Abstract
At onset of systemic sclerosis (SSc), T cells have been found to oligoclonally expand in the skin, presumably in response to auto-antigens, but the T cell repertoire has not been evaluated at a later stage. To determine whether a perpetuating immune response contributes to the pathogenesis of stable SSc, the T cell repertoire was analysed in patients with diffuse (d) or limited (l) SSc, and compared to patients with primary Raynaud's phenomenon (RP) or healthy volunteers (Ctrl). The T cell repertoire (total, CD4 or CD8 sorted blood T cells) was analysed by qualitative and quantitative immunoscope (14 BV families analysed) in 11 untreated dSSc and 11 untreated lSSc, 10 RP and 11 Ctrl. To better detect in vivo activated cells, repertoire analysis was also performed on sorted CD4 T cells after in vitro culture with IL-2. In parallel, 6 skin biopsies from SSc patients were analysed. After 7–8 years of disease evolution, SSc patients did not show detectable clonal T cell expansions in the skin, even after tentative expansion from the biopsy with IL-2. Total T cell, sorted CD4 and CD8 T cell repertoires from the blood of patients with SSc did not show significant perturbation as compared to patients with RP and Ctrl. After IL-2 culture for 7 days, blood CD4 T cells from the patients did not preferentially expand as compared to RP and Ctrl. These findings suggest that antigen-driven immune responses may play a lesser role in established SSc than at disease onset.
Keywords: T cell repertoire, scleroderma, Raynaud's phenomenon
Introduction
Systemic sclerosis (SSc) is considered an autoimmune disease of unknown aetiology characterized by excessive fibrosis of the skin and others organs. Pathological studies have shown a proliferative small-vessel vasculopathy and fibrosis associated to perivascular and interstitial T cell infiltration. Expression of HLA class II molecules on these T cells indicated recent activation in one study [1]. These activated T cells were found to produce cytokines, cause cytotoxicity and contribute to tissue damage, and it was concluded that they may have a causal relationship to fibrosis. The precise stimulus for T cell activation has not yet been identified. Recent data demonstrated the presence of oligoclonal expansions of αβ T cells at onset of SSc in humans [2] and also in Tsk2 scleroderma-like mice [3], suggesting an antigen-driven immune response. Also, γδ T-cell repertoire biases were observed in peripheral blood from patients with an association of primary biliary cirrhosis and SSc [4], and restricted TCR CDR3 lengths were detected in CD8 T cells from SSc bronchoalveolar lavage fluid [5]. Moreover, augmented serum and skin levels of chemokines [6,7], increased cell adhesion molecules [8,9] and higher levels of IL-2R in suction blister fluid in the skin of patients with SSc have been demonstrated [10]. Additionally, it has been suggested that disease-specific, activated T cell from patients with SSc produced Th2 cytokines [11,12]. Together, these observations suggested antigen-driven activation and expansion of T cells in patients with SSc. Nevertheless, progression of SSc may also be ascribed to nonimmune mechanisms such as endothelial cell injury with a high production of vasoconstrictor peptides that stimulates extracellular matrix production [13,14] or abnormal regulation of transcription of genes encoding various collagens that increases fibrosis [15,16]. Therefore, the role of antigen-driven clonal expansion of T cells in perpetuating the disease remains elusive.
Antigen priming takes place in secondary lymphoid organs, yielding specific T cells to expand and skewing of the T cell repertoire. The resulting oligoclonal T cell expansions may recirculate and be detected in peripheral blood. This was shown to be the case in several autoimmune diseases such as multiple sclerosis [17] or polymyositis [18,19]. If an antigen-specific T cell activation continues to play a major role in the progression of SSc, a skewed T cell repertoire should therefore be detected. Thus, we investigated the T cell repertoire by a global and sensitive method, i.e. immunoscope, in blood and skin from patients with established SSc in comparison to patients with Raynaud phenomenon (RP) or to healthy controls (Ctrl).
Patients and methods
Patients and skin biopsies
Eleven patients with diffuse SSc (dSSc; 10 women, 1 man; mean age ± SEM: 48·0 ± 3·8 years) and 11 with limited SSc (lSSc; 8 women, 3 men; mean age ± SEM: 54·0 ± 1·6 years) who fulfilled the American Rheumatism Association criteria for SSc [20]. All patients were categorized as dSSc or lSSc according to the classification of LeRoy et al. [21]. The average disease duration was 7 and 8 years for dSSc and lSSc patients, respectively (Table 1). Patients did not take any immunosuppressive or corticosteroid therapy. Among patients with SSc, skin biopsies were obtained from 3 dSSc and 3 lSSc patients. Ten patients suffering primary RP (7 women, 3 men; mean age ± SEM: 46 ± 3·1 years) according to Leroy & Medsger criteria [12] and 11 healthy volunteers (9 women, 2 men; mean age ± SD: 52·0 ± 2·4 years) were also enrolled into the study. This study was approved by the local ethics committee (Hôpital Saint Antoine, Paris, France). All patients and Ctrl provided informed consent.
Table 1.
Patients’ characteristics and analysis of CD4 and CD8 T cell subsets.
| dSSc (n = 11) | lSSc (n = 11) | RP (n = 10) | Ctrl (n = 11) | |
|---|---|---|---|---|
| Age (years)(mean ± SEM) | 48 ± 4 | 54 ± 2 | 46 ± 3 | 52 ± 2 |
| Sex ratio (female/male) | 10/1 | 8/3 | 7/3 | 9/2 |
| Duration of the disease (mean ± SEM; years) | 7 ± 1·6 | 8 ± 1·3 | – | – |
| Total lymphocyte counts (109/l) | 42 ± 7 | 55 ± 9 | 44 ± 4 | 47 ± 6 |
| CD4+ (% ± SEM) | 48 ± 2 | 52 ± 2 | 38 ± 4 | 45 ± 2 |
| CD8+ (% ± SEM) | 19 ± 2* | 24 ± 2 | 27 ± 3 | 29 ± 2 |
| CD4+ HLA-DR+ (% ± SEM) | 3 ± 1 | 3 ± 1 | 4 ± 1 | 3 ± 1 |
| CD4+ CD25+ (% ± SEM) | 2 ± 1 | 4 ± 1 | 5 ± 1 | 3 ± 1 |
| CD8+ HLA-DR+ (% ± SEM) | 4 ± 1 | 5 ± 1 | 5 ± 1 | 5 ± 1 |
| CD8+ CD25+ (% ± SEM) | 2 ± 2 | 1 ± 0·3 | 1 ± 1 | 0 ± 0·3 |
dSSc, diffuse systemic sclerosis; lSSc, limited systemic sclerosis; RP, primary Raynaud's phenomenon: Ctrl, controls (healthy volunteers).
P < 0·05 as compared to Ctrl (Mann–Whitney test).
Peripheral blood cell preparation
Peripheral blood (40 ml) from all patients and Ctrl was collected in heparinized tubes and PBMC were isolated by centrifugation on Ficoll-hypaque (density: 1·077 g/ml), washed with RPMI medium. Then, 7 × 106 cells were used for RNA extraction and further immunoscope analysis. In parallel, PBMC were incubated with anti-CD4 magnetic-embedded monoclonal antibodies (Miltenyi Biotech, Bergischgladbach, Germany), washed and sorted. The CD4+ fraction was divided, one half for cell culture and the other half for RNA extraction. The purity of cell preparations was determined by FACS analysis (FACSCalibur; BD Biosciences, Mountain View, CA, USA) and was routinely >95%.
Skin biopsy
Skin biopsy was divided into 3 pieces: the first was snapped in OCT (Tissutek, Osaka, Japan) for haematoxin and eosin staining and immunohistochemical analyses; the second was cultured in RPMI with human AB serum (10%), glutamine (1%) and antibiotics; and the third was stored immediately in Rnable (Eurobio, Ulysse, France) for further RNA preparation.
Histopathological and immunohistochemical examinations
Snap-frozen 6 µm cryostat sections of skin specimen from patients with SSc were stained with haematoxylin and eosin. Alternatively, sections were fixed in acetone for 10 min and then incubated with anti-CD3 (1 : 20) monoclonal antibody (Becton Dickinson, Mountain View, CA, USA). Cryostat sections were incubated with biotinylated rabbit antimouse Ig (Dako, Glostrup, Denmark), then with Avidin-Biotin complex and peroxidase substrate (Dako). Sections were counterstained by haematoxylin.
CD4 T cell culture and skin culture
CD4 T cells were purified from total peripheral blood by positive selection using magnetic beads (MACS; Miltenyi Biotech). CD4 T cells were then cultured with IL-2 (600 UI/ml; Chiron, Amstedam, Holland) for 7 days. Then, RNA was extracted for further immunoscope analysis.
Immunoscope analysis
Analysis was performed in 14 different BV families that represent more than 70% of the T cell repertoire of healthy subjects [22]. Briefly, cellular RNA was reverse transcribed into cDNA using oligo(dT) and moloney murine leukaemia reverse transcriptase (RT) (Life technologies, Rockville, MD, USA). After phenol/chloroform extraction, a quantity of cDNA corresponding to 300 ng of total RNA was amplified by PCR in 50 µl reaction tubes using one primer for each of the 14 BV studied and a common BC primer (Genset, Paris, France). The final concentration was 0·5 µm for each primer, 0·2 mm dNTP (except for 0·4 mm dUTP), 2 mm MgCl2 in PCR buffer (Boeringher Mannheim, Mannheim, Germany), in the presence of 2·5 U of Taq polymerase (Boeringher Mannheim). The amplification was performed on a thermal cycler (Thermo Hybaid, Ashford, UK) with an initial denaturation step at 94°C for 5 min and then 40 of the following cycles: 94°C for 1 min, 60°C for 1 min, 72°C for 2 min, and a final step at 72°C for 10 min. Amplification was verified by agarose gel electrophoresis. Each BV-BC PCR product was subjected to run-off cycles primed with a nested fluorophore-labelled BC primer in presence of Taq polymerase. Each run-off product was denatured and loaded on a gel for fluorescence analysis using an Applied Biosystems 377 sequencer (Perkin Elmer, Norwalk, CT, USA). Raw data were analysed with immunoscope 3·01b software (Loginserm, Paris, France) [23]. The method was validated in our previous series of experiments using cultured T cells [24], or fresh blood T cells or muscle biopsies from patients with autoimmune myositis [19].
The method for quantifying T cell repertoire perturbation was adapted from Gorochov et al. [25]. Briefly, CDR3 length profiles were translated into a p distribution pk(i) for each peak i (i = 1–8) of a given BVk as a function of the area under the curve (expressed in relative fluorescence intensity, RFI) with normalization so that Σipk(i) = 100%. The extent of perturbation Dk(i) for sample and prefk(i) values were calculated from a reference distribution derived from repertoire analysis of eight cord blood samples. The perturbation Dk for a given BVk was then calculated as Σi| Dk(i) |/2, so that Dk = 0 or 100% for a p distribution equal to or completely nonoverlapping with the reference, respectively. The mean of individual BVk perturbations yielded the average perturbation D = Σ kDk/n for all n BVk families (n = 14). Quality assurance of data processing was provided by parallel data entry by two independent investigators followed by separate calculation using two independently programmed Excel spreadsheet (Microsoft, Redman, WA, USA).
Statistical analysis
Statistical analyses were performed using Statview software (SAS institute, Cary, NC). The Mann–Whitney U-test was used to compare data (α = 0·05 and β = 0·20).
Results
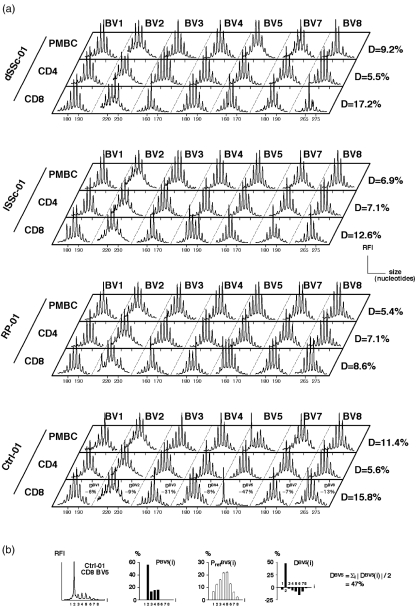
Immunoscope analysis of unperturbed T cell repertoires yields Gaussian-like distributions of CDR3 mRNA lengths that are separated by a 3-nucleotide length corresponding to in-frame transcripts. Accumulation of oligoclonal T cell expansions manifest as peaks of given lengths that accumulate above the Gaussian-like background of polyclonal T cells. Visual analysis of total T cell, CD4 and CD8 T cells immunoscope profiles did not reveal noticeable qualitative differences between dSSC, lSSC, RP and Ctrl groups (Fig. 1a). In order to allow a more precise quantitative analysis and perform statistical analyses, we quantified the extent of the perturbation of the T cell repertoire (Fig. 1b). This was performed by calculating a perturbation index (D) whose reference values for healthy controls is in the order of 10% or lower [19], higher values reflecting accumulation of clonal expansions. Using this method, there was no statistically significant difference between the average perturbations (mean of all 14 BV analysed) of T cell repertoires from the different groups (dSSc, lSSc, RP, Ctrl) (Fig. 2). When each BV family was analysed separately, the comparison of the 4 groups did not reveal any overrepresentation of a given BV family between patients with SSc and those with RP nor between SSc and Ctrl (not shown).
Fig. 1.
Qualitative T cell repertoire analysis and method for quantifying the extent of T cell repertoire perturbations. (a) Examples of immunoscope profiles of unsorted PBMC, and CD4 and CD8 T cells from dSSC, lSSc, RP patients and Ctrl. Only the first 7 BV (out of the 14 BV families analysed) are displayed. RFI, relative fluorescence intensity. (b) To quantify repertoire perturbations, the CDR3 profiles for each BV family is translated into a probability distribution PBV(i) for each peak i (i = 1–8). The extent of perturbation DBV(i) for each peak i is computed by the distance between PBV(i) values from the sample and PrefBV(i) values from a reference distribution (mean ± SEM). The perturbation DBV is then calculated as Σi |DBV(i)|/2. The mean of DBV perturbations for all 14 BV families yields the average perturbation D = Σk DBVk/14. An example of calculation is given for BV5 in CD8 T cells from Ctrl-01. All DBV results for this sample are displayed in (a). Average D-values (computed on 14 BV families) are also given in (a) for all displayed samples.
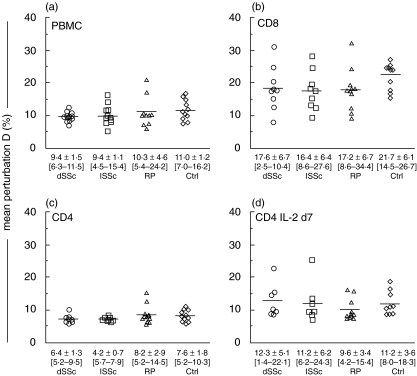
Fig. 2.
Quantitative T cell repertoire analysis. Perturbation indices D of unsorted (a) PBMC, (b) CD8 and (c) CD4 sorted T cells, and (d) IL-2-cultured CD4 T cells are given for dSSc, lSSc, RP and Ctrl groups. Mean ± SD [range] of D indices for the different cell subsets are displayed for each group. Horizontal bar indicates mean. Inter-group differences were not statistically significant (Mann–Whitney test).
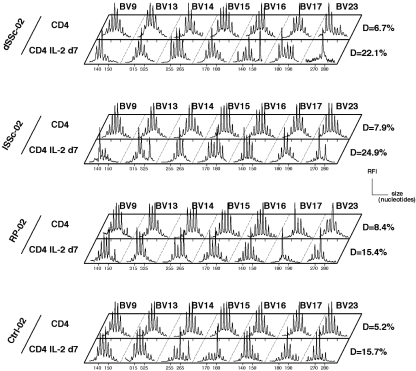
To augment sensitivity of our assay and possibly detect minor expansions of T cells that would have been previously activated in vivo and thus become IL-2-responsive, we also attempted to amplify CD4 T cells by culturing them with IL-2 for 7 days. In these conditions, we observed a raise in perturbation indices as compared to CD4 T cell repertoires before culture, which reflected the oligoclonal expansion of preactivated cells (Fig. 3). Nevertheless, the different groups behaved similarly and there was no preferential expansion in dSSc or lSSc patients as compared to RP or healthy volunteers. These results are in line with immunophenotypic analyses of blood T cells that did not reveal a significantly higher level of circulating T cells with an activated (HLA-DR+ or CD25+) phenotype in SSc patients as compared to controls (Table 1). It may be noted that a moderate decrease of CD8 T cell counts was observed in this series of dSSc patients. The lack of blood T cell repertoire perturbation could reflect the lack of detectable ongoing immune response in the skin of stable SSc patients in contrast to what was described at disease onset [2]. Therefore, we analysed skin biopsies in a group of 6 SSc patients. Histological analysis revealed that only one of them (dSSc-03) had a moderate mononuclear skin infiltrate and anti-CD3 staining of this biopsy indicated that T cells represented only a minor fraction of this infiltrate (Fig. 4a). In contrast, all tested patients had skin fibrosis, which was consistent with the diagnosis of SSc (Fig. 4b). RT-PCR amplification of 14 BV families from these skin biopsies RNA remained negative, except for one BV in one patient. This was also the case after attempts to amplify T cells from the biopsies by a step of in vitro culture with IL-2 (not shown). Together, these results suggest that a significant ongoing T cell response is unlikely to be prominent in late SSc in contrast to what was described at disease onset.
Fig. 3.
Repertoire analysis of CD4 T cells after culture with IL-2. Example of immunoscope profiles of CD4 T cells (before culture and d7 postculture) from dSSC, lSSc, RP and Ctrl. Only the last 7 BV (out of the 14 BV families analysed) are displayed. Perturbation indices D are given for all samples.
Fig. 4.
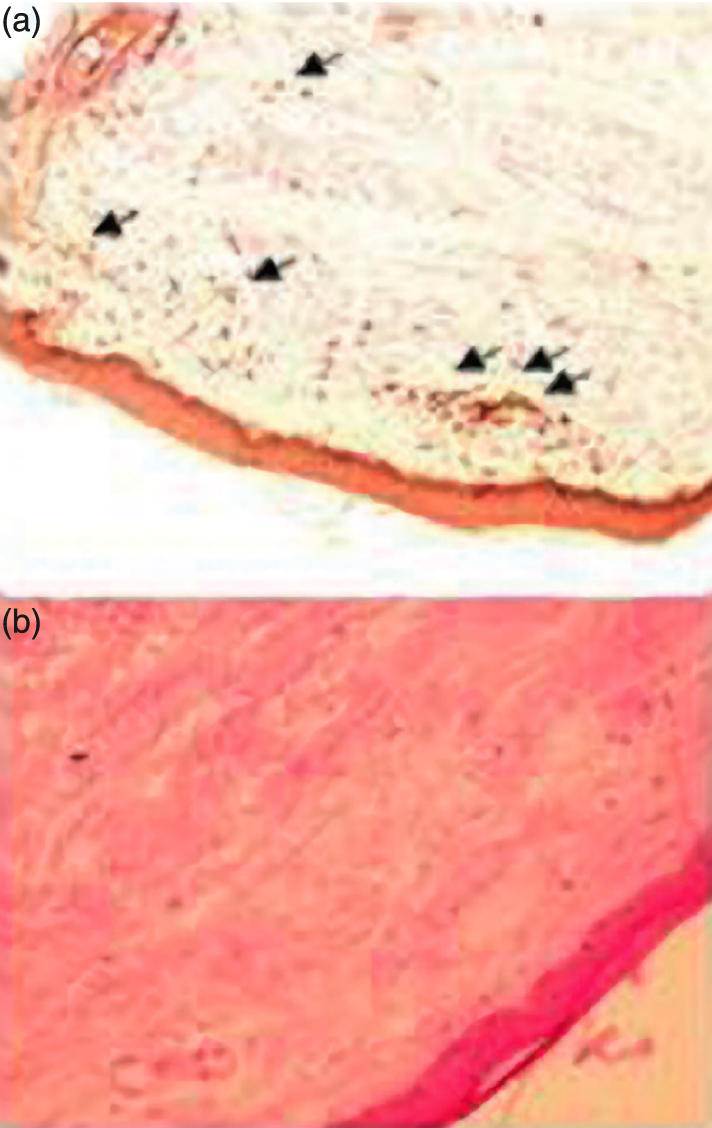
Histological analysis of skin sections. (a) Anti-CD3 immunohistochemical analysis of skin section from patient dSSc-03 shows a discrete T cell infiltrate. Original magnification ×200. (b) Hematoxin and eosin staining of skin section from patient dSSc-04 shows thickened collagen bundles characteristic of SSc. Original magnification ×200.
Discussion
These results show that oligoclonal T cell expansions – measured by the index of repertoire perturbation D – can not be detected, neither in blood nor in skin from patients suffering from evolved stable SSc without immunosuppressive therapy. The level of perturbations was higher in the CD8 than the CD4 subset – which is an expected finding since CD8 clonal expansions can physiologically accumulate with age in healthy subjects [26]– but repertoire perturbations in SSc patients were undistinguishable from that of RP and Ctrl whatever the T cell subset considered.
The immunoscope method detects heterogeneity of CDR3 length distributions and its sensitivity to detect TCR clonality may be lower than that of other methods such as SSCP analysis for instance. Nevertheless, by providing a global representation of the T cell repertoire, immunoscope has proved to be a sensitive method to detect clonal expansions associated with antigen-driven immune responses [27]. For instance, we and others have recently shown that immunoscope accurately detects muscle-infiltrating CD8 T cell clones that recirculate in the blood of patients with polymyostis [18,19]. The present findings do not formally rule out that CD4 T cells play a role in the perpetuation of fibroblast activation in stable SSc. Indeed, it is possible that the CD4 T cell response is more diverse than the CD8 T cell response, which would explain why CD4 T cell expansions are difficult to detect by repertoire analysis. In this line, a recent study in patients with multiple sclerosis, a disease where CD4 T cells are very likely to play a significant role, failed to identify CD4 clonal expansion whereas brain-infiltrating CD8 clones were shown to persist in the cerebrospinal fluid and also in the blood [28]. Also, it may be that the CD4 responses in SSc are indeed antigen-specific but below the level of detection of our assay (late disease may not be associated with such high frequencies as early disease). Finally, our data do not exclude that the CD4 T cell response may be fluctuating, as also observed in multiple sclerosis [17].
Since CD4 T cell expansions are difficult to detect, we reasoned that a step of in vitro culture in the presence of IL-2 could amplify in vivo activated cells whereas naive unstimulated T cells would die in culture. Indeed, this procedure allowed the detection of oligoclonal expansions that manifested by an augmented level of perturbation after culture (Fig. 3). Nevertheless, the different groups of patients behaved similarly in this assay. This rendered further unlikely that in vivo preactivated T cells play a major role in stable SSc. Finally, in contrast to what observed at disease onset [17], we did not detect T cell infiltrates in skin biopsies of SSc patients, neither by histological nor TCR molecular analyses.
There is convincing evidence that T cells play a role in the pathogenesis of SSc at disease onset. T cells are found in the vascular infiltrates, oligoclonal expansions suggest antigen-driven immune response [2,3], T cells from SSc patients produce cytokines that promote collagen synthesis by fibroblasts, and CD4 T cells are presumably required for autoantibody production in SSc [29]. In contrast, the results presented herein show that fibrosis progression evolves independently of a detectable T cell response. Therefore, it is possible that nonimmune mechanisms may be prominent at this stage. These mechanisms may include autonomous production of collagen by fibroblasts independently of a T cell help that may be required at disease onset, and is likely to reflect the poor clinical efficacy of steroid therapy in stable SSc patients.
Acknowledgments
This study was supported by Association des Sclérodermiques de France, Assistance Publique-Hôpitaux de Paris and Centre National de la Recherche Scientifique.
References
- 1.Prescott RJ, Freemont AJ, Jones CJ, Hoyland J, Fielding P. Sequential dermal microvascular and perivascular changes in the development of scleroderma. J Pathol. 1992;166:255–63. doi: 10.1002/path.1711660307. [DOI] [PubMed] [Google Scholar]
- 2.Sakkas LI, Xu B, Artlett CM, Lu S, Jimenez SA, Platsoucas CD. Oligoclonal T cell expansion in the skin of patients with systemic sclerosis. J Immunol. 2002;168:3649–59. doi: 10.4049/jimmunol.168.7.3649. [DOI] [PubMed] [Google Scholar]
- 3.Wooley PH, Sud S, Langendorfer A, et al. T cells infiltrating the skin of Tsk2 scleroderma-like mice exhibit T cell receptor bias. Autoimmunity. 1998;27:91–8. doi: 10.3109/08916939809008039. [DOI] [PubMed] [Google Scholar]
- 4.Mayo MJ, Jenkins RN, Combes B, Lipsky PE. Association of clonally expanded T cells with the syndrome of primary biliary cirrhosis and limited scleroderma. Hepatology. 1999;29:1635–42. doi: 10.1002/hep.510290637. [DOI] [PubMed] [Google Scholar]
- 5.Yurovsky VV, Wigley FM, Wise RA, White B. Skewing of the CD8+ T-cell repertoire in the lungs of patients with systemic sclerosis. Hum Immunol. 1996;48:84–97. doi: 10.1016/0198-8859(96)00091-2. [DOI] [PubMed] [Google Scholar]
- 6.Yamamoto T, Eckes B, Hartmann K, Krieg T. Expression of monocyte chemoattractant protein-1 in the lesional skin of systemic sclerosis. J Dermatol Sci. 2001;26:133–9. doi: 10.1016/s0923-1811(00)00169-9. [DOI] [PubMed] [Google Scholar]
- 7.Yamamoto T, Eckes B, Krieg T. High expression and autoinduction of monocyte chemoattractant protein-1 in scleroderma fibroblasts. Eur J Immunol. 2001;31:2936–41. doi: 10.1002/1521-4141(2001010)31:10<2936::aid-immu2936>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 8.Gruschwitz MS, Hornstein OP, von Den DP. Correlation of soluble adhesion molecules in the peripheral blood of scleroderma patients with their in situ expression and with disease activity. Arthritis Rheum. 1995;38:184–9. doi: 10.1002/art.1780380206. [DOI] [PubMed] [Google Scholar]
- 9.Sato S. Abnormalities of adhesion molecules and chemokines in scleroderma. Curr Opin Rheumatol. 1999;11:503–7. [PubMed] [Google Scholar]
- 10.Sondergaard K, Stengaard-Pedersen K, Zachariae H, Heickendorff L, Deleuran M, Deleuran B. Soluble intercellular adhesion molecule-1 (sICAM-1) and soluble interleukin-2 receptors (sIL-2R) in scleroderma skin. Br J Rheumatol. 1998;37:304–10. doi: 10.1093/rheumatology/37.3.304. [DOI] [PubMed] [Google Scholar]
- 11.Sakkas LI, Tourtellotte C, Berney S, Myers AR, Platsoucas CD. Increased levels of alternatively spliced interleukin 4 (IL-4delta2) transcripts in peripheral blood mononuclear cells from patients with systemic sclerosis. Clin Diagn Laboratory Immunol. 1999;6:660–4. doi: 10.1128/cdli.6.5.660-664.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.LeRoy EC, Medsger TA., Jr Raynaud's phenomenon: a proposal for classification. Clin Exp Rheumatol. 1992;10:485–8. [PubMed] [Google Scholar]
- 13.Morelli S, Ferri C, Polettini E, et al. Plasma endothelin-1 levels, pulmonary hypertension, and lung fibrosis in patients with systemic sclerosis. Am J Medical. 1995;99:255–60. doi: 10.1016/s0002-9343(99)80157-0. [DOI] [PubMed] [Google Scholar]
- 14.Abraham DJ, Vancheeswaran R, Dashwood MR, et al. Increased levels of endothelin-1 and differential endothelin type A and B receptor expression in scleroderma-associated fibrotic lung disease. Am J Pathol. 1997;151:831–41. [PMC free article] [PubMed] [Google Scholar]
- 15.Mori Y, Chen SJ, Varga J. Expression and regulation of intracellular SMAD signaling in scleroderma skin fibroblasts. Arthritis Rheum. 2003;48:1964–78. doi: 10.1002/art.11157. [DOI] [PubMed] [Google Scholar]
- 16.Saitta B, Gaidarova S, Cicchillitti L, Jimenez SA. CCAAT binding transcription factor binds and regulates human COL1A1 promoter activity in human dermal fibroblasts: demonstration of increased binding in systemic sclerosis fibroblasts. Arthritis Rheum. 2000;43:2219–29. doi: 10.1002/1529-0131(200010)43:10<2219::AID-ANR9>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 17.Muraro PA, Bonanni L, Mazzanti B, et al. Short-term dynamics of circulating T cell receptor V beta repertoire in relapsing-remitting MS. J Neuroimmunol. 2002;127:149–59. doi: 10.1016/s0165-5728(02)00105-4. [DOI] [PubMed] [Google Scholar]
- 18.Nishio J, Suzuki M, Miyasaka N, Kohsaka H. Clonal biases of peripheral CD8 T cell repertoire directly reflect local inflammation in polymyositis. J Immunol. 2001;167:4051–8. doi: 10.4049/jimmunol.167.7.4051. [DOI] [PubMed] [Google Scholar]
- 19.Benveniste O, Cherin P, Maisonobe T, et al. Severe perturbations of the blood T cell repertoire in polymyositis, but not dermatomyositis patients. J Immunol. 2001;167:3521–9. doi: 10.4049/jimmunol.167.6.3521. [DOI] [PubMed] [Google Scholar]
- 20.Subcommittee for Scleroderma Criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Preliminary criteria for the classification of systemic sclerosis (scleroderma) Arthritis Rheum. 1980;23:581–90. doi: 10.1002/art.1780230510. [DOI] [PubMed] [Google Scholar]
- 21.LeRoy EC, Black C, Fleischmajer R, et al. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol. 1988;15:202–5. [PubMed] [Google Scholar]
- 22.Roglic M, Macphee RD, Duncan SR, Sattler FR, Theofilopoulos AN. T cell receptor (TCR) BV gene repertoires and clonal expansions of CD4 cells in patients with HIV infections. Clin Exp Immunol. 1997;107:21–30. doi: 10.1046/j.1365-2249.1997.d01-886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pannetier C, Cochet M, Darche S, Casrouge A, Zoller M, Kourilsky P. The sizes of the CDR3 hypervariable regions of the murine T-cell receptor beta chains vary as a function of the recombined germ-line segments. Proc Natl Acad Sci USA. 1993;90:4319–23. doi: 10.1073/pnas.90.9.4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Movassagh M, Boyer O, Burland MC, Leclercq V, Klatzmann D, Lemoine FM. Retrovirus-mediated gene transfer into T cells: 95% transduction efficiency without further in vitro selection. Hum Gene Ther. 2000;11:1189–200. doi: 10.1089/10430340050015239. [DOI] [PubMed] [Google Scholar]
- 25.Gorochov G, Neumann AU, Kereveur A, et al. Perturbation of CD4+ and CD8+ T-cell repertoires during progression to AIDS and regulation of the CD4+ repertoire during antiviral therapy. Nat Med. 1998;4:215–21. doi: 10.1038/nm0298-215. [DOI] [PubMed] [Google Scholar]
- 26.Posnett DN, Sinha R, Kabak S, Russo C. Clonal populations of T cells in normal elderly humans: the T cell equivalent to ‘benign monoclonal gammapathy’. J Exp Med. 1994;179:609–18. doi: 10.1084/jem.179.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pannetier C, Even J, Kourilsky P. T-cell repertoire diversity and clonal expansions in normal and clinical samples. Immunol Today. 1995;16:176–81. doi: 10.1016/0167-5699(95)80117-0. [DOI] [PubMed] [Google Scholar]
- 28.Skulina C, Schmidt S, Dornmair K, et al. Multiple sclerosis: brain-infiltrating CD8+ T cells persist as clonal expansions in the cerebrospinal fluid and blood. Proc Natl Acad Sci USA. 2004;101:2428–33. doi: 10.1073/pnas.0308689100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jimenez SA, Derk CT. Following the molecular pathways toward an understanding of the pathogenesis of systemic sclerosis. Ann Intern Med. 2004;140:37–50. [PubMed] [Google Scholar]