Abstract
Asthma is a serious health problem and during the last decade various experimental models of asthma have been developed to study the pathogenesis of this disease. In this study we describe a new mouse model of asthma that uses the spores of Alternaria alternata and Cladosporium herbarum, two allergenic molds recognized as common inducers of rhinitis and asthma in humans. Here we demonstrate that A. alternata and C. herbarum spores are immunogenic when injected into BALB/c mice, and induce the production of specific IgM and IgG1 antibodies and strongly increase IgE serum levels. To induce the allergic response, mice were sensitized by two intraperitoneal (i.p.) injections and then intranasaly (i.n.) challenged with A. alternata and C. herbarum spores. Bronchoalveolar lavages (BALs) from these mice contained numerous macrophages, neutrophils, eosinophils and lymphocytes whereas neutrophils were the predominant BAL inflammatory cells in nonsensitized mice. Histological studies demonstrated an influx of eosinophils in peri-vascular and peri-bronchial areas and the presence of numerous epithelial goblet cells only in sensitized mice. Increased expression of mRNA specific for various chemokines (eotaxin, MIP-1α, MIP-2) and chemokine receptors (CCR-1, CCR-2 and CCR-5) was observed in the lungs of nonsensitized mice challenged with the spores. Expression of CCR-3 mRNA in the lungs and Th2 cytokine (IL-4, IL-5 and IL-13) secretion in the BAL was additionally observed in sensitized and challenged mice. Finally we demonstrate through whole-body plethysmography that mold spore sensitization and challenge induce the development of an airway hyperreactivity in response to nebulized methacholine.
Keywords: allergy, eosinophils, neutrophils, lung immunology/disease, animal models/studies, mice/rats
Introduction
Asthma is a major cause of chronic illness, affecting as many as 25% of schoolchildren in regions of high prevalence [1]. In asthmatic patients, the penetration of allergens into the lungs leads to an airway inflammation characterized by a peri-bronchial infiltration of CD4+ T cells, macrophages, eosinophils and neutrophils and their presence in bronchoalveolar lavages (BALs). Asthmatic patients also present a goblet cell metaplasia/hyperplasia and characteristic modifications of the airway wall including epithelial hyperplasia, thickening of the basement membrane, subepithelial fibrosis and increased airway smooth muscle mass [2,3]. It is believed that these modifications finally lead to airway hyperreactivity (AHR) to specific and non specific stimuli although the exact mechanisms of AHR pathogenesis are still unclear.
Indoor allergens are represented by allergens from dust mites, cockroaches and animal dander while plant pollen are typical outdoor allergens [4]. Fungal spores are universal atmospheric components and are also recognized as important causes of respiratory allergies. More than 80 genera of fungi have been associated with symptoms of respiratory allergy and the prevalence of this kind of allergy is estimated at 20–30% among atopic individuals and up to 6% in the general population [5,6].
Despite the clinical importance and the worldwide abundant release of fungal spores, few investigations have focused on the pathophysiology of fungal asthma. So far, most experimental models of asthma have been developed in rodents and have used ovalbumin (OVA) as a surrogate of allergen. These models have been valuable tools for the study of inflammatory mechanisms of asthma. However the relevance of allergic bronchopulmonary models that use protein antigens like OVA that bear little relationship with aeroallergen present in daily environment is debatable and it is likely that the use of common aeroallergens that trigger human asthma might be more valuable. Therefore, some investigators have used crude extracts or purified allergens from ragweed [7], mites [8], or cockroach [9] as immunizing agents.
In an effort to establish a new mouse model of lung hypersensitivity, we decided to use the spores of two well-recognized allergenic molds Alternaria alternata and Cladosporium herbarum. In contrast to Aspergillus which is a opportunistic pathogen causing allergic and invasive diseases [10], spores from Alternaria and cladosporium do not colonize the lungs and are rapidly cleared. Spores from these two molds are generally considered to be important causes of both allergic rhinitis and asthma and exposure to airborne spores of A. alternata might trigger severe asthma and represent a risk factor for respiratory failure [11]. In this paper we describe a new mouse model of mold spore-induced lung allergy and show that spores from A. alternata and C. herbarum are strong inducers of IgE synthesis, allergic airway inflammation and airway hyperreactivity.
Materials and methods
Mold cultures, spore production and extract preparation
Alternaria alternata (strain18586) was obtained from the BCCMTM/IHEM (Institute of Public Health, Brussels, Belgium). Cladosporium herbarum (strain 19275) was obtained from the BCCMTM/MUCL (Université Catholique de Louvain, Louvain, Belgium). These molds were cultured at 27°C on potato dextrose agar (Difco) plates for one week before gently harvesting the spores with a cell scraper. Spores were diluted in PBS and counted with a haemocytometer.
Mold extracts were prepared as previously described [12] with slight modifications. Mold cultures were grown for 3 weeks at 27°C in flasks containing 250 ml of Czapek's medium. Mold pellicles were harvested and homogenized in 0·4% NH4HCO3+ polyvinyl polypyrrolidone (Sigma) with an ultra-thurax. The homogenates were then agitated for 3 h at 4°C. Extracts were centrifuged twice 30 min at 20 000 g, dialysed against PBS and stored at −20°C in 50% glycerol.
Animals and immunizations
Female BALB/c mice were obtained from the Elevage Janvier or from the breeding facilities of the Pasteur Institute of Brussels and maintained under standard laboratory conditions. For immunogenicity experiments, mice were immunized by intraperitoneal (i.p) injections of the spores diluted in PBS or emulsified in Aluminium hydroxyde (Alum) (Imject Alum, Pierce) in a final volume of 300 µl. Mice were also immunized with 20 µg of mold extract emulsified in Alum. Animals were boosted one month after the first injection. Mice were bled before and 15 days after each immunization in the retro-orbital plexus and antibodies in the sera were analysed by ELISA. To induce the allergic lung inflammation, mice were sensitized by i.p. immunization with 2 × 106 spores emulsified in Alum on day 0 and day 7. Control mice were immunized with PBS + Alum. On day 13, 14 and 15 mice were challenged i.n. with 2 × 105 spores. Mice were lightly anaesthetized with halothane (Belamont). When the mice were unresponsive but breathing comfortably, 100 µl of the spore solution was directly applied on the nostrils. The animals were allowed to slowly inhale the liquid and were then recovered in a supine position. In all experiments, data shown are representative of at least two different experiments. Results were analysed using the paired student t-test. Significant P-values are indicated.
Bronchoalveolar lavages
Mice were euthanized by cervical dislocation 24 h after the last challenge. The trachea was exposed and incised. A needle (1·2 × 40 mm) was inserted into the trachea and BAL fluid was harvested by lavaging the lungs twice with 1 ml of PBS. Total cell counts were determined with an haemacytometer. Differential cell counts were obtained by counting at least 500 cells on cytospin slides stained with Diff-Quick (Dade Behring).
Histological analysis
Tissues for histopathological examination were collected 24 h after the last i.n. instillation. Mice were euthanized by cervical dislocation, the trachea was canulated and the lungs were inflated with 10% buffered formalin. After fixation overnight, the lungs were embedded in paraffin. Tissues were sliced and 5 µm sections were stained with Hematoxylin-Eosin-Safran (HES) for light microscopy examination of the lung inflammation or with Periodic Acid Shiff (PAS) for the detection of mucus-producing cells.
ELISA
Serum IgE levels in sera were determined using a sandwich ELISA. Plates were coated with a rat antimouse IgE mAb (LO-ME-2, IMEX, UCL, Brussels, Belgium) and saturated. Serial twofold dilutions of serum or purified monoclonal mouse IgE (LB-4, IMEX, UCL, Brussels, Belgium) were applied for 2 h. Then peroxidase labelled rat antimouse IgE (LO-ME-3) was used. Finally the plates were washed and developed by the addition of 100 µl of TMB (Immunopure TMB substrate kit, Pierce). The reaction was stopped with 50 µl of 2 N H2SO4 and O.D. was read at 450 nm with an automatic Multiskan Reader MCC/340 (Titertek). Serum titre was converted to IgE concentration by comparison with titre of purified LB-4 standard. Similar immunoglobulin concentrations for serum were calculated when serum and standard were titred at any point of the linear part of the titration curve.
Cytokines were measured in the bronchoalveolar lavage supernatants (BALs) obtained 24 h after the last i.n. inoculation. The levels of IL-4, IL-5 and IL-13 were quantified by ELISA using commercial kits according to the instructions provided by the manufacturers (R & D systems, Minneapolis, USA).
Chemokine mRNA quantification: Rnase protection assay (RPA)
The levels of mRNA encoding chemokines and chemokine receptors in the lungs were quantified using a RPA. Lungs were removed 24 h after the last i.n. instillation. Total Lung RNA was obtained using the total RNA isolation kit (Pharmingen, San Diego, CA, USA) according to the instructions provided by the manufacturer. The levels of specific mRNA transcripts were then evaluated by RPA using the Riboquant kit and the mCK-1 and mCR-5 Riboquant multiprobe template set (Pharmingen). 32P labelled riboprobes were synthetized by in vitro transcription. For each experiment 8 µg of whole lung RNA was incubated at 56°C for 16 h with the labelled riboprobes. Unbound riboprobes not hybridized to the mRNAs were removed by adding RNAse followed by phenol/chlorophorm-isoamyl alcohol extraction. Protected riboprobes binding to the lung mRNA were then separated by electrophoresis over a Novex precast 6% TBE-urea gel (Invitrogen). After gel transfer to a trans-blot 0·45 µm nitrocellulose sheet (Biorad), the gels were dried and exposed to a Kodak X-OMAT AR film in cassettes at −80°C during 24–48 h for development of autoradiographs.
Measurement of airway reactivity
AHR was measured within 24 h after the last spore inoculation, in response to methacholine inhalation using a whole-body plethysmography system (EMKA, Paris, France). Mice were placed in the plethysmograph box and allowed to acclimatize for at least 10 min before analysis. Control measurements were obtained over a period of 5 min directly after a 3 min nebulization of PBS generated by an ultrasonic nebulizer (LS Syst’AM, France). Afterwards, increasing concentrations of methacholine (12·5, 25 and 50 mg/ml) in PBS were nebulized into the plethysmograph box for 3 min. Immediately after each nebulization the enhanced pause (Penh), a dimensionless index that reflects changes in the amplitude of the pressure waveform and expiratory time, were recorded and averaged for 5 min. Penh measurements have been validated by Hamelmann et al. [13] in regard to identification of AHR since the heightened increase in Penh with methacholine challenge in OVA-sensitized/challenged mice was accompanied by a parallel enhancement of in lung airway resistance (RL) responses to the methacholine with a high correlation between Penh and RL.
Results
Injection of A. alternata or C. herbarum spores into BALB/c mice induces a specific IgG1 response and a polyclonal IgE production
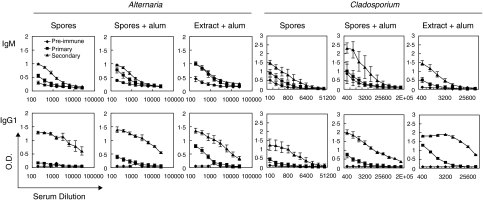
To determine the amount of mold spores required to induce an antibody response, BALB/c mice were injected twice with 2 × 104, 2 × 105, 2 × 106 or 2 × 107A. alternata or C. herbarum spores without any adjuvant or in the presence of Alum. As a positive control, mice were also injected with 20 µg of mold extract precipitated in Alum. A weak specific antibody production was observed in the groups injected with 2 × 104 or 2 × 105 spores only in the secondary response. In the other two groups, antibody levels were low in the primary response while a strong production occurred after the boost injection. Analysis of the isotypes revealed that, independently of the adjuvant, the secondary response was dominated by the production of IgG1 antibodies specific for A. alternata or C. herbarum antigens (Fig. 1). IgG2a, IgG2b or IgG3 were not detected at all (data not shown).
Fig. 1.
IgM and IgG1 antibody responses induced by mold-spore immunization. BALB/c mice were immunized i.p. twice with a one-month interval between immunization with 2 × 106A. alternata or C. herbarum spores either in PBS or emulsified in Alum, or 20 µg of mold extract in Alum. Mice were bled before the immunization or 15 days after each injection. Data represent the mean ± SEM of the A450 values of four to five individual mice per group.
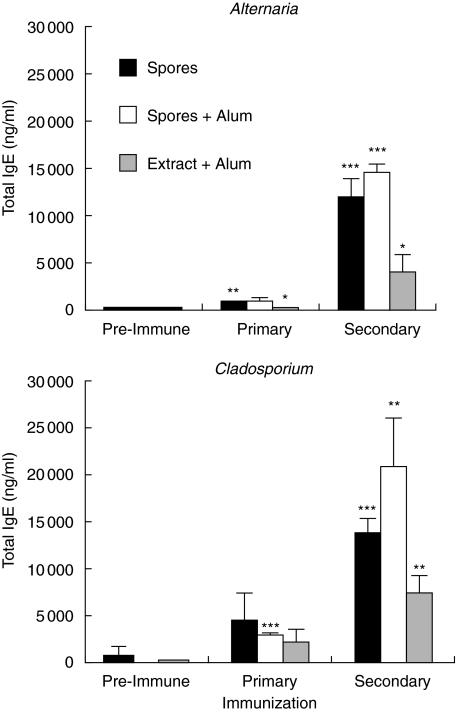
Total IgE production was also monitored in mice injected with 2 × 106 spores (Fig. 2). Serum level of IgE before the immunization was below 1 µg/ml. As observed with the specific antibodies, only a slight increase in the IgE serum levels was observed after the first injection while a strong IgE production occurred after the boost injection. Serum IgE levels up to 15 µg/ml were obtained in the groups that received the A. alternata or the C. herbarum spores in the presence or even in the absence of Alum. Mice immunized with the extract showed a moderate increase in their IgE serum levels.
Fig. 2.
Polyclonal IgE secretion induced by mold-spore immunization. BALB/c mice were immunized i.p. twice with a one-month interval between immunization with 2 × 106A. alternata or C. herbarum spores in PBS (▪) or emulsified in Alum ( ) or 20 µg of mold extract in Alum (□). Mice were bled before the immunization or 15 days after each injection. Data represent the mean ± SD of four to five individual mice per group. Significant differences (*P < 0·1. **P < 0·05. ***P < 0·01) between values of primary or secondary response versus preimmune response.
) or 20 µg of mold extract in Alum (□). Mice were bled before the immunization or 15 days after each injection. Data represent the mean ± SD of four to five individual mice per group. Significant differences (*P < 0·1. **P < 0·05. ***P < 0·01) between values of primary or secondary response versus preimmune response.
Pulmonary inflammation after sensitization and i.n. instillation of A. alternata or C. herbarum spores
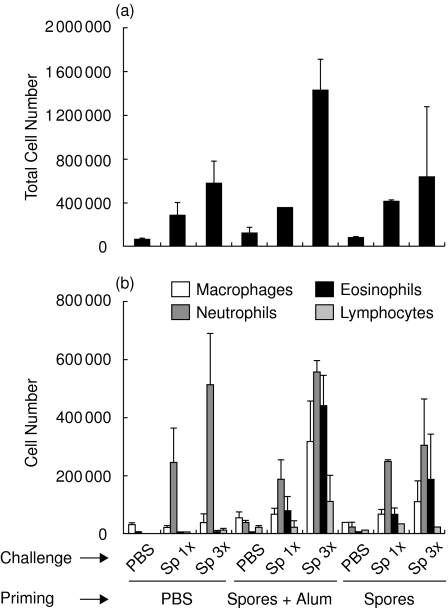
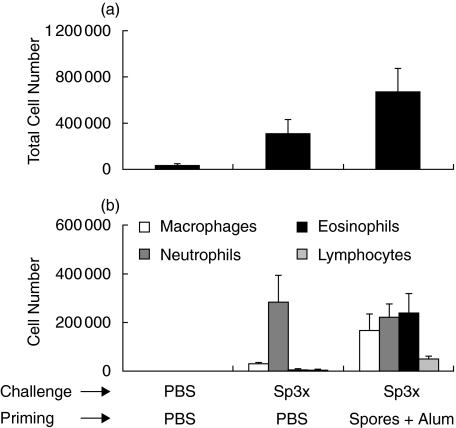
Alternaria and cladosporium sensitization is strongly correlated with the development of allergic disease, especially asthma. The aim of this study was to establish a novel murine model of allergic airway inflammation using whole mold spores instead of immunizing the mice with recombinant or purified allergens. To characterize pulmonary inflammation in this mouse model, BALB/c mice were first sensitized twice i.p. with 2 × 106A. alternata spores in the presence or absence of Alum. Control mice were injected with PBS. Afterwards mice were challenged i.n. with 2 × 105A. alternata spores once or three times or with PBS only. Twenty-four hours later, BALs were obtained and analysed for the presence of macrophages, neutrophils, eosinophils and lymphocytes. Figure 3 shows that the total number of cells obtained in BALs increased with the number of challenges, the maximum being reached in the group of mice sensitized with the spores in the presence of Alum. Analysis of the different cell subpopulations showed that i.n. inoculation of the spores in nonsensitized mice leads to the accumulation of a high number of neutrophils in the BALs. In contrast, upon challenge with the spores, BALs obtained from mice sensitized with the spores (in the presence or in the absence of adjuvant) were more heterogeneous, presenting an increased number of macrophages, neutrophils, eosinophils and lymphocytes (Fig. 3). This increase in the number of eosinophils in the airways has been considered as a hallmark of allergic asthma [3].
Fig. 3.
Inflammatory cell recruitment in the lungs of A. alternata sensitized and/or challenged mice. BALB/c mice were not sensitized (PBS) or were primed with 2 × 106A. alternata spores in PBS or emulsified in Alum. Mice were thereafter challenged i.n. with PBS or 2 × 105 spores once or three times. (a) BALs were collected 24 h later and the total cell number in each BAL was determined. (b) Differential cell count of the inflammatory subpopulation in the BALs 24 h after the last challenge.
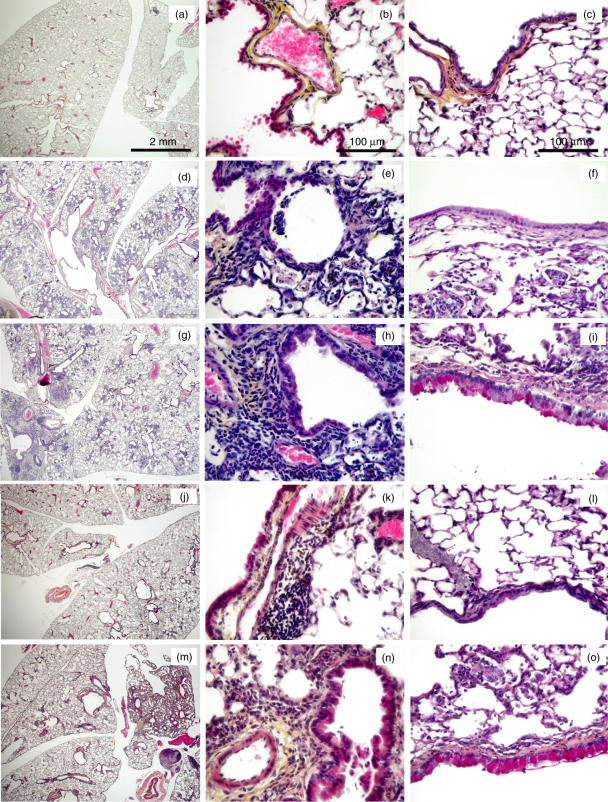
Eosinophil recruitment in the lungs of alternaria-sensitized and challenged mice was further studied by histological analysis. Mice were either not sensitized or sensitized with the spores emulsified in Alum and then challenged i.n. three times with the spores. Control mice were not sensitized and challenged i.n. with PBS. Lungs were processed for histology 24 h after the last challenge. In control mice, the pulmonary histology was normal demonstrating no inflammation at all (Fig. 4a,b). Mice challenged with the spores showed an important lung inflammation located in the peri-vascular, peri-bronchial areas and also in the lung parenchyma for some mice (Fig. 4d,e,g,h). In nonsensitized mice, we observed a strong accumulation of neutrophils along with some lymphocytes (Fig. 4e). However large number of eosinophils in inflammatory foci were only observed in mice previously sensitized with the spores (Fig. 4h). To assess the degree of goblet cell hyperplasia, lung sections were stained with PAS. Mice sensitized and challenged with the spores showed numerous PAS-positive cells in the large airways and an important thickening of the epithelium (Fig. 4i). Nonsenzitized mice challenged with the spores and control mice demonstrated no thickening of the airway wall and minimal goblet cells in the airway epithelium (Fig. 4c,f)
Fig. 4.
Histopathological changes in the lungs of A. alternata and C. herbarum sensitized and/or challenged mice. BALB/c mice were challenged i.n. with PBS (a–c), or A. alternata spores (d–f) or C. herbarum spores (j–l) or were sensitized i.p. and challenged i.n. with A. alternata spores (g–i) or C. herbarum spores (m–o). The first two columns show lung sections stained with Hematoxylin-Eosin-Safran and the last column shows lung sections stained with Periodic Acid Shiff.
The inflammatory cell response from mice treated with the C. herbarum or A. alternata spores was very similar although the numbers of inflammatory cells in the BALs were constantly lower in response to the former mold. Intranasal inoculation of C. herbarum spores in nonsensitized mice induced only the accumulation of neutrophils into the BALs. However, BALs from mice sensitized and challenged with these spores showed a similar accumulation of macrophages, neutrophils and eosinophils and a lower amount lymphocytes (Fig. 5). Histological examination of lung sections from C. herbarum spores-challenged mice demonstrated an accumulation of eosinophils around the vessels and the bronchioles, a thickening of the airway epithelia and the presence of goblet cells in sensitized mice (Fig. 4j–o). Nonsensitized mice showed minimal inflammation foci which were devoid of eosinophils (Fig. 4k), no epithelial thickening and no PAS positive cells (Fig. 4l).
Fig. 5.
Inflammatory cell recruitment in the lungs of C. herbarum sensitized and/or challenged mice. BALB/c mice were not sensitized (PBS) or were primed with 2 × 106C. herbarum spores in PBS or emulsified in Alum. Mice were thereafter challenged i.n. with PBS or 2 × 105 spores three times. (a) BALs were collected 24 h later and the total cell number in each BAL was determined. (b) Differential cell count of the inflammatory subpopulation in the BALs 24 h after the last challenge.
Analysis of the chemokine response in the lungs
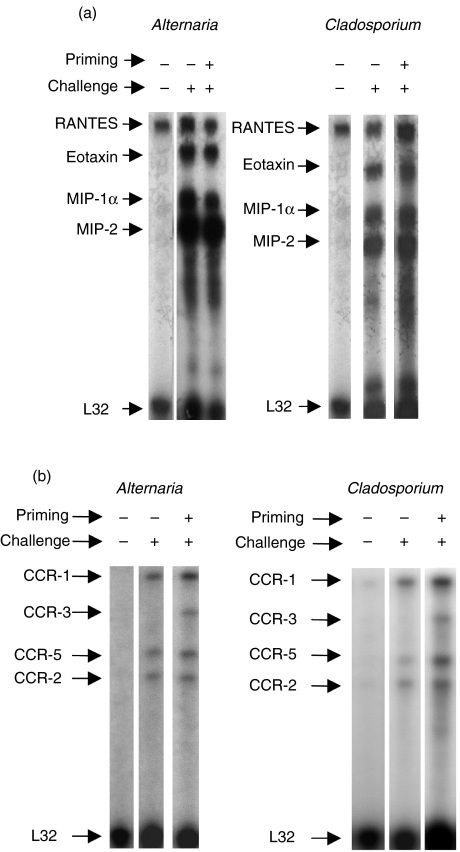
Chemokines are a group of cytokines that promote the leucocyte recruitment to inflammatory sites, stimulate leucocyte exocytosis and activation of inflammatory immunocompetent cells through specific receptors on target cells. One of the most important chemokines for the recruitment of eosinophils is eotaxin that binds to its specific receptor CCR-3. We compared the expression of chemokines and chemokine receptors using a ‘Ribonuclease protection assay’ in the lungs of nonsensitized and sensitized mice challenged with A. alternata and C. herbarum spores (Fig. 6). In control unchallenged mice a low expression of RANTES and no expression at all of chemokine receptors was found. Upon i.n. inoculation, a similar up-regulation of the expression of eotaxin, MIP-1α, MIP-2 and MCP-1 was found independently of the sensitization status. No lymphotoxin, MIP-1β, IP-10 or TCA-3 were detected. However, a differential expression of chemokine receptors was observed in nonsensitized and sensitized mice. I.n. A. alternata or Cladosporium spore challenge up-regulated the mRNA level coding for CCR-1, CCR-2 and CCR-5 while CCR-3 mRNA was additionally up-regulated in the sensitized and challenged group. mRNA coding for CCR-1b or CCR4 were below detection level.
Fig. 6.
RNase protection assay analysis of transcripts for chemokines and chemokines receptors. BALB/c mice were challenged i.n. with PBS, A. alternata or C. herbarum spores or were sensitized and then i.n. challenged with mold spores. 24 h after the last challenge lung RNAs were prepared and the levels of mRNA encoding chemokines and chemokines receptors were evaluated by RPA as described in Materials and Methods. One representative mouse (of five) per group is presented. The level of expression of the housekeeping gene L32 is used as an internal control.
Analysis of the Th2 cytokine response in the lungs
Th2 cytokines secreted by T cells play a key regulatory role in the induction of allergic airway inflammation. IL-4 and IL-13 are required for IgE production and goblet cell hyperplasia and IL-5 is essential for the development of tissue eosinophilia. The presence of IL-4, IL-5 and IL-13 was analysed by ELISA in the BALs from control PBS sensitized/challenged mice and nonsensitized or sensitized mice challenged with the spores one day after the last i.n. inoculation. As shown in Table 1, a low secretion of IL-13 and IL-4 was observed in control PBS-treated mice while IL-5 was not detected at all. In nonsensitized mice, i.n. inoculation of the spores did not significantly induce any secretion of these cytokines. However IL-13, IL-4 and IL-5 were abundantly found in the BALs from mice sensitized and challenged with A. alternata and C. herbarum spores one day after the last challenge.
Table 1.
Th2 cytokine detection in the BALs.†
| Treatment | IL-4 (pg/ml) | IL-5 (pg/ml) | Il-13 (pg/ml) |
|---|---|---|---|
| PBS/PBS | 2 ± 0.5 | ND | 16 ± 5.7 |
| PBS/Alt Sp | 2 ± 0.9 | ND | 28 ± 4.1 |
| Alt Sp/Alt Sp | 35 ± 9.1* | 40 ± 14* | 150 ± 65.7 |
| PBS/Clado Sp | 2 ± 0.8 | ND | 40 ± 15.2 |
| Clado Sp/Clado Sp | 16 ± 5 | 23 ± 5.6 | 197 ± 74.8 |
Statistically significant at P < 0.1.
As described in Material and methods BALB/c mice were not sensitized or sensitized with spores and then challenged i.n. with spores. 24h later, BALs were performed and Th2 cytokine content in BALs was analyzed by ELISA. Values shown as mean ± SD of 4 to 5 mice. ND, not detected.
Development of airway hyperreactivity after A. alternata or C. herbarum spore sensitization and i.n. instillation
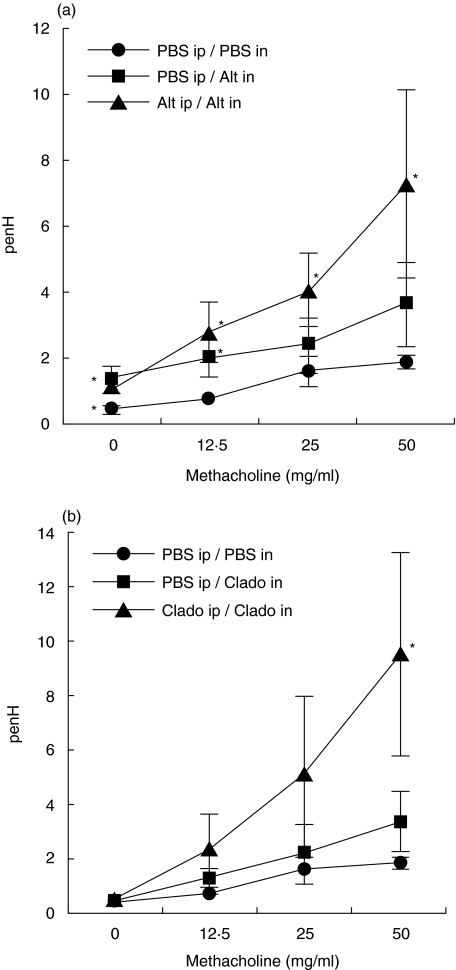
Whole body plethysmography was used to analyse the airway reactivity of mice sensitized or not and then challenged with A. alternata or C. herbarum spores. As shown in Fig. 7, mice challenged i.n. with A. alternata spores demonstrated, independently of their sensitization status, an increased basal Penh value (PBS nebulization) as compared to the PBS sensitized and challenged mice. In contrast, challenge with C. herbarum spores did not increase the basal Penh values. I.n. inoculation of the mold spores in nonsensitized mice did not significantly alter the overall response to the nebulization of increasing doses of methacholine as compared to the PBS sensitized/challenged mice. However mice sensitized and challenged with both A. alternata and C. herbarum showed increased Penh values upon nebulization of methacholine clearly demonstrating that sensitization and challenge with these mold spores induced the development of an AHR.
Fig. 7.
Mold spore sensitized and challenged mice develop an airway hyperreactivity to inhaled methacholine. (a) BALB/c mice (5 per group) were not sensitized and then challenged i.n. with PBS (•) or A. alternata spores (▪). Mice were also sensitized i.p. and then challenged i.n. with the spores (▴). (b) BALB/c mice (5 per group) were not sensitized and then challenged i.n. with PBS or C. herbarum spores. Mice were also sensitized i.p. and then challenged i.n. with the spores. Experiments were performed 24 h after the last challenge. Results are expressed as means ± SD. *Significant difference (P < 0·1) between values of mold spore challenged groups versus PBS challenged group.
Discussion
So far, most experimental model of allergic asthma have been developed in rodents and have used proteins like OVA or purified or recombinant allergens. In this study we developed a new mouse model of allergic lung inflammation using the spores of A. alternata and C. herbarum. Mold spores have several advantages over the generally used proteins or allergens. Molds can easily be cultured and their spores can be obtained very rapidly in high purity. More importantly, mold spores are complex particules composed of numerous proteins and allergens to which allergic people are commonly exposed. Our results demonstrate that A. alternata and C. herbarum spores have the ability to induce a type-2 antibody reponse characterized by a strong production of polyclonal IgE immunoglobulins and specific IgG1 antibodies. Since the production of IgE by B cells is a T cell-dependent phenomenon requiring the production of Th2 cytokines, mold spores not only induce an activation B cells but also of Th2 cells, even in the absence of an adjuvant. The factors responsible for the synthesis of antibodies (and Th2 cytokines) were also present in the soluble protein extract from these molds. Helminth infection [14] or injection of anti-IgD antibodies [15,16] also induces a striking serum elevation of specific IgG1 and nonspecific IgE antibodies regulated by IL-4 and IL-13 secretion [17]. Moreover some purified helminth proteins have intrinsic B-cell activation or adjuvant properties [18,19] and it is likely that some similar strong Th2 promoting factors are also present on the spores and in the extracts of Alternaria alternata and C. herbarum.
We also showed in this study that mice sensitized and then i.n. challenged with mold spores develop an allergic lung inflammation. One can speculate that some constituents of the spores can be released in the airways and subsequently picked up by APCs as for instance alveolar macrophages leading to the local activation of immune cells (in the airways or in the draining lymph nodes). Indeed, large A. alternata spores are clearly visible on lung sections from challenged mice and these spores are also constantly present in the BALs. Alternatively alveolar macrophages might engulf the spores, process them and then trigger immune cells. This is consistent with the absence of C. herbarum spores (which are smaller) on the lung sections or in the BALs.
The lung inflammation induced in mold spore sensitized and challenged mice was characterized by the appearance in the BALs of high numbers of macrophages, neutrophils and eosinophils and a smaller population of lymphocytes. The presence of eosinophils in the airways has been recognized as a defining feature of human asthma and of mouse models of allergic respiratory inflammation. Indeed inflammatory cells recovered in the BALs from OVA sensitized/challenged mice are mainly eosinophils besides lymphocytes and macrophages [20,21].
In addition to eosinophils, increased numbers of neutrophils have also been found in the airways and in the BALs of patients suffering from asthma [22,23] but the role of neutrophils in asthma and the factors regulating their influx and activation remains completely unknown [24,25].
In our model, mice challenged with mold spores developed a neutrophilic lung inflammation, neutrophils representing more than 90% of the cells in the BALs of nonsensitized mice and more or less 50% of the cellular content of the BALs obtained from sensitized mice. A similar accumulation of neutrophils in the BALs was observed in BALB/c mice immunized and challenged with an aquous extract from a house dust sample containing endotoxin and cockroach allergens [26]. Since the concentration of LPS in our spore preparations was very low (between 3 and 6 U/ml analysed with the QCL-1000 LAL, Biowhittaker, corresponding to 0·9–1·8 U instilled to each mouse) it seems unlikely that the high number of neutrophils into the BALs of mice inoculated with the spores was due to LPS contamination.
Activation of innate immunity and the recruitment of neutrophils might be triggered by some pathogen-associated molecular patterns (PAMPs) on the surface of the spores. Interestingly, dectin-1, a major receptor for fungal β-glucan, highly expressed on the surface of alveolar macrophages and neutrophils [27], has recently been shown to collaborate with Toll-like receptor-2 (TLR-2) in the production of inflammatory mediators such as TNF-α[28,29]. Recognition of β-glucan released from the spores by dectin-1 on alveolar macrophages could therefore trigger the release of TNF-α and initiate the inflammatory response in the lungs. TNF-alpha could then act via an autocrine or/and a paracrine pathway to stimulate epithelial cells to release other cytokines and chemokines, which are directly chemotactic to leucocytes. We found that MIP-1α and MIP-2 mRNA were strongly up-regulated after inoculation of the spores. These chemokines are strongly chemotactic for neutrophils, induce their activation and degranulation and could be secreted by epithelial cells through their activation by TNF-α, leading to an amplification of the inflammatory response [30]. Since neutrophils also express dectin-1, the binding of β-glucans could also directly sustain the recruitment and the activation of these cells.
Eotaxin, the main chemoattractant for eosinophils was also strongly expressed in the lungs upon i.n. inoculation of the spores. However eotaxin expression was not sufficient to recruit eosinophils since lung eosinophilia and lung CCR-3 expression required a previous sensitization with the spores. This sensitization step induces an activation of B cells and triggers the production of specific antibodies which could participate in the recruitment of allergic inflammatory cells into the lungs. The importance of these antibodies in our model is not known yet and requires further examination but we have already observed that specific antibodies were very low at the time of the i.n. spore challenges (data not shown) arguing against an important function of specific antibodies. Moreover, in the OVA model, the use of B-cell deficient mice has clearly shown that specific antibodies are not involved in the development of lung inflammation and AHR [31]. The sensitization step also induces a systemic activation of Th2 cells. In these sensitized mice, i.n. challenges with the spores induced the recruitment of already activated Th2 cells into the lungs, their local re-activation and finally the secretion of IL-4, IL-5 and IL-13 as confirmed by the presence of these cytokines into the BALs of sensitized and challenged mice. These cytokines have been previously shown in various models to be responsible for several characteristics of asthma. We also found a correlation between the presence of these cytokines in the BALs, the goblet cell hyperplasia and a thickening of the lung epithelium in mice sensitized and challenged with the spores. Conversely nonsensitized and challenged mice demonstrated no goblet cell hyperplasia and no epithelium thickening and their BALs were devoid of Th2 cytokines.
Finally we found a correlation between the lung eosinophilia, the production of Th2 cytokines in the lungs and the development of AHR. This AHR developed similarly in mice sensitized and challenged with both A. alternata and C. herbarum spores although the basal Penh values from mice challenged with the A. alternata spores were constantly higher than those of mice challenged with PBS or with C. herbarum spores. This phenomenon could be due to the higher number of neutrophils recruited into the lungs of A. alternata challenged mice as compared to C. herbarum spores challenged mice, but also to their more diffuse distribution in the lung parenchyma.
In summary, we have shown that mold spores are strong inducers of Th2 type responses and chemokine secretion in the lungs of BALB/c mice. Mice sensitized and challenged i.n. with mold spores develop an allergic lung inflammation characterized by the presence of high numbers of eosinophils but also neutrophils, the local secretion of Th2 cytokines, a goblet cell hyperplasia and an AHR. This model, using relevant particulate allergens, could be an interesting tool to study the mechanisms leading to the development of allergic lung diseases and maybe design new ways for their control.
Acknowledgments
This work was partially supported by the Fond National de la Recherche Scientifique (FNRS), grant n°3·4616·04.
References
- 1.Kay AB. Allergy and allergic diseases. Second of two parts. N Engl J Med. 2001;344:109–13. doi: 10.1056/NEJM200101113440206. [DOI] [PubMed] [Google Scholar]
- 2.Arm JP, Lee TH. The pathobiology of bronchial asthma. Adv Immunol. 1992;51:323–82. doi: 10.1016/s0065-2776(08)60491-5. [DOI] [PubMed] [Google Scholar]
- 3.Busse WW, Lemanske RF, Asthma N, Engl J. Medical. 2001;344:350–62. doi: 10.1056/NEJM200102013440507. [DOI] [PubMed] [Google Scholar]
- 4.Kay AB. Allergy and allergic diseases. first of two parts. N Engl J Med. 2001;344:30–7. doi: 10.1056/NEJM200101043440106. [DOI] [PubMed] [Google Scholar]
- 5.Kurup VP, Shen H, Banerjee B. Respiratory fungal allergy. Microbes Infect. 2000;2:1101–10. doi: 10.1016/s1286-4579(00)01264-8. [DOI] [PubMed] [Google Scholar]
- 6.Zureik M, Neukirch C, Leynaert B, Liard R, Bousquet J, Neukirch F. Sensitisation to airborne moulds and severity of asthma: cross sectional study from the European Community respiratory health survey. B M J. 2002;325:411–4. doi: 10.1136/bmj.325.7361.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sur S, Wild JS, Choudhury BK, Sur N, Alam R, Klinman DM. Long term prevention of allergic lung inflammation in a mouse model of asthma by CpG oligodeoxynucleotides. J Immunol. 1999;162:6284–93. [PubMed] [Google Scholar]
- 8.Clarke AH, Thomas WR, Rolland JM, Dow C, O'Brien RM. Murine allergic respiratory responses to the major house dust mite allergen Der p 1. Int Arch Allergy Immunol. 1999;120:126–34. doi: 10.1159/000024230. [DOI] [PubMed] [Google Scholar]
- 9.Campbell EM, Kunkel SL, Strieter RM, Lukacs NW. Temporal role of chemokines in a murine model of cockroach allergen-induced airway hyperreactivity and eosinophilia. J Immunol. 1998;161:7047–53. [PubMed] [Google Scholar]
- 10.Soubani AO, Chandrakar PH. The clinical spectrum of pulmonary aspergillosis. Chest. 2002;121:1988–99. doi: 10.1378/chest.121.6.1988. [DOI] [PubMed] [Google Scholar]
- 11.Black PN, Udy AA, Brodie SM. Sensitivity to fungal allergens is a risk factor for life-threatening asthma. Allergy. 2000;55:501–4. doi: 10.1034/j.1398-9995.2000.00293.x. [DOI] [PubMed] [Google Scholar]
- 12.Paris S, Fitting C, Latgé JP, Herman D, Guinnepain MT, David B. Comparison of conidial and mycelial allergens of Alternaria alternata. Int Arch Allergy Appl Immunol. 1990;92:1–8. doi: 10.1159/000235216. [DOI] [PubMed] [Google Scholar]
- 13.Hamelmann E, Schwarze J, Takeda K, Oshiba A, Larsen GL, Irvin CG, Gelfand EW. Noninvasive measurement of airway responsiveness in allergic mice using barometric plethysmography. Am J Respir Crit Care Med. 1997;156:766–75. doi: 10.1164/ajrccm.156.3.9606031. [DOI] [PubMed] [Google Scholar]
- 14.Zakroff SG, Beck L, Platzer EG, Spiegelberg HL. The IgE and IgG subclass responses of mice to four helminth parasites. Cell Immunol. 1989;119:193–201. doi: 10.1016/0008-8749(89)90235-9. [DOI] [PubMed] [Google Scholar]
- 15.Goroff DK, Holmes JM, Bazin H, Nisol F, Finkelman FD. Polyclonal activation of the murine immune system by an antibody to IgD.XI contribution of membrane IgD crosslinking to the generation of an in vivo polyclonal antibody response. J Immunol. 1991;146:18–25. [PubMed] [Google Scholar]
- 16.Denis O, Macedo-Soares F, Latinne D, Nisol F, Bazin H. In vivo study of mIgM and mIgD cross-linking on murine B cells. Scand J Immunol. 1994;39:625–32. doi: 10.1111/j.1365-3083.1994.tb03423.x. [DOI] [PubMed] [Google Scholar]
- 17.Finkelman FD, Urban JF. The other side of the coin: The protective role of the Th2 cytokines. J Allergy Clin Immunol. 2001;107:772–80. doi: 10.1067/mai.2001.114989. [DOI] [PubMed] [Google Scholar]
- 18.Lee TD, Xie CY. IgE regulation by nematodes. the body fluid of Ascaris contains a B-cell mitogen. J Allergy Clin Immunol. 1995;95:1246–54. doi: 10.1016/s0091-6749(95)70082-x. [DOI] [PubMed] [Google Scholar]
- 19.Holland MJ, Harcus YM, Riches PL, Maizels RM. Proteins secreted by the parasitic nematode Nippostrongylus brasiliensis act as adjuvants for Th2 responses. Eur J Immunol. 2000;30:1977–87. doi: 10.1002/1521-4141(200007)30:7<1977::AID-IMMU1977>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka H, Masuda T, Tokuoka S, Takahashi Y, Komai M, Nagao K, Nagai H. Time course study on the development of allergen-induced airway remodeling in mice: the effect of allergen avoidance on established airway remodeling. Inflamm Res. 2002;51:307–16. doi: 10.1007/pl00000309. [DOI] [PubMed] [Google Scholar]
- 21.Lloyd CM, Gonzalo JA, Coyle AJ, Gutierrez-Ramos JC. Mouse models of allergic airway disease. Adv Immunol. 2001;77:263–95. doi: 10.1016/s0065-2776(01)77019-8. [DOI] [PubMed] [Google Scholar]
- 22.Norzila MZ, Fakes K, Henry RL, Simpson J, Gibson PG. Interleukin-8 secretion and neutrophil recruitment accompanies induced sputum eosinophil activation in children with acute asthma. Am J Respir Crit Care Med. 2000;161:769–74. doi: 10.1164/ajrccm.161.3.9809071. [DOI] [PubMed] [Google Scholar]
- 23.Ordonez CL, Shaughnessy TE, Matthay MA, Fahy JV. Increased neutrophil numbers and IL-8 levels in airway secretions in acute severe asthma: Clinical and biologic significance. Am J Respir Crit Care Med. 2000;161:1185–90. doi: 10.1164/ajrccm.161.4.9812061. [DOI] [PubMed] [Google Scholar]
- 24.Ennis M. Neutrophils in asthma pathophysiology. Curr Allergy Asthma Rep. 2003;3:159–65. doi: 10.1007/s11882-003-0029-2. [DOI] [PubMed] [Google Scholar]
- 25.Sampson AP. The role of eosinophils and neutrophils in inflammation. Clin Exp Immun. 2000;30(Suppl. 1):22–7. doi: 10.1046/j.1365-2222.2000.00092.x. [DOI] [PubMed] [Google Scholar]
- 26.Kim J, Merry AC, Nemzek JA, Bolgos GL, Siddiqui J, Remick DG. Eotaxin represents the principal eosinophil chemoattractant in a novel murine asthma model induced by house dust containing cockroach allergens. J Immunol. 2001;167:2808–15. doi: 10.4049/jimmunol.167.5.2808. [DOI] [PubMed] [Google Scholar]
- 27.Taylor PR, Brown GD, Reid DM, Willment JA, Martinez-Pomares L, Gordon S, Wong SY. The beta-glucan receptor, dectin-1, is predominantly expressed on the surface of cells of the monocyte/macrophage and neutrophil lineages. J Immunol. 2002;169:3876–82. doi: 10.4049/jimmunol.169.7.3876. [DOI] [PubMed] [Google Scholar]
- 28.Gantner BN, Simmons RM, Canavera SJ, Akira S, Underhill DM. Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2. J Exp Med. 2003;197:1107–17. doi: 10.1084/jem.20021787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown GD, Herre J, Williams DL, Willment JA, Marshall AS, Gordon S. Dectin-1 mediates the biological effects of beta-glucans. J Exp Med. 2003;197:1119–24. doi: 10.1084/jem.20021890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tao F, Kobzik L. Lung macrophage–epithelial cell interactions amplify particle-mediated cytokine release. Am J Respir Cell Mol Biol. 2002;26:499–505. doi: 10.1165/ajrcmb.26.4.4749. [DOI] [PubMed] [Google Scholar]
- 31.Hamelmann E, Tadeda K, Oshiba A, Gelfand EW. Role of IgE in the development of allergic airway inflammation and airway hyperresponsiveness: a murine model. Allergy. 1999;54:297–305. doi: 10.1034/j.1398-9995.1999.00085.x. [DOI] [PubMed] [Google Scholar]