmune mice are genetically linked and inherited as dominant traits. Immunology. 1989;68:209–14. [PMC free article] [PubMed] [Google Scholar]
- 8.Carlsten H, Holmdahl R, Tarkowski A, Nilsson LA. Estradiol suppression of delayed-type hypersensitivity in autoimmune (NZB/NZW) F1 mice is a trait inherited from the healthy NZW parental strain. Immunology. 1989;67:205–9. [PMC free article] [PubMed] [Google Scholar]
- 9.Josefsson E, Tarkowski A, Carlsten H. Anti-inflammatory properties of estrogen. I. In vivo suppression of leukocyte production in bone marrow and redistribution of peripheral blood neutrophils. Cell Immunol. 1992;142:67–78. doi: 10.1016/0008-8749(92)90269-u. [DOI] [PubMed] [Google Scholar]
- 10.Rachon D, Mysliwska J, Suchecka-Rachon K, Wieckiewicz J, Mysliwski A. Effects of estrogen deprivation on interleukin-6 production by peripheral blood mononuclear cells of postmenopausal women. J Endocrinol. 2002;172:387–95. doi: 10.1677/joe.0.1720387. [DOI] [PubMed] [Google Scholar]
- 11.Green S, Walter P, Greene G, et al. Cloning of the human estrogen receptor cDNA. J Steroid Biochem. 1986;24:77–83. doi: 10.1016/0022-4731(86)90035-x. [DOI] [PubMed] [Google Scholar]
- 12.Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci USA. 1996;93:5925–30. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shim GJ, Wang L, Andersson S, et al. Disruption of the estrogen receptor beta gene in mice causes myeloproliferative disease resembling chronic myeloid leukemia with lymphoid blast crisis. Proc Natl Acad Sci USA. 2003;100:6694–9. doi: 10.1073/pnas.0731830100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shim GJ, Kis LL, Warner M, Gustafsson JA. Autoimmune glomerulonephritis with spontaneous formation of splenic germinal centers in mice lacking the estrogen receptor alpha gene. Proc Natl Acad Sci USA. 2004;101:1720–4. doi: 10.1073/pnas.0307915100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krege JH, Hodgin JB, Couse JF, et al. Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proc Natl Acad Sci USA. 1998;95:15677–82. doi: 10.1073/pnas.95.26.15677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci USA. 1993;90:11162–6. doi: 10.1073/pnas.90.23.11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vidal O, Lindberg MK, Hollberg K, et al. Estrogen receptor specificity in the regulation of skeletal growth and maturation in male mice. Proc Natl Acad Sci USA. 2000;97:5474–9. doi: 10.1073/pnas.97.10.5474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Islander U, Erlandsson MC, Hasseus B, et al. Influence of estrogen receptor alpha and beta on the immune system in aged female mice. Immunology. 2003;110:149–57. doi: 10.1046/j.1365-2567.2003.01704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carlsten H, Verdrengh M, Taube M. Additive effects of suboptimal doses of estrogen and cortisone on the suppression of T lymphocyte dependent inflammatory responses in mice. Inflamm Res. 1996;45:26–30. doi: 10.1007/BF02263501. [DOI] [PubMed] [Google Scholar]
- 20.Offner H, Adlard K, Zamora A, Vandenbark AA. Estrogen potentiates treatment with T-cell receptor protein of female mice with experimental encephalomyelitis. J Clin Invest. 2000;105:1465–72. doi: 10.1172/JCI9213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Erlandsson MC, Ohlsson C, Gustafsson JA, Carlsten H. Role of estrogen receptors alpha and beta in immune organ development and in estrogen-mediated effects on thymus. Immunology. 2001;103:17–25. doi: 10.1046/j.1365-2567.2001.01212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kousteni S, Bellido T, Plotkin LI, et al. Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: dissociation from transcriptional activity. Cell. 2001;104:719–30. [PubMed] [Google Scholar]
- 23.Kousteni S, Chen JR, Bellido T, et al. Reversal of bone loss in mice by nongenotropic signaling of sex steroids. Science. 2002;298:843–6. doi: 10.1126/science.1074935. [DOI] [PubMed] [Google Scholar]
- 24.Kousteni S, Han L, Chen JR, et al. Kinase-mediated regulation of common transcription factors accounts for the bone-protective effects of sex steroids. J Clin Invest. 2003;111:1651–64. doi: 10.1172/JCI17261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Centrella M, McCarthy TL, Chang WZ, Labaree DC, Hochberg RBE. stren (4-estren-3{alpha},17{beta}-diol) is a prohormone that regulates both androgenic and estrogenic transcriptional effects through the androgen receptor. Mol Endocrinol. 2004;18:1120–30. doi: 10.1210/me.2003-0491. [DOI] [PubMed] [Google Scholar]
- 26.Moverare S, Dahllund J, Andersson N, et al. Estren is a selective estrogen receptor modulator with transcriptional activity. Mol Pharmacol. 2003;64:1428–33. doi: 10.1124/mol.64.6.1428. [DOI] [PubMed] [Google Scholar]
- 27.Lindberg MK, Erlandsson M, Alatalo SL, et al. Estrogen receptor alpha, but not estrogen receptor beta, is involved in the regulation of the OPG/RANKL (osteoprotegerin/receptor activator of NF-kappa B ligand) ratio and serum interleukin-6 in male mice. J Endocrinol. 2001;171:425–33. doi: 10.1677/joe.0.1710425. [DOI] [PubMed] [Google Scholar]
- 28.Frey-Wettstein M, Craddock CG. Testosterone-induced depletion of thymus and marrow lymphocytes as related to lymphopoiesis and hematopoiesis. Blood. 1970;35:257–71. [PubMed] [Google Scholar]
- 29.Viselli SM, Reese KR, Fan J, Kovacs WJ, Olsen NJ. Androgens alter B cell development in normal male mice. Cell Immunol. 1997;182:99–104. doi: 10.1006/cimm.1997.1227. [DOI] [PubMed] [Google Scholar]
- 30.Benten WP, Becker A, Schmitt-Wrede HP, Wunderlich F. Developmental regulation of intracellular and surface androgen receptors in T cells. Steroids. 2002;67:925–31. doi: 10.1016/s0039-128x(02)00055-7. [DOI] [PubMed] [Google Scholar]
- 31.Holmdahl R, Jansson L, Meyerson B, Klareskog L. Estrogen-induced suppression of collagen arthritis. I. Long-term estradiol treatment of DBA/1 mice reduces severity and incidence of arthritis and decreases the anti-type II collagen immune response. Clin Exp Immunol. 1987;70:372–8. [PMC free article] [PubMed] [Google Scholar]
- 32.D’Elia HF, Larsen A, Mattsson LA, et al. Influence of hormone replacement therapy on disease progression and bone mineral density in rheumatoid arthritis. J Rheumatol. 2003;30:1456–63. [PubMed] [Google Scholar]