Abstract
Our laboratory has demonstrated that down-regulation of proliferation and cytokine synthesis by CD4+ T cells in mice fed diets rich in n-3 polyunsaturated fatty acids (PUFA) is highly dependent on the involvement of the co-stimulatory molecule, CD28. It has been reported that the inhibitory cytokine interleukin (IL)-10 acts directly on T cells which up-regulate IL-10 receptor (IL-10R) expression following stimulation via CD28 by efficiently blocking proliferation and cytokine production. Thus, it was hypothesized that dietary n-3 PUFA would suppress T cell function through the effects of IL-10. The proliferation of purified splenic CD4+ T cells activated in vitro with anti-CD3 and anti-CD28 (αCD3/CD28) from conventional mice (C57BL/6) fed either a control corn oil (CO)-enriched diet devoid of n-3 PUFA, docosahexaenoic acid (DHA; 22 : 6) or eicosapentaenoic acid (EPA; 20 : 5) for 14 days was suppressed by dietary DHA and EPA. Surprisingly, a similar trend was seen in IL-10 gene knock-out (IL-10–/–) mice fed dietary n-3 PUFA. IL-10R cell surface expression was also significantly down-regulated on CD4+ T cells from both the C57BL/6 and IL-10–/– mice fed dietary n-3 PUFA after 72 h of in vitro stimulation with αCD3/CD28. Enzyme-linked immunosorbent assay (ELISA) measurements revealed that C57BL/6 mice fed DHA had significantly reduced interferon (IFN)-γ and IL-10 levels 48 h post-activation. However, CD4+ T cells from IL-10–/– mice fed dietary n-3 PUFA produced significantly greater levels of IFN-γ than the CO-fed group. Our data suggest that in the absence of IL-10, CD4+ T cells from n-3 PUFA-fed mice may up-regulate IFN-γ. Suppressed CD4+ T cells from n-3 PUFA-fed C57BL/6 mice may use mechanisms other than IL-10 to down-regulate T cell function.
Keywords: co-stimulatory molecules, helper T cells, mice, nutrition
Introduction
Interleukin (IL)-10 is a major regulatory cytokine of inflammatory responses. It was described originally as a mouse Th2 cell factor, inhibiting cytokine synthesis by Th1 cells [1]. However, increasing evidence suggests that IL-10 acts as a general inhibitor of proliferative and cytokine responses of both Th1 and Th2 cells in vitro and in vivo[2–4]. IL-10 is released by mononuclear phagocytes [2,3], natural killer cells and by both Th1- and Th2-type lymphocytes [4]. Its production is tightly regulated, as excess IL-10 leads to the inability to control infectious pathogens, while insufficient IL-10 leads to the pathology secondary to tissue injury. The immunosuppressive potency of IL-10 depends on the timing of IL-10 and IL-10 receptor (IL-10R) expression, and the IL-10 suppressive activity can diminish during immune and inflammatory responses [5,6].
The co-stimulatory signal induced by complexing CD28 with specific monoclonal antibodies (MoAbs) or by interaction with B7 counter-receptors enhances the antigen-dependent T cell proliferation and cytokine production [7,8]. It has been shown that IL-10 elicits tolerance in T cells by selective inhibition of the CD28 co-stimulatory pathway and thereby controls suppression and development of antigen-specific immunity. IL-10 inhibited only T cells stimulated by the engagement of low numbers of T cell receptors, i.e. conditions which require CD28 co-stimulation [9,10]. IL-10 inhibited CD28 tyrosine phosphorylation, the initial step of the CD28 signalling pathway, and consequently the phosphatidylinositol 3-kinase p85 binding to CD28 [9]. In addition, Akdis et al. demonstrated that stimulation of CD45RO+ memory T cells from healthy human subjects up-regulated IL-10 receptor (IL-10R) expression, rendering the cells more susceptible to IL-10-mediated suppression. The IL-10-induced selective inhibition of the CD28 co-stimulatory pathway acts as a decisive mechanism in determining whether a T cell will contribute to an immune response or become anergic.
Previous results from our laboratory show that T lymphocytes from mice fed diets enriched in n-3 polyunsaturated fatty acids (PUFA), docosahexaenoic acid (DHA; 22 : 6) and eicosapentaenoic acid (EPA; 20 : 5), found in fish oil (FO), produced significantly less IL-2, but only when cells were activated with αCD3/CD28 and not when activated with αCD3/PMA (phorbol ester) [11]. Furthermore, dietary n-3 PUFA significantly enhanced the expression of CD28 on the surface of CD4+ T cells (Ly et al., submitted for publication).
On this background, we hypothesized that the suppressive effects of diet would be mediated by IL-10 and its relationship with CD28. To investigate this hypothesis, we have determined the influence of dietary DHA and EPA on the proliferative response, kinetics of IL-10R expression and anti-inflammatory cytokine production of purified splenic CD4+ T cells from conventional C57BL/6 and IL-10 gene knock-out (IL-10–/–) mice. Surprisingly, all responses were similar in both mouse groups with the exception of cytokine production. Dietary n-3 PUFA significantly reduced interferon (IFN)-γ production in conventional mice while dramatically up-regulating extracellular IFN-γ in IL-10–/– mice. Therefore, we conclude that dietary n-3 PUFA suppress CD4+ T cell functions through mechanisms which do not involve IL-10. Furthermore, our results suggest that dietary n-3 PUFA may elicit the normally proinflammatory cytokine, IFN-γ, to serve as an immunosuppressive cytokine in IL-10-deficient cells.
Methods
Diets and animals
Female, pathogen-free, young (12–14 g) C57BL/6 mice were purchased from the Frederick National Cancer Research Facility (Frederick, MD, USA). IL-10–/– mouse breeder pairs were a generous gift from Dr Daniel Berg (University of Iowa). The colony was maintained at the Laboratory Animal Resources and Research facility at Texas A&M University where all breeders were genotyped according to protocol (Jackson Laboratory, Bar Harbor, ME, USA) (data not shown). Female and male IL-10–/– (1–2 months of age) and C57BL/6 mice were assigned to one of three semipurified diets: 5% corn oil (CO) (control diet containing no n-3 PUFA), 1% DHA + 4% CO (DHA), or 1% EPA + 4% CO (EPA), for 14 days. Diets were analysed by gas chromatography prior to feeding, aliquoted, and stored at −80°C. Fresh diet was provided daily to prevent lipid peroxidation. There was no significant difference in food intake between dietary groups and weight gain was similar in all groups (data not shown). The purified diets met National Research Council nutrition requirements and varied only in lipid composition as described previously [12,13]. The vitamin E levels in the diets were approximately equal (mean ± s.e.m. = 169·2 ± 4·4 mg/kg diet) and exceeded the minimum requirement (22 mg vitamin E/kg diet). DHA (88·9% as 22 : 6, n-3) and EPA (94·1% as 20 : 5, n-3) were obtained in ethyl ester form from Martek Biosciences (Columbia, MD, USA) and Laxdale Ltd (UK), respectively. Corn oil (57·3% as 18 : 2, n-6) was obtained from Degussa Bioactives (Champaign, IL, USA) [11].
Isolation and preparation of splenic lymphocytes
Mice were killed by CO2asphyxiation. Spleens were placed in RPMI complete medium [RPMI-1640 with 25 mmol/l HEPES (Irvine Scientific, Santa Ana, CA, USA) supplemented with 2·5% fetal bovine serum (FBS) + 2·5% homologous mouse serum (HMS) [14], 1 × 105µ/l penicillin and 100 mg/l streptomycin (Irvine Scientific), 2 mmol/l l-glutamine and 10 µmol/l 2-mercaptoethanol]. Spleens were dispersed with glass homogenizers and passed through a 149-µm wire mesh filter to create single-cell suspensions. Cells were subsequently washed with RPMI complete medium before T cell enrichment as described previously [13].
CD4+ T cell purification
Total lymphocytes were initially enriched by density gradient centrifugation using Lympholyte-M (Cedarlane, Canada) in accordance with the manufacturer's protocol. The resulting cell fraction from each spleen was incubated with an antibody cocktail provided by the manufacturer, loaded onto a negative-selection mouse CD4+ T cell purification column (R&D Systems, Minneapolis, MN, USA), and incubated for 10 min at room temperature (RT). Non-adherent cells were eluted for purity and viability analysis, proliferation, FACS analysis and real-time polymerase chain reaction (PCR) assays. The purity of the CD4+ T cell population was analysed by flow cytometry (FACSCalibur; Becton-Dickinson, Bedford, MA, USA) using anti-CD4 antibody conjugated to fluorescein isothiocyanate (Pharmingen, San Diego, CA, USA) and determined to be 90·3 ± 1·4% (n = 3) [13].
T cell proliferation
Purified CD4+ T cells were cultured in triplicate at 2 × 105 cells per well (200 µl total) in 96-well round-bottomed microtitre plates (Falcon, Becton-Dickinson). Cells were cultured in the presence of 1 µg/ml plate-bound purified hamster antimouse CD3 monoclonal antibody (BD Pharmingen) and 5 µg/ml soluble purified hamster antimouse CD28 monoclonal antibody (BD Pharmingen) in complete medium. These concentrations were determined by preliminary experiments to induce proliferation without compromising viability [11,13]. Cells were incubated at 37°C in an atmosphere of 5% CO2 in air for 72 or 96 h. For the final 6 h, 1·0 µCi [3H]-thymidine/well (New England Nuclear, North Bellerica, MA, USA) was added to the cultures. Cells were harvested on a 96-well cell harvester (Packard Instrument Co., Meridien, CT, USA) and cellular thymidine uptake was measured using a liquid scintillation counter (Beckman Coulter, Fullertan, CA, USA). Results are expressed as net disintegrations per minute (DPM).
T cell activation for flow cytometry
Purified CD4+ T cells were cultured at 1–5 × 106 cells per well (2 ml total volume) in 24-well flat-bottomed microtitre plates (Falcon, Becton-Dickinson). Cells were cultured in the presence of 1 µg/ml anti-CD3 with 5 µg/ml anti-CD28 (BD Pharmingen) at 37°C in an atmosphere of 5% CO2 in air for the times indicated [11].
Immunofluorescence flow cytometry
For quantitative surface receptor staining, 106 CD4+ T cells from activated and control cultures were labelled with anti-IL-10R1 (131.Ba; PE, red) (BD Pharmingen)-labelled MoAb (4 µg/ml) and processed as described previously (Ly et al., submitted for publication).
Enzyme-linked immunosorbent assay (ELISA) analysis
CD4+ T cells from C57BL/6 and IL-10–/– mice were cultured in triplicate for 48 h. The supernatants were analysed for IFN-γand IL-10 protein using mouse immunoassay (ELISA) kits (R&D Systems).
Statistical analysis
Data were analysed by two-way anova for main treatment effects using Superanova statistical software (Berkeley, CA, USA). A difference between means was tested using Duncan's multiple range test. Significant and highly significant differences were defined as P < 0·05 and P < 0·01, respectively, for all tests.
Results
Dietary n-3 PUFA suppress CD4+ T cell proliferation in C57BL/6 and IL-10–/– mice
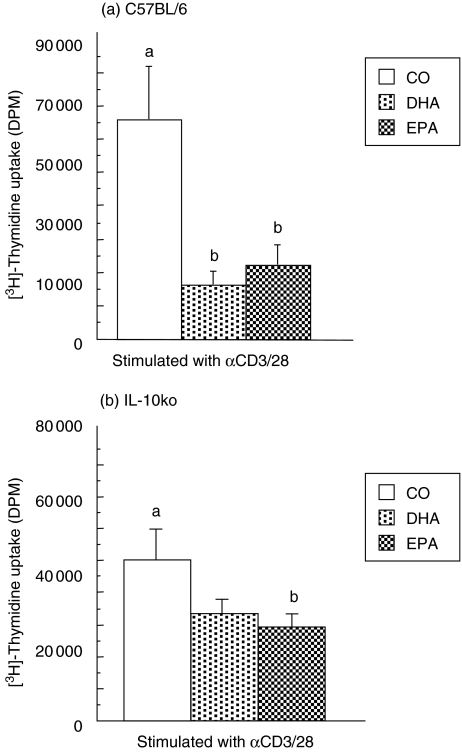
To determine the role of IL-10 in diet-mediated immunosuppression, purified splenic CD4+ T cells from conventional C576BL/6 and IL-10–/– mice fed diets containing n-3 polyunsaturated fatty acids (PUFA), DHA or EPA or corn oil (CO; devoid of n-3 PUFA) for 14 days were activated with antibodies to CD3 and CD28 (αCD3/CD28) for 72 h. Surprisingly, cell proliferation was suppressed significantly in both the C57BL/6 (Fig. 1a) and IL-10–/– mice (Fig. 1b) fed dietary n-3 PUFA. Both dietary n-3 DHA and EPA significantly reduced T cell proliferation in the C57BL/6 mice (Fig. 1a), whereas only EPA had a statistically significant effect on CD4+ T cells from the IL-10–/– mice (Fig. 1b). Therefore, the absence of IL-10 does not alter the suppressive effect of dietary n-3 PUFA on polyclonal T cell activation involving co-stimulation through CD28. These results were contrary to our hypothesis that dietary n-3 PUFA would not reduce CD4+ T cell proliferation in IL-10–/– mice, thereby demonstrating a role for the IL-10 cytokine in the suppressive effects of diet.
Fig. 1.
Dietary DHA and EPA down-regulate murine CD4+ T cell proliferation. Purified splenic CD4+ T cells from (a) C57BL/6 and (b) IL-10–/– mice fed CO, DHA or EPA were activated with antibodies to surface receptors CD3 and CD28 and cellular uptake of [3H]-thymidine was measured 72 h post-activation. Results are expressed as mean ± s.e.m. of the net disintegrations per minute (DPM), n = 5. CD4+ T cells cultured with media alone produced less than 600 DPM (data not shown). Different letters denote highly significant differences found between diet groups (P < 0·01). (b) DHA versus CO-fed IL-10–/– mice, P = 0·058. All cells were cultured in the presence of 2·5% homologous mouse serum (HMS) + 2·5% FBS.
Dietary n-3 PUFA suppress IL-10 receptor (IL-10R) protein expression on the surface of CD4+ T cells from C57BL/6 and IL-10–/– mice
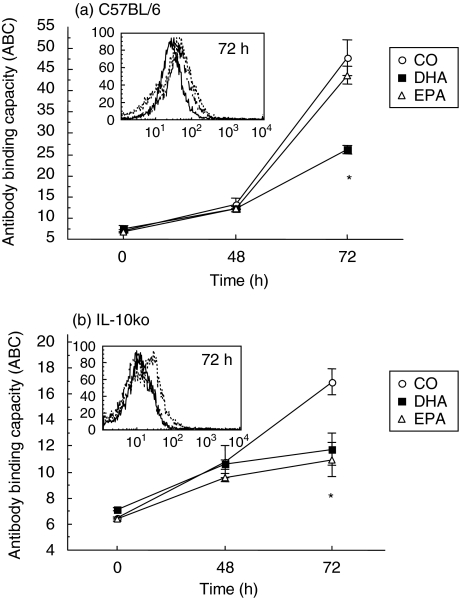
To elucidate the influence of dietary n-3 PUFA on IL-10R expression, kinetic analyses were performed on purified CD4+ T cells from conventional and IL-10–/– mice fed the three diets. As expected, activation of CD4+ T cells with αCD3/CD28 up-regulated cell surface expression of the IL-10R (Fig. 2). Cells from C57BL/6 mice fed DHA had significantly lower levels (∼40%) of IL-10R molecules on a per-cell basis than those fed the CO (control) and EPA diet after 72 h of in vitro stimulation (P < 0·01; Fig. 2a inset). Similarly, IL-10–/– mice fed dietary n-3 PUFA displayed markedly reduced levels of IL-10R molecules 72 h post-activation (∼35%; Fig. 2b, inset). In this instance, both DHA and EPA-fed groups significantly influenced the expression of the IL-10R. These results indicate that dietary n-3 PUFA significantly and differentially reduce the temporal expression of cell surface-expressed IL-10R in both conventional and IL-10–/– mice.
Fig. 2.
Dietary n-3 PUFA suppress IL-10R surface protein expression. Time-course analyses of the expression of IL-10R molecules were carried out in splenic CD4+ T cells cultured in the presence of HMS from (a) C57BL/6 and (b) IL-10–/– mice fed CO, DHA, or EPA. The y-axis represents the antibody binding capacity (ABC) equivalent sites for the IL-10R after culture with αCD3/CD28. Values represent means ± s.e.m. of cultures from n = 5 mice. *Highly significant differences were found between DHA and/or EPA and CO-fed mice (P < 0·01) Inset: histogram represents the mean fluorescence of anti-IL10R-PE. CO (dotted line), DHA (solid line) or EPA (dashed line).
Dietary n-3 PUFA differentially modulate IFN-γ and IL-10 cytokine production in CD4+ T cells
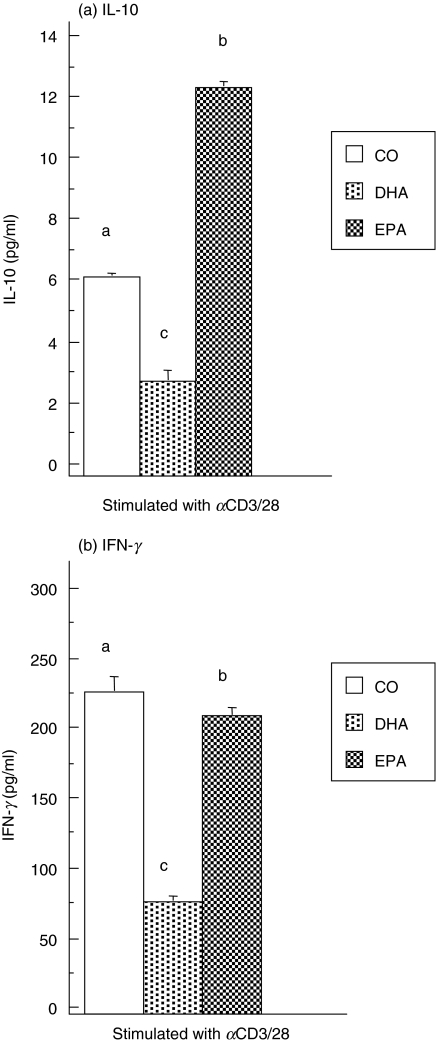
To investigate further the mechanism by which dietary n-3 PUFA suppress CD4+ T cell function in conventional and IL-10–/– mice, the production of the anti-inflammatory cytokine, IL-10, in cell culture supernatants was measured. It has been reported recently that the proinflammatory IFN-γ cytokine possesses anti-inflammatory properties and actively regulates IL-10 activity [15,16]. Thus, it was of interest to measure IFN-γ production as a potential anti-inflammatory mediator. Purified CD4+ T cells were stimulated with αCD3/CD28 for 48 h and cell supernatants were collected. Quantitative determination of IL-10 concentrations from C57BL/6 mice revealed that the DHA-fed group produced significantly less (∼50%) IL-10 than those fed the CO diet, whereas the EPA-fed group secreted dramatically more (twofold) of the suppressive cytokine (Fig. 3a). These results confirm that the anti-inflammatory cytokine IL-10 does not play a role in the suppression of CD4+ T cell proliferation by DHA from C57BL/6 mice (Fig. 1a). On the other hand, the immunosuppressive EPA diet may utilize IL-10 by up-regulating its expression. ELISA analysis of IFN-γ production in the same CD4+ T cell culture supernatants showed that both DHA (P < 0·01) and EPA (P = 0·01) diets significantly reduced IFN-γ production (Fig. 3b). This suggests strongly that IFN-γ does not mediate the suppressive effects of dietary n-3 PUFA on the T cell response.
Fig. 3.
Dietary n-3 PUFA differentially modulate CD4+ T cell IFN-γ and IL-10 production in C57BL/6 mice. Purified splenic CD4+ T cells from mice fed the three diets were activated with αCD3/CD28 in the presence of 2·5% HMS + 2·5% FBS for 48 h. (a) IL-10 and (b) IFN-γ in culture supernatant fluids were quantified by ELISA as described in the Materials and Methods. CD4+ T cells cultured with media alone produced less than 10 pg/ml of IFN-γ and 2 pg/ml of IL-10 (data not shown). Values from n = 5 mice represent the mean ± s.e.m. in pg/200 000 cells. Different letters denote highly significant differences found between diet groups (P < 0·01).
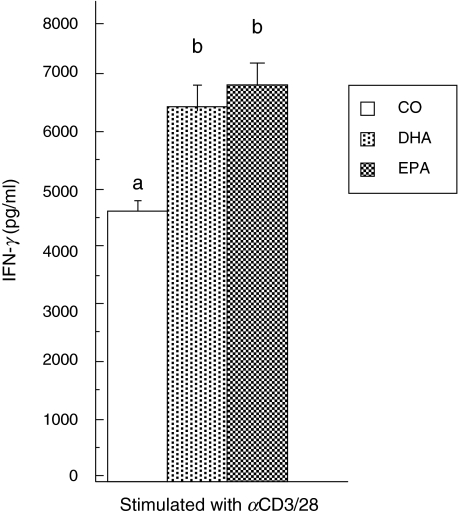
The production of IFN-γ was also quantified in cell supernatants from IL-10–/– mice. Figure 4 illustrates that dietary n-3 PUFA feeding significantly up-regulated IFN-γ production by CD4+ T cells (>40%). Overall, the levels of IFN-γ in the supernatants of IL-10–/– CD4+ T cells were 20–100-fold higher than those observed under identical culture conditions in CD4+ T cells from conventional C57BL/6 mice (Fig. 3b). Our results indicate that T cells from IL-10–/– mice may utilize the anti-inflammatory properties of IFN-γ to down-regulate T cell function (Fig. 1b). This may occur to compensate for the lack of available IL-10 within the cytokine network.
Fig. 4.
Dietary n-3 PUFA enhance IFN-γ production in CD4+ T cells from IL-10–/– mice. Purified splenic CD4+ T cells from mice fed the three diets were activated with αCD3/CD28 in the presence of 2·5% HMS + 2·5% FBS for 48 h. IFN-γ production in culture supernatant fluids was quantified by ELISA as described in the Materials and methods. CD4+ T cells cultured with media alone produced less than 200 pg/ml of IFN-γ (data not shown). Values from n = 5 mice represent the mean ± s.e.m. in pg/200 000 cells. Different letters denote highly significant differences found between diet groups (P < 0·01).
Discussion
The immunosuppressive role of IL-10 has been demonstrated by several human and mouse studies [17–20]. Inflammatory bowel disease and other exaggerated inflammatory responses exhibited by IL-10–/– mice indicated that a critical in vivo function of IL-10 is to limit inflammatory responses [21–23]. Moreover, inhibition of graft-versus-host disease by IL-10 and allograft rejection in human leucocyte antigen-mismatched bone-marrow transplantation in severe combined immunodeficient patients gives further evidence for a key role of this cytokine in the induction and maintenance of anergy [24].
The biological effects of cytokines are mediated through cell surface receptors. These receptors transduce the binding of their cytokines into cytoplasmic signals that eventually trigger a cascade of intracellular responses. The functional receptor complex of IL-10 consists of at least two subunits of IL-10R1 and IL-10R2, both of which have been characterized and shown to play critical roles in determining whether cells respond to IL-10 [25–29].
Our data in this current study strongly suggest that the anti-inflammatory effects of diets enriched in n-3 PUFA are probably not mediated by IL-10. Figures 1 and 2 illustrate that dietary n-3 PUFA continued to suppress CD4+ T lymphocyte responses in the absence of endogenous IL-10. Conventional C57BL/6 mice fed EPA may utilize the inhibitory IL-10 cytokine to suppress T cell function (Figs 1a and 3a), although the IL-10R expression levels remained unaltered (Fig. 2a). However, in similar mice fed the DHA diet, alternative mechanisms may explain the suppressive effect of diet as both IFN-γ and IL-10 cytokine production were down-regulated (Fig. 3).
Beside the inhibitory effect of IL-10, other mechanisms acting on co-stimulatory pathways have been demonstrated to render T cells unresponsive to an antigenic trigger. Blocking of the CD28–B7 interaction by CTLA-4 leads to an inhibition of xenogeneic graft rejection of pancreatic islets in mice [30]. It has been shown that CTLA-4 forms a multimolecular complex with T cell receptor (TCR)ξ and an SH2-containing tyrosine phosphatase (SHP-2), leading to a direct dephosphorylation of TCRξ and a subsequent inhibition of the TCR signalling pathway [31]. We demonstrated recently that dietary n-3 PUFA feeding significantly up-regulated both CD28 and CTLA-4 protein expression on the surface of murine CD4+ T cells (Ly et al., submitted for publication), thereby disrupting the balance between these two signals to favour reduced T cell activation. These findings are relevant to elucidate the mechanism(s) by which diet reduces T cell function, which do not involve IL-10 (Figs 1 and 2), in conventional mice. Further studies will be needed to determine the extent to which CTLA-4 plays a role in diet-mediated immunosuppression.
Interestingly, dietary DHA and EPA exhibited somewhat different immunomodulatory properties. In this study, both EPA and DHA significantly reduced CD4+ T cell proliferation in C57BL/6 mice but only EPA significantly suppressed this response in IL-10–/– mice (Fig. 1; DHA P = 0·058). Measurement of IL-10R expression levels revealed that conventional mice fed DHA had significantly lower levels than the CO-fed group, while both DHA and EPA significantly reduced IL-10R cell surface molecules in IL-10–/– mice (Fig. 2; t = 72 h post-activation). Furthermore, our results indicate that the EPA diet reduced IFN-γ but enhanced IL-10 production, while the DHA diet down-regulated both cytokines in C57BL/6 mice (Fig. 3). Although both experimental diets up-regulated production of IFN-γ in a similar manner (Fig. 4; IL-10–/– mice), it is clear that EPA and DHA have unique effects on certain T lymphocyte responses in our model. Consistent with these current findings, we reported recently that dietary DHA and EPA differentially altered co-stimulatory regulation as DHA significantly enhanced the cell surface expression of CD28, while dietary EPA up-regulated the expression of CTLA-4 (Ly et al., submitted for publication). The mechanisms responsible for the differential effects of EPA and DHA on T lymphoctye responses are unclear. EPA and DHA may conceivably have different effects on membrane raft stability because DHA is thought to adopt a more folded conformation in membranes and has been shown to exclude phospholipase D from lipid rafts [32]. We have demonstrated recently an effect of dietary n-3 PUFA on T cell lipid raft composition in our model [14,33].
Production of IFN-γ in response to infection is the hallmark of innate and adaptive immunity [34]. IFN-γ up-regulates a variety of proinflammatory parameters such as IL-12, IL-15, tumour necrosis factor (TNF)-α, iNOS and caspase-1 [35–38]. IL-10 and IFN-γ have opposing effects during an active phase of an immune or inflammatory response, characterized by high levels of IFN-γ production and modest IL-10 activity such that pathogens can be cleared effectively [5,39]. These proinflammatory characteristics of IFN-γ contradict certain aspects of its biological activity. Treatment of rheumatoid arthritis in mouse and human studies was associated with a reduction of leucocyte influx into the synovium, less synovial hyperplasia and erosion and improved clinical status [40,41]. Similarly, administration of IFN-γ markedly reduced the incidence of disease in a rat model of insulin-dependent diabetes mellitus [42]. The mechanisms by which the proinflammatory IFN-γ may exert its anti-inflammatory properties has been reviewed recently [15]. The data reviewed in this study demonstrate that IFN-γ redirects inflammatory responses by inhibiting production of proinflammatory IL-1 and IL-8 by up-regulating the production of cytokine antagonists such as IL-1Ra and IL−18 BP, inducing expression of the suppressors of cytokine signalling (SOCS) and inducing apoptosis in leucocytes and local resident cells. The biological triggers responsible for shifting the role of IFN-γ in response to immunological stimuli have not been elucidated. However, these anti-inflammatory properties of the principally proinflammatory cytokine IFN-γ may explain, in part, its enhanced production in suppressed T cells from IL-10–/– mice fed dietary n-3 PUFA (Figs 1b and 4). A clear indication of this compensatory role for IFN-γis the overall increase in IFN-γ production in lymphocytes from IL-10–/–versus C57BL/6 mice (20–100-fold; Figs 3b and 4). Further experiments will be necessary to demonstrate the anti-inflammatory role of IFN-γ in IL-10–/– mice fed dietary n-3 PUFA.
Acknowledgments
We thank Dr Daniel J. Berg, University of Iowa College of Medicine, for providing the IL-10 null breeder mice. We would also like to thank Martek Biosciences (Columbia, MD, USA) and Laxdale (UK) for donating the purified DHA and EPA ethyl esters, respectively. This study was supported, in part, by NIH grant no. DK53055 and P30-ES09106 and USDA grant 2003-35200-13338.
References
- 1.Fiorentino DF, Bond MW, Mosmann TR. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989;170:2081–95. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fiorentino DF, Zlotnik A, Mosmann TR, Howard M, O'Garra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 1991;147:3815–22. [PubMed] [Google Scholar]
- 3.de Waal Malefyt R, Abrams J, Bennett B, Figdor CG, de Vries JE. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991;174:1209–20. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Del Prete G, De Carli M, Almerigogna F, Giudizi MG, Biagiotti R, Romagnani S. Human IL-10 is produced by both type 1 helper (Th1) and type 2 helper (Th2) T cell clones and inhibits their antigen-specific proliferation and cytokine production. J Immunol. 1993;150:353–60. [PubMed] [Google Scholar]
- 5.Pajkrt D, Camoglio L, Tiel-van Buul MC, et al. Attenuation of proinflammatory response by recombinant human IL-10 in human endotoxemia: effect of timing of recombinant human IL-10 administration. J Immunol. 1997;158:3971–7. [PubMed] [Google Scholar]
- 6.Avdiushko R, Hongo D, Lake-Bullock H, Kaplan A, Cohen D. IL-10 receptor dysfunction in macrophages during chronic inflammation. J Leukoc Biol. 2001;70:624–32. [PubMed] [Google Scholar]
- 7.Linsley PS, Brady W, Grosmaire L, Aruffo A, Damle NK, Ledbetter JA. Binding of the B cell activation antigen B7 to CD28 costimulates T cell proliferation and interleukin 2 mRNA accumulation. J Exp Med. 1991;173:721–30. doi: 10.1084/jem.173.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harding FA, McArthur JG, Gross JA, Raulet DH, Allison JP. CD28-mediated signalling co-stimulates murine T cells and prevents induction of anergy in T-cell clones. Nature. 1992;356:607–9. doi: 10.1038/356607a0. [DOI] [PubMed] [Google Scholar]
- 9.Akdis CA, Blaser K. Mechanisms of interleukin-10-mediated immune suppression. Immunology. 2001;103:131–6. doi: 10.1046/j.1365-2567.2001.01235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joss A, Akdis M, Faith A, Blaser K, Akdis CA. IL-10 directly acts on T cells by specifically altering the CD28 co-stimulation pathway. Eur J Immunol. 2000;30:1683–90. doi: 10.1002/1521-4141(200006)30:6<1683::AID-IMMU1683>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 11.Arrington JL, McMurray DN, Switzer KC, Fan YY, Chapkin RS. Docosahexaenoic acid suppresses function of the CD28 costimulatory membrane receptor in primary murine and Jurkat T cells. J Nutr. 2001;131:1147–53. doi: 10.1093/jn/131.4.1147. [DOI] [PubMed] [Google Scholar]
- 12.Fowler KH, Chapkin RS, McMurray DN. Effects of purified dietary n-3 ethyl esters on murine T lymphocyte function. J Immunol. 1993;151:5186–97. [PubMed] [Google Scholar]
- 13.Arrington JL, Chapkin RS, Switzer KC, Morris JS, McMurray DN. Dietary n-3 polyunsaturated fatty acids modulate purified murine T-cell subset activation. Clin Exp Immunol. 2001;125:499–507. doi: 10.1046/j.1365-2249.2001.01627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muhl H, Pfeilschifter J. Dietary n-3 polyunsaturated fatty acids promote activation-induced cell death in Th1-polarized murine CD4+ T cells. Int Immunopharmacol. 2003;3:1247–55. [Google Scholar]
- 15.Herrero C, Hu X, Li WP, et al. Anti-inflammatory properties of pro-inflammatory interferon-gamma. J Immunol. 2003;171:5034–41. [Google Scholar]
- 16.Go NF, Castle BE, Barrett R, et al. Reprogamming of IL-10 activity and signaling by IFN-gamma. J Exp Med. 1990;172:1625–31. [Google Scholar]
- 17.MacNeil IA, Suda T, Moore KW, Mosmann TR, Zlotnik A. Interleukin 10, a novel B cell stimulatory factor: unresponsiveness of X chromosome-linked immunodeficiency B cells. J Immunol. 1990;145:4167–73. [Google Scholar]
- 18.Rousset F, Garcia E, Defrance T, et al. IL-10, a novel growth cofactor for mature and immature T cells. Proc Natl Acad Sci USA. 1992;89:1890–3. [Google Scholar]
- 19.Chen WF, Zlotnik A. Interleukin 10 is a potent growth and differentiation factor for activated human B lymphocytes. J Immunol. 1991;147:528–34. [Google Scholar]
- 20.Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. IL-10: a novel cytotoxic T cell differentiation factor. Cell. 1993;75:263–74. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 21.Berg DJ, Kuhn R, Rajewsky K, et al. Interleukin-10-deficient mice develop chronic enterocolitis. J Clin Invest. 1995;96:2339–47. [Google Scholar]
- 22.Berg DJ, Leach MW, Kuhn R, et al. Interleukin-10 is a central regulator of the response to LPS in murine models of endotoxic shock and the Shwartzman reaction but not endotoxin tolerance. J Exp Med. 1995;182:99–108. doi: 10.1172/JCI118290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bacchetta R, Bigler M, Touraine JL, et al. Interleukin 10 but not interleukin 4 is a natural suppressant of cutaneous inflammatory responses. J Exp Med. 1994;179:493–502. [Google Scholar]
- 24.Ho AS, Liu Y, Khan TA, Hsu DH, Bazan JF, Moore KW. High levels of interleukin 10 production in vivo are associated with tolerance in SCID patients transplanted with HLA mismatched hematopoietic stem cells. Proc Natl Acad Sci USA. 1993;90:11267–71. [Google Scholar]
- 25.Liu Y, Wei SH, Ho AS, de Waal Malefyt R, Moore KW. A receptor for interleukin 10 is related to interferon receptors. J Immunol. 1994;152:1821–9. [PubMed] [Google Scholar]
- 26.Kotenko SV, Krause CD, Izotova LS, Pollack BP, Wu W, Pestka S. Expression cloning and characterization of a human IL-10 receptor. EMBO J. 1997;16:5894–903. doi: 10.1093/emboj/16.19.5894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spencer SD, Di Marco F, Hooley J, et al. Identification and functional characterization of a second chain of the interleukin-10 receptor complex. J Exp Med. 1998;187:571–8. doi: 10.1084/jem.187.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ding Y, Qin L, Zamarin D, et al. The orphan receptor CRF2-4 is an essential subunit of the interleukin 10 receptor. J Immunol. 2001;167:6884–92. [Google Scholar]
- 29.Lenschow DJ, Zeng Y, Thistlethwaite JR, et al. Differential IL-10R1 expression plays a critical role in IL-10-mediated immune regulation. Science. 1992;257:789–92. [Google Scholar]
- 30.Lee KM, Chuang E, Griffin M, et al. Long-term survival of xenogeneic pancreatic islet grafts induced by CTLA4lg. Science. 1998;282:2263–6. doi: 10.1126/science.1323143. [DOI] [PubMed] [Google Scholar]
- 31.Diaz O, Berquand A, Dubois M, et al. Molecular basis of T cell inactivation by CTLA-4. J Biol Chem. 2002;277:39368–78. doi: 10.1074/jbc.M202376200. [DOI] [PubMed] [Google Scholar]
- 32.Switzer KC, Fan YY, Wang N, McMurray DN, Chapkin RS. The mechanism of docosahexaenoic acid-induced phospholipase D activation in human lymphocytes involves exclusion of the enzyme from lipid rafts. J Lipid Res. 2004;45:1482–92. [Google Scholar]
- 33.Fan YY, McMurray DN, Ly LH, Chapkin RS. Dietary (n-3) polyunsaturated fatty acids remodel mouse T-cell lipid rafts. J Nutr. 2003;133:1913–20. doi: 10.1093/jn/133.6.1913. [DOI] [PubMed] [Google Scholar]
- 34.Billiau A. Interferon-gamma: biology and role in pathogenesis. Adv Immunol. 1996;62:61–130. doi: 10.1016/s0065-2776(08)60428-9. [DOI] [PubMed] [Google Scholar]
- 35.Yoshida A, Koide Y, Uchijima M, Yoshida TO. IFN-gamma induces IL-12 mRNA expression by a murine macrophage cell line, J774. Biochem Biophys Res Commun. 1994;198:857–61. doi: 10.1006/bbrc.1994.1122. [DOI] [PubMed] [Google Scholar]
- 36.Hayes MP, Freeman SL, Donnelly RP. IFN-gamma priming of monocytes enhances LPS-induced TNF production by augmenting both transcription and MRNA stability. Cytokine. 1995;7:427–35. doi: 10.1006/cyto.1995.0058. [DOI] [PubMed] [Google Scholar]
- 37.Xie QW, Nathan C. Promoter of the mouse gene encoding calcium-independent nitric oxide synthase confers inducibility by interferon-gamma and bacterial lipopolysaccharide. Trans Assoc Am Physicians. 1993;106:1–12. [PubMed] [Google Scholar]
- 38.Tamura T, Ueda S, Yoshida M, Matsuzaki M, Mohri H, Okubo T. Interferon-gamma induces Ice gene expression and enhances cellular susceptibility to apoptosis in the U937 leukemia cell line. Biochem Biophys Res Commun. 1996;229:21–6. doi: 10.1006/bbrc.1996.1752. [DOI] [PubMed] [Google Scholar]
- 39.Hart PH, Ahern MJ, Smith MD, Finlay-Jones JJ. Comparison of the suppressive effects of interleukin-10 and interleukin-4 on synovial fluid macrophages and blood monocytes from patients with inflammatory arthritis. Immunology. 1995;84:536–42. [PMC free article] [PubMed] [Google Scholar]
- 40.Wahl SM, Allen JB, Ohura K, Chenoweth DE, Hand AR. IFN-gamma inhibits inflammatory cell recruitment and the evolution of bacterial cell wall-induced arthritis. J Immunol. 1991;146:95–100. [PubMed] [Google Scholar]
- 41.Rockey DC, Maher JJ, Jarnagin WR, Gabbiani G, Friedman SL. Inhibition of rat hepatic lipocyte activation in culture by interferon-gamma. Hepatology. 1992;16:776–84. doi: 10.1002/hep.1840160325. [DOI] [PubMed] [Google Scholar]
- 42.Nicoletti F, Zaccone P, Di Marco R, et al. Paradoxical antidiabetogenic effect of gamma-interferon in DP-BB rats. Diabetes. 1998;47:32–8. doi: 10.2337/diab.47.1.32. [DOI] [PubMed] [Google Scholar]