Abstract
The surface density of the triggering receptors (e.g. NKp46 and NKp30) responsible for natural killer (NK) cell-mediated cytotoxicity determines the ability of NK cells to kill susceptible target cells. In this study, we show that prolactin up-regulates and cortisol down-regulates the surface expression of NKp46 and NKp30. The prolactin-mediated activation and the cortisol-mediated inhibition of natural cytotoxicity receptor (NCR) surface expression reflects gene regulation at the transcriptional level. NKp46 and NKp30 are the major receptors involved in the NK-mediated killing of K562, a human chronic myelogenous leukaemia cell line. Accordingly, the prolactin dramatically increased the NK-mediated killing of the K562 cell line, whereas cortisol abolished this activity. Our data suggest a mechanism by which prolactin activates the lytic function of NK cells, and cortisol inhibits the NK-mediated attack.
Keywords: cortisol, cytotoxicity, NCR, NK cells, prolactin
Introduction
Cortisol, the most well-known adrenocortical hormone in humans, suppresses the immune system and directly inhibits natural killer (NK) cell activity [1–3]. Conversely, prolactin, a 24 kDa single chain hormone secreted by the anterior pituitary is an immunostimulatory ‘cytokine’[4,5] that directly affects NK cell function through the prolactin receptor expressed on human NK cells [6]. We previously measured the ex vivo NK cell cytotoxicity against Plasmodium falciparum-infected erythrocytes (pRBCs) and the K562 cell line in the function of cortisol and prolactin concentrations of plasma samples collected from primiparous and multiparous women at the time of delivery. NK cell activity was highest in multiparous women with high prolactin concentrations, and an inverse correlation was found between the magnitude of NK cell cytotoxicity and cortisol production in primiparous women [7]. However, there is currently little evidence indicating that prolactin and cortisol affect NK cell-mediated cytolysis of pRBCs.
In humans, NK cell recognition and functions are regulated by multiple cell surface molecules, including inhibitor receptors that bind to HLA class I molecules and deliver inhibitory signals to NK cells to prevent NK cell-mediated attack of autologous normal cells [8,9]. In the absence of sufficient signalling by their HLA class I-specific inhibitory receptors, human NK cells are activated and display potent cytotoxicity against cells that are either HLA class I-negative or -deficient. This indicates that the NK receptors that induce cytotoxicity recognize ligands other than HLA class I molecules on target cells. Thus, NK cell triggering is considered to be a mainly non-major histocompatibilty complex (MHC)-restricted mechanism. The recent identification of a group of NK-specific triggering surface molecules led to a series of studies on the functional characteristics of such receptors. NKp46, NKp44 and NKp30 are the first three members of the natural cytotoxicity receptor (NCR) family to be described [10–12]. These receptors are found only on NK cells. NKp30 acts together with NKp46 and/or NKp44 to induce NK-mediated cytotoxicity against most target cells. There is a strict correlation between NCR density and NK cell-mediated cytolytic activity [13,14]. Thus, NK cells with a high NCR surface density (NCRbright) display strong cytolytic activity, whereas those with a low NCR surface density (NCRdull) are poorly cytolytic or even non-cytolytic against most target cells [15].
Given the major role of the NCRs in triggering the natural cytotoxic activity of NK cells [16–18], we decided to assess whether the increased NK cell function observed in P. falciparum-infected multiparous women may be related to prolactin-induced increased expression of NCR molecules. For this purpose, we analysed the expression of NCR molecules and transcripts, cytolytic activity against the K562 cell line of unstimulated peripheral blood mononuclear cell (PBMC)-derived purified NK cells, and PBMC-derived purified NK cells that had been activated in vitro with prolactin or cortisol.
Materials and methods
NK cell purification and cell culture
A magnetic cell sorting (MACS) system for human leucocytes (Miltenyi Biotec, Gladbach, Germany) was used to isolate NK cells from peripheral blood samples donated by healthy volunteers to the Tübingen University Hospital blood bank. The blood was diluted 1 : 1 in RPMI-1640, deposited on a Ficoll density gradient (Biochrom, Berlin, Germany) and centrifuged at 400 g for 30 min. The PBMCs were removed and washed twice in RPMI-1640. The cell pellet was then resuspended in 40 µl of MACS labelling buffer [phosphate-buffered saline (PBS) containing 2 mm EDTA and 0·5% fetal calf serum (FCS) (Gibco-BRL, Karlshrue, Germany)] per 107 total cells and incubated with 10 µl of biotin–antibody cocktail containing biotin-conjugated monoclonal antibodies (MoAbs) directed against CD3, CD4, CD14, CD15, CD19, CD36, CD123 and glycophorin A (Miltenyi Biotec). After 10 min at 4°C, 30 µl of MACS buffer and 20 µl of antibiotin microbeads were added per 107 total cells. Cells were mixed and incubated for an additional 15 min at 4°C. The cells were washed with MACS buffer by adding 10× labelling volume and centrifuged at 300 g for 10 min to eliminate unbound antibodies. They were then passed through the Super MACSTM LS separation columns (Miltenyi Biotec) placed in a magnetic field to retain labelled cells for subsequent elution. The eluted cells (i.e. NK cells) were washed, resuspended in phosphate buffer (PBS: 0·15 mm sodium chloride/10 mm phosphate sodium, pH 7·4) and analysed in a flow cytometer to determine their number and viability. The controls consisted of double-labelled NK cells. The following antibodies were used: CD56-FITC (clone B159) and CD16-PE (clone 3G8). Both antibodies were from Southern Biotech (Birmingham, AL, USA). The negative isotype controls were the IgG1-FITC/IgG2a-PE mixture of mouse dual TAGTM clone MOPC-21/UPC-10 (Sigma Aldrich, Taufkirchen, Germany). Cells (106) were incubated at 4°C in 250 µl of PBS containing 1% FCS and an optimal antibody concentration according to the manufacturer's instructions. After 20 min of labelling, cells were washed three times in PBS and submitted directly to FACS analysis. FACScan analysis of CD56+/CD16+ and CD56+/CD16– cells showed that the resulting populations of NK cells were >97% pure.
Endotoxin-free recombinant human interleukin 2 (rhIL-2) (2·25 × 106 U/mg) and recombinant human interleukin 15 (rhIL-15) (3·4 × 106 U/mg) were purchased from Biosource (Nurtigen, Germany). Recombinant human prolactin and cortisol were purchased from Sigma. Purified NK cells were cultured at 37°C in a 5% CO2 incubator in complete RPMI-1640 medium in 12-well plates (106 cell/ml). Cells were examined after 16 h in culture with prolactin (2 µm), cortisol (2 µm), rhIL-2 (50 U/ml) or rhIL-15 (50 ng/ml).
Semi-quantitative reverse transcription-PCR (RT-PCR)
NKp46 and NKp44 transcripts were analysed by RT-PCR as described previously [19]. Briefly, total RNA was extracted from NK cells using RNAeasy Minikit 50 (Qiagen GmbH, Hilden, Germany). The primers for the amplification of NKp46, NKp44 and β-actin were designed to be 18–24 nucleotides long and to be 100% identical to the regions of the gene encoding characteristic extracellular regions of the molecules. The primers used were: 5′-ACT CCA TCA TGA AGT GTG ACG-3′ (forward) and 5′-CAT ACT CCT GCT TGC TGA TCC-3′ (reverse) for β actin; 5′-TCC AAG GCT CAG GTA CTT CAA AG-3′ (forward), and 5′-GGG CGG GTA CTG GCA TCT-3′ (reverse) for NKp44; and 5′- ACG GGA CTC CAG AAA GAC CAT-3′ (forward), and 5′-CAG GCC CAT CCG AAG GA-3′ (reverse) for NKp46. These primers were produced by Thermo Electron GmbH (Ulm, Germany). Both sets of primers and probes spanned an intron, so that they detected NKp44 and NKp46 cDNA but not genomic DNA. RT-PCR was performed using a Perkin-Elmer thermal cycler (Emeryville, France). The PCR conditions were: 94°C for 2 min, 10 cycles of 94°C for 30 s, 55°C for 30 s and 68°C for 3 min, and 25 cycles of 94°C for 30 s, 55°C for 30 s and 68°C for 3 min with 5 s of elongation of each cycle, and at the end a prolonged elongation at 68°C for 5 min. PCR samples were separated in 1·5% agarose gel and visualized under UV light. Relative intensities of individual bands were quantified by densitometric scanning.
Flow cytometry
For membrane staining, purified NK cells were suspended in ice-cold PBS supplemented with 0·1% azide and 1% FCS. The cells (2 × 105 in 100 µl) were then incubated on ice for 30 min with FITC-labelled anti-CD56 MoAb (clone B-159) (10 µg/ml) and PE-labelled anti-NKp46 (clone BAB281), anti-NKp44 (clone Z231) or anti-NKp30 (clone Z25) MoAbs (Beckman-Coulter, Krefeld, Germany). Negative controls were isotype-matched mouse MoAbs directed against irrelevant surface molecules (i.e. IgG1a and IgG2b). Stained cells were washed twice in PBS and analysed directly by flow cytometry using three-colour cytometer (Becton Dickinson, Heildelberg, Germany) as described previously [20]. Briefly, samples were assessed immediately after single (PE-labelled MoAbs alone) or double staining (PE-labelled MoAbs plus FITC-labelled anti-CD56) and results were acquired and analysed using the cellquest 3·3 and winmid2·8 softwares. We counted 10 000 events per sample, and the mean fluorescence intensity (MFI) was defined as the difference between the geometric mean of the fluorescence emitted by all the purified NK cells and the geometric mean of the fluorescence emitted by the purified NK cells that do not express the NCRs (background).
51Chromium release cytotoxicity assay
We used K562 cells, a NK-sensitive cell line, as target cells in a cytotoxicity assay. K562 cells were cultured in RPMI-1640 with 10% FCS supplemented with 100 U/ml penicillin, 100 µg/ml streptomycin, 2 mm glutamine and 1 mm sodium pyruvate (Gibco-BRL) in cell culture flasks (Polylabo, Strasbourg, France). The culture medium was changed every 2 days. The assay was performed using purified NK cells that had previously been cultured alone or with prolactin, cortisol, rhIL-2 or rhIL-15. The K562 cells (3 × 106) were incubated with 100 µCi of 51Cr (aqueous solution of sodium chromate: Na251CrO4) (NEN, Berlin, Germany) in 1·5 ml of complete medium for 1 h at 37°C in a humidified 5% CO2 atmosphere. The NK cells were mixed with 51Cr-labelled K562 target cells at effector (E) to target (T) ratios of 50 : 1, 25 : 1, 10 : 1, 5 : 1 and 1 : 1 as described previously [21]. After 4 h, the supernatants were harvested and analysed on a gamma counter (Cobra Auto-Gamma, Canbera Packard, New Zealand). The percentage of specific lysis was calculated as follows: % specific lysis = [counts per million (cpm) exp. – cpm spont./cpm max. – cpm spont)] × 100. The spontaneous release was less than 20% of the maximal release. Each point represents the average of triplicate values. All values were within 5% of their mean.
Statistical analysis
Analyses were performed using StatView for Windows 5·01 (SAS Institute Inc., Cary, NC, USA). Paired continuous variables were compared by using the non-parametric Wilcoxon signed-rank test. Unpaired data were compared using the Mann–Whitney U-test. Multiple comparisons were made using the Kruskall–Wallis test. Differences were considered significant if two-tailed P-values were <0·05.
Ethical clearance
This study was approved by the ethics review committee of the University of Tübingen. Informed consent was obtained from all blood donors.
Results
Prolactin increases the cytolytic activity of NK cells from healthy donors
Previous experiments using NK cells collected at the time of delivery from pregnant women living in area in which P. falciparum malaria is endemic showed that the NK cell-mediated killing of pRBCs and K562 (a MHC-I-negative NK cell-sensitive cell line) is down-regulated in primiparous women with a high plasma cortisol concentration. We also found that NK cells from women with higher plasma prolactin concentrations exhibit stronger cytolytic activity than those from women with higher plasma cortisol concentrations. As cortisol inhibits NK cell function [1–3] and prolactin activates NK cells [4,5], it was crucial to determine how prolactin affects NK cell cytotoxicity.
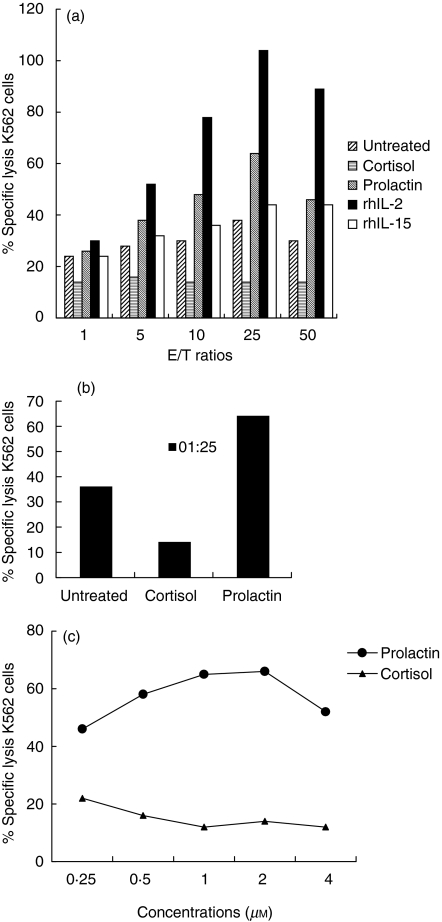
To address this question, we directly activated NK cells. We tested unstimulated NK cells and prolactin-, cortisol-, rhIL-2- or rhIL-15-treated cells to assess NK cell activity in a standard 51chromium release assay. Prolactin-treated NK cells exhibited stronger cytolytic activity against K562 than untreated-NK cells, whereas cortisol treatment decreased cytolytic activity (Fig. 1a). Cytolytic activity was also higher following treatment with rhIL-2 a well-known NK cell activator. However, NK cell cytotoxicity was not altered by rhIL-15 treatment. Cytotoxic activity was maximal with an E:T ratio of 25 : 1. At this ratio, prolactin-treated NK cells displayed significantly stronger natural cytotoxicity than their cortisol-treated counterparts (Fig. 1b). To characterize further the observed effects of prolactin and cortisol on NK cell cytotoxicity, we treated purified NK cells with various concentrations of prolactin and cortisol before carrying out the cytotoxicity assays (E : T ratio 25 : 1). The effects of prolactin and cortisol varied in a dose-dependent manner (Fig. 1c). As the concentration of cortisol increased, the percentage of K562 cells lysed decreased. Conversely, the percentage of target cells lysed increased together with the concentrations of prolactin. The maximal activating and inhibitory effects were obtained with concentrations between 1 and 2 µm of the two hormones. Numerous studies have shown that using specific MoAbs to mask NKp30, NKp44 and NKp46 on NK cells inhibits the cytolysis of several targets due to the inability of the NCRs expressed on NK cells to interact with their putative ligands on target cells [8,12–14]. Finally, after 5 min at 100°C, prolactin was no longer able to stimulate NK cells (data not shown). These results indicate that prolactin has a direct effect on NK cell cytolytic activity, that this effect does not require other mediating molecules to stimulate NK cells and that NCRs, are involved in NK-mediated lysis of K562. These findings also confirm that cortisol directly inhibits NK cell function, as reported by others [1,2].
Fig. 1.
Effect of prolactin and cortisol treatment on the functions of peripheral blood purified NK cells. (a) NK cells were tested in a 4-h 51Cr-release assay against K562 a human chronic myelogenous leukaemia cell line at 1 : 1, 5 : 1, 10 : 1, 25 : 1 and 50 : 1 effector : target cell ratio. Cells were treated with prolactin, cortisol, rhIL-2 or rhIL-15 for 16 h before assays or left untreated. The increase in 51Cr-release over baseline reflects the ability of a given stimulus to trigger the lytic machinery. Results are expressed as means of absolute values ± s.d. of six individual experiments. (b) NK cells were used after treatment with or without prolactin and cortisol: prolactin-enhanced and cortisol-inhibited NK cell cytotoxicity against the K562 cell line at an E : T ratio of 25 : 1. This experiment is representative of six performed with similar results; s.d. for all data were less than 5%. (c) Cytolytic activity after treatment of NK cells with increasing concentrations (0·25 µm, 0·5 µm, 1 µm, 2 µm and 4 µm) of prolactin or cortisol. Percentage of specific lysis was measured at an E : T ratio of 25 : 1 after 4 h. This experiment is representative of six performed with similar results; s.d. for all data was less than 5%.
Surface expression of NCRs on NK cells is up-regulated by prolactin
We next studied whether the increase in NK cell cytolytic activity observed after prolactin treatment was correlated with surface expression of activating receptors.
The mechanism by which NK cells lyse virus-infected, parasitized and tumour cells while sparing normal cells has been elucidated. They express MHC class-I-specific inhibitory receptors [killer inhibitory receptors (KIRs)] that block NK cell function by interacting with their ligands [22]. Thus, the expression of insufficient MHC molecules at the surface renders cells susceptible to NK cell lysis. Although the activating counterparts of KIRs have been identified [killer activating receptors (KARs)], their contribution to NK cell triggering is clearly limited to MHC class I+ target cells [23]. However, one of the major functions of NK cells is to kill cells that do not express MHC class I molecules, thus implying that receptors recognizing non-MHC ligands play a major role in NK cell triggering.
NCRs and NKG2D are recognition structures that mediate NK cell activation. The effects of NCRs or NKG2D engagement induced by cross-linking anti-NCR MoAb or recombinant NKG2D protein ligands are similar [22]. As de novo expression of NCRs plays a role in the marked increase in cytolytic activity [24], as K562 is a NK cell line that does not express HLA class I molecule [25] and as the contribution of NKG2D to the lysis of K562 is negligible [26], we then analysed the expression of NCRs on the surface of peripheral blood NK cells with or without treatment. This was done by cytofluometry using MoAb specific for NKp46, NKp44 and NKp30.
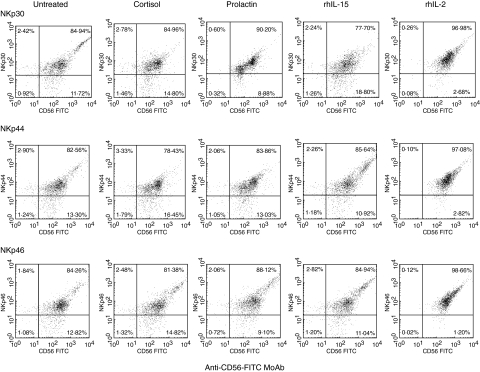
This confirmed the results of the functional study, showing that after 16 h in complete medium untreated control cells expressed a small amount of NCRs (weak fluorescence), and that NKp30 and NKp46 were more abundant than NKp44 (Table 1). This is consistent with previous studies that showed that NKp30 and NKp46 are expressed on the surfaces of both activated and nonactivated cells, whereas the NKp44 receptor is expressed preferentially after in vitro activation [11]. Resting cells from all 19 donors tested exhibited a NCRbright phenotype (MFI > 10) when stained with anti-NKp30, anti-NKp44 or anti-NKp46 MoAbs. Some stimulated cells were NCRbright, whereas others were NCRdull. This suggested that the NCRsbright phenotype was modified by 16 h in vitro culture. Direct single staining with specific anti-NKp30, anti-NKp44 or anti-NKp46 MoAb confirmed that NCRs were expressed on the surface of resting NK cells (Fig. 2a). The expression profile differed according to the stimulus used. Treatment with rhIL-2 increased the expression of all three NCRs whereas cortisol treatment had the opposite effect. Treatment with rhIL-15 increased the expression of NKp44, whereas prolactin favoured the expression of NKp30 and NKp46 (data not shown). Direct double staining using anti-CD56 MoAb and anti-NCRs gave similar results, and revealed that 85% of control NK cells expressed NCR antigens (Fig. 2). After stimulation with rhIL-2, only 3% of NK cells remained NCR-negative, whereas stimulation with cortisol slightly increased the percentage of NCR-negative NK cells. Prolactin decreased the number of NKp30 and NKp46 negative NK cells and rhIL-15 reduced only the number of NKp44-negative NK cells.
Table 1.
Typical NCRs-specific fluorescence intensity values obtained with control peripheral blood purified NK cells from healthy donors and used for calculation of MFI values.
| Fluorescence intensity | ||||
|---|---|---|---|---|
| Untreated controls purified NK cells | ||||
| Cell type (dot plot quadrant)* | NKp30 | NKp44 | NKp46 | |
| Double-stained cells | 115·57 | 137·40 | 141·70 | |
| Purified NK cells which do not express NCR | 55·33 | 91·74 | 95·37 | |
| Mean** | 60·24 | 45·66 | 56·33 | |
Cells were obtained from the upper and lower right quadrants of a dot plot obtained from flow cytometric determinations.
Mean was defined as the difference between the geometric mean of the fluorescence emitted by the purified NK cells that express the specific NCRs analysed (NKp46, NKp44 or NKp30) and the geometric mean of the fluorescence emitted by the purified NK cells that do not express the NCRs (background).
Fig. 2.
Expression of NKp30, NKp44 and NKp46 on peripheral blood NK cells from healthy human donors. Freshly purified peripheral blood NK cells were cultured for 16 h with or without prolactin, cortisol, rhIL-2 or rhIL-15. They were then analysed by direct two-colour immunofluorescence and FACS analysis with PE-conjugated MoAbs against NKp30, NKp44 and NKp46 in combination with FITC-conjugated anti-CD56 MoAb. The dot plots were divided into quadrants representing unstained cells (lower left), cells with only red fluorescence (upper left), cells with red and green fluorescence (upper right) and cells with only green fluorescence (lower right).
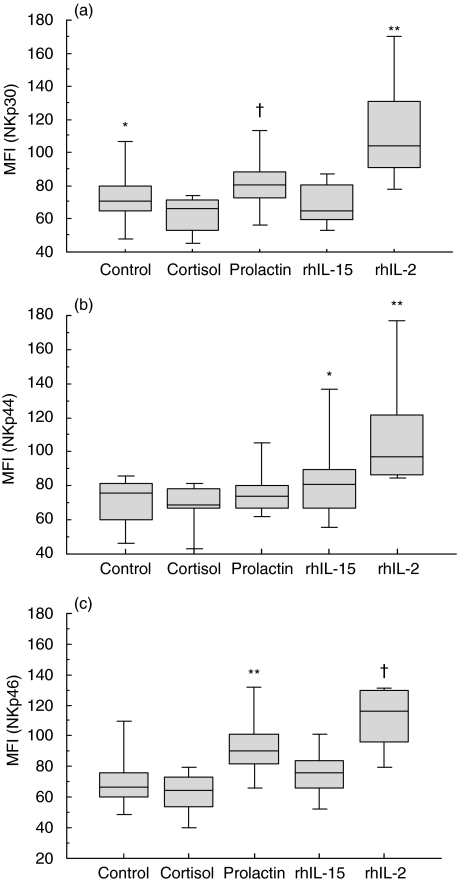
Overall, NKp46 phenotype was expressed significantly (P < 0·001) in both treated and untreated cells (Fig. 3c). A similar trend was also observed with NKp30 (P = 0·002) (Fig. 3a), whereas NKp44 was less strongly expressed (P = 0·004) (Fig. 3b). When we excluded the results obtained with rhIL-2 and rhIL-15, the expression levels of NKp44 did not differ significantly between untreated, cortisol- and prolactin-treated cells. Anti-NKp46 and anti-NKp30 staining was significantly stronger in prolactin-treated cells than in untreated cells [median = 90 MFI arbitrary units (a.u.) (82–101) versus 67 MFI a.u. (60–76), p = 0·01 for NKp46 and 81 MFI a.u. (73–89) versus 71 MFI a.u. (65–80), p = 0·07 for NKp30]. The anti-NKp30 MoAb staining was stronger in control NK cells than in cortisol-treated NK cells, whereas no difference was observed between these two groups with anti-NKp44 and anti-NKp46 MoAbs. Untreated control cells and rhIL-15-treated NK cells expressed similar levels of NKp30 [71 MFI a.u. (65–80) versus 64·7 MFI a.u. (60–81)] and NKp46 [76 MFI a.u. (60–82) versus 76 MFI a.u. (66–84)] on their surface. Furthermore, rhIL-15 up-regulated only NKp44 expression [81 MFI a.u. (67–90) versus 76 MFI a.u. in control cells (60–81)] (P = 0·04).
Fig. 3.
Mean fluorescence intensity (MFI) of human peripheral blood NK cells expressing each indicated NCR in untreated cells and cells treated with prolactin, cortisol, rhIL-2 or rhIL-15. (a) Expression of NKp30 in the treated and untreated cells. Overall analysis: P = 0·002. *P = 0·006 for comparison of control and cortisol-treated cells, †P = 0·07 for comparison of prolactin-treated and control cells, **P = 0·02 for comparison of rhIL-2- or rhIL-15-treated cells. (b) Expression of NKp44 in treated and untreated cells. Overall analysis: P = 0·004. *P = 0·04 for comparison of rhIL-15-treated and untreated cells, **P = 0·07 for comparison of rhIL-2- and rhIL-15-treated cells. (c) Expression of NKp46 in treated and untreated cells. Overall analysis P < 0·001. *P = 0·26 for comparison of untreated cells and cortisol-treated cells, **P = 0·01 for comparison of prolactin-treated cells and untreated cells, †P = 0·01 for comparison of rhIL-2- and rhIL-15-treated cells. The boxes illustrate the 25th−75th percentiles, the horizontal lines the medians and the vertical lines the 10th and 90th percentiles.
NKp30, NKp44 and NKp46 expression was strongest with rhIL-2. A further fine analysis of the percentage of double-stained cells revealed that untreated cells are mainly CD56dimNCRdull, with a small percentage of CD56brightNCRbright. Cortisol treatment decreases the CD56brightNCRbright cells for NKp30, NKp44 but not for NKp46. Prolactin increases the percentage of CD56intNCRint only for NKp30. IL-2 reduces the percentage of CD56dimNCR– and increases the percentage of CD56brightNCRbright for all NCRs.
These findings demonstrated that prolactin increases expression of lysis receptors, particularly NKp46, whereas cortisol decreased this expression. They also showed that rhIL-15 does not play a major role in the induction of the surface expression of NKp30 and NKp46. Finally, they show that rhIL-2 is a potent inducer of NKp30, NKp44 and NKp46 phenotypes.
Levels of NKp46 and NKp44 transcripts in resting and activated NK cells
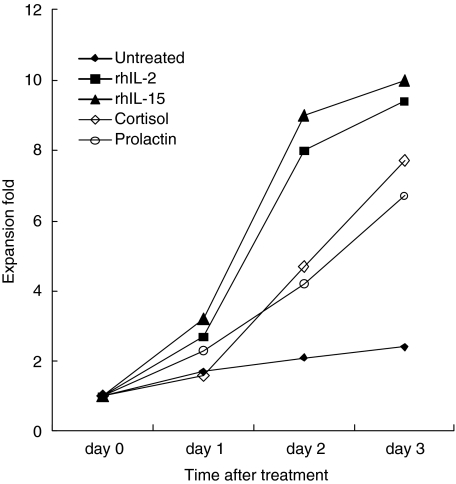
We next used RT-PCR to determine whether the increased expression of NCRs on NK cells after in vitro culture was correlated with the level of the NCRs gene transcripts. In control cells NKp46 and NKp44 transcripts were expressed (data not shown). We next investigated the effect of prolactin and cortisol on NKp46 and NKp44 expression in NK cells. It is important to notice that no significant difference in cell proliferation was observed between prolactin- and cortisol-treated NK cells (Fig. 4). No T cells were present in the cultures, as assessed by lack of reactivity with anti-CD3 MoAb (data not shown). In 3 days, the number of NK cells typically increased by between seven- and 10-fold. Slight difference in NK cells proliferation was observed between cultures stimulated with prolactin, cortisol and untreated cultures. More than 95% of cells were viable, even following treatment with 100 µm prolactin. Prolactin increased the number of NKp46 and NKp44 transcripts (data not shown). Cortisol significantly altered the number of NCRs transcripts present. RhIL-15 increased the NKp44 more than the NKp46 transcript. RhIL-2 was the most potent NCR transcript inducer. These findings confirm that prolactin directly enhances NK cell cytolytic activity, at least partly, by increasing the expression of NCRs.
Fig. 4.
Growth of NK cells after exposure to prolactin, cortisol, rhIL-2 or rhIL-15. Peripheral blood NK cells (5 × 106) were cultured at 37°C in a 5% CO2 incubator. Cells were harvested and counted at the indicated times. Cell viabilities were determined by the trypan blue exclusion test and were >95% for all preparations. All time-points represent means of six independent experiments.
Discussion
In this report, we provide the first experimental evidence that the surface expression and function of the triggering receptors responsible for NK-mediated recognition and killing of tumour cells is regulated by hormones. Prolactin selectively up-regulates the surface expression of the NKp46 and NKp30. In contrast, cortisol down-regulates the expression of NKp30. The ability of prolactin-treated NK cells to lyse K562 was increased as a consequence of the up-regulation of NKp46 and NKp30. Conversely, the down-regulation of the NCRs by cortisol was correlated with strongly reduced NK cytolytic activity in a direct killing assay. The up-regulation of NKp46 surface expression by prolactin is clearly a result of an increase in NKp46 transcript levels. In the same way, the increased expression of NKp46 and NKp44 antigens induced by rhIL-2 is associated with an increase in the levels of their mRNAs. Our data are interesting, because NKp30 acts together with NKp46 and NKp44 to induce cytotoxic activity against a variety of target cells [14]. The results obtained with rhIL-15 were consistent with those of Sivori and colleagues [13], who showed that lysis of certain target cells was only marginally NKp46- and/or NKp44-dependent, as MoAb-mediated masking of these molecules did not interfere significantly with cytotoxicity. By activating NKp44 but not NKp30 or NKp46, rhIL-15 did not increase NK cell cytolytic activity significantly.
These results have important implications for the understanding of the involvement of NK cells in the susceptibility of pregnant women to P. falciparum malaria. Indeed, pregnant women living in endemic areas are most susceptible to malaria between the second trimester and the early postpartum period [27], and their corticosteroid concentration is elevated from the second trimester of pregnancy, with highest levels in primiparous and P. falciparum-infected women [28]. The cortisol concentrations are about two or three times higher than non-pregnant values at delivery and return to normal non-pregnant levels in the early postpartum period (i.e. between the first and the sixth week postpartum) [28,29]. We have discussed previously the effects of cortisol concentrations and the decrease in NK cell cytotoxicity in increased susceptibility to maternal malaria [7,30]. As prolactin concentrations are elevated in multiparous pregnant women and as cortisol concentrations are elevated in primiparous pregnant women [7], it would be interesting to evaluate the NCR phenotype in pregnant women. A recent study that looked at the effect of prolactin on NK cells in vivo in an animal model and its implications for NK cell immunotherapy suggested that recombinant human prolactin may be an important regulator of NK cells and a potential biological product for immunotherapy [31].
Our findings imply that other as yet undefined factors are responsible for the modulation of NKp46, NKp44 and NKp30. The surface density of NCRs is directly correlated with the magnitude of NK cell cytolytic activity against several target cell types [32]. NCRs play an important role in NK cell cytotoxicity, as indicated by the ability of anti-NCR MoAbs to block specifically the NK mediated killing of most tumour cells and infected cells [10,13,32,33]. As cytotoxicity against suitable targets is maximal with mature NK cells that express both NCRs and NKG2D, as their cytolytic activity is mediated by all these triggering receptors, as occurs in mature cells [22], and as the sum of the interactions of activating, inhibitory and adhesion molecules with their respective ligands determines the outcome of the NK cell/target cell contact, we asked why the cortisol-treated NK cells did not lyse target cells. This may be due to the lack of the expression of NCRs, as shown here, and to the possible down-regulation of NKG2D proteins. Thus, it will be of interest to measure NKG2D levels following hormone stimulation.
We show here for the first time that resting peripheral blood NK cells from healthy donors express low levels of NKp44. However, we found that expression of NKp44 on untreated NK cells might indicate that purified NK cells obtained by Miltenyi Biotech's purification system are activated as Vitale et al. [34] found in clonal NK cells, that NKp44 was expressed only on in vitro activated cells. Furthermore, analysis of NK cell clones derived from two groups of donors, characterized by either a NKp46bright or a NKp46dull phenotype, showed that these phenotypes were stable and were not modified by in vitro stimulation. We extended these observations to all three NCRs and showed that NKp30bright, NKp44bright and NKp46bright phenotypes could change after 16 h in vitro culture. In light of our results, after treatment of NK cells, NCR expression seems to be proportional to CD56 expression. As CD56bright cells are agranular and weakly cytotoxic, and as NCRs are triggering molecules, the increase of cytotoxicity observed after prolactin treatment and obviously after IL-2 treatment could be related to the high NCR expression. Our study also shows that pretreatment of NK cells with rhIL-2 results in a significant increase in the levels of NKp46 and NKp44 transcripts, and the surface expression of the corresponding proteins, which in turn leads to strong cytolytic activity. However rhIL-15 had little or no effect on the NKp46 gene transcript or protein expression on NK cells and thus did not alter NK cytotoxicity compared to untreated cells. This is not surprising, as the killing of autologous antigen-presenting cells is correlated with the amount of NKp46 and NKp30 expressed on the cell surface and as cytolysis is abrogated when these two NCRs are masked [35].
IL-15 and IL-2 have similar biological properties in vitro, consistent with them sharing a receptor (R) signalling component (IL-2/IL-15Rβγc) [36]. However, specificity for IL-15 rather than IL-2 is conferred by unique α-chain receptors that complete the IL-15Rαβγ and IL-2Rαβγ heterotrimeric high-affinity receptor complexes and thereby allow differential responsiveness, depending on the ligand and high-affinity receptor expressed. Multiple complex post-transcriptional regulatory mechanisms tightly control IL-15 expression. Based on the complex regulation and the differential patterns of IL-15 and IL-15Rα expression, it is likely that the critical in vivo functions of this receptor/ligand pair differ from those of IL-2 and IL-2α. This is in agreement with our own results and may explain the different effects of rhIL-2 and rhIL-15 on the levels of NCR gene transcripts. Our findings also support the recently reported differences between IL-2 and IL-15 in functioning on human NK cells [6].
NCRs are also expressed at very low levels in NK cells from individuals with NK cell expansions, suggesting that these receptors are not involved in the expansion process [37]. We found that rhIL-2 and rhIL-15 increased the ex vivo proliferation of NK cells, whereas prolactin and cortisol did not. These findings are in agreement with previous in vivo studies that showed that excess cortisol and prolactin in humans affect the machinery responsible for cytotoxicity while sparing NK cell integrity and viability [37,38]. Studies by Lanier and coworkers provide evidence that resting NK cells proliferate when cultured with certain stimulator cells and IL-2, and that NK cell proliferation occurs at different phases of the immune response with the particular cytokine milieu influencing the repertoire of NK cell-secreted cytokines [39–42]. We show that after 3 days of incubation, there is little difference in the expansion fold between NK cells incubated with prolactin and those incubated with cortisol (Fig. 4). However, the difference in killing effect noted 1 day after these two treatments could be explained, at least in part, by the ability of prolactin to induce the expression of NCRs on the surface of NK cells within minutes or hours while cortisol down-regulates this expression. Killing experiments performed after 3 days of incubation confirm the results obtained after 16 h of incubation (data not shown) supporting this hypothesis.
Prolactin receptors (PRL-R) are distributed throughout the immune system and are members of the cytokine receptor superfamily. PRL-R signal transduction is mediated by a complex array of signalling molecules, including Janus kinase 2 (Jak2) [43]. The interactions between IL-15 and IL-2 and their receptors in various cell types lead to a series of signalling events that are similar and include activation of the Jak/Stat pathway [44]. IL-2/IL-15Rβ is associated with Jak1 and γc is associated with Jak3, resulting in STAT3 and STAT5 phosphorylation, respectively, after ligation with IL-15 or IL-2 [45,46]. Thus, the slight difference between the PRL-R signal transduction compared to those of IL-2 and IL-15 might be explained by the difference in the magnitude of effects.
The use of K562, a (MHC)-I negative NK cell-sensitive cell line, as a target instead of pRBC was justified by the fact that healthy erythrocytes are one of only two classes (with neurones) of healthy cells that fail to express normal levels of MHC molecules and yet are resistant to NK cell attack. Moreover, parasitized erythrocytes become sensitive to NK cytolysis [21,47]. NK activation by pRBC is entirely dependent upon direct contact between the two cells, suggesting that specific receptor–ligand interactions occur between NK cells and pRBCs [21,48]. As NK cells up- and down-regulate NCRs in response to prolactin and cortisol, respectively, we hypothesize that differences in NCR expression between individuals may affect responses to pRBCs in vivo in the same way that defective NK cell function in HIV-1-infected patients was attributed to a defect in NCR expression [49].
In conclusion, the opposite effects of prolactin and cortisol on NCR gene and phenotype expression and NCR-mediated lysis explain at least one of the possible mechanisms responsible for the defective NK cell function in P. falciparum-infected primiparous pregnant women. As other triggering NCR co-receptors are expressed on NK cells, including NKp80 as well as the triggering NKG2D molecule [15], further work is needed to determine whether some of these receptors are also affected during infection. In addition, it is important to determine if and to what extent the impairment of NCR is responsible for the impaired control of P. falciparum growth in these women. Finally, further work will help to elucidate whether the defective NCR expression in P. falciparum infection is associated with parasitaemia or with disease progression and whether it can be corrected by antimalarial chemotherapy.
Acknowledgments
This study was financed by a grant from Deutscher Akademischer AustauchDienst (DAAD) (to M. K. B.-A.) and by the Fortüne Program, contract no. (1317-0-0) of the Medical Faculty of the University of Tübingen. None of the authors has a commercial or other association that might pose a conflict of interest.
References
- 1.Callewaert DM, Moudgil VK, Radcliff G, Waite R. Hormone specific regulation of natural killer cells by cortisol: direct inactivation of the cytotoxic function of cloned human NK cells without an effect on cellular proliferation. FEBS Lett. 1991;285:108–10. doi: 10.1016/0014-5793(91)80736-m. [DOI] [PubMed] [Google Scholar]
- 2.Gatti G, Cavallo R, Sartori ML, et al. Inhibition by cortisol of human natural killer (NK) cell activity. J Steroid Biochem. 1987;26:49–58. doi: 10.1016/0022-4731(87)90030-6. [DOI] [PubMed] [Google Scholar]
- 3.Zhou J, Olsen S, Moldovan J, et al. Glucocorticoid regulation of natural cytotoxicity: effects of cortisol on the phenotype and function of a cloned human natural killer cell line. Cell Immunol. 1997;178:108–16. doi: 10.1006/cimm.1997.1138. [DOI] [PubMed] [Google Scholar]
- 4.Jara L, Lavalle J, Fraga C, et al. Prolactin, immunoregulation and autoimmune disease. Semin Arthritis Rheum. 1983;20:273–84. doi: 10.1016/0049-0172(91)90028-x. [DOI] [PubMed] [Google Scholar]
- 5.Nagy E, Berczi I, Friesen HG. Regulation of immunity in rats by lactogenic and growth hormones. Acta Endocrinol. 1983;102:351–7. doi: 10.1530/acta.0.1020351. [DOI] [PubMed] [Google Scholar]
- 6.Sun R, Li AL, Wie HM, Tian ZG. Expression of prolactin receptor and response to prolactin stimulation of human NK cell lines. Cell Res. 2004;14:67–73. doi: 10.1038/sj.cr.7290204. [DOI] [PubMed] [Google Scholar]
- 7.Bouyou-Akotet MK, Issifou S, Meye JF, et al. Depressed natural killer cell cytotoxicity against Plasmodium falciparum-infected erythrocytes during first pregnancies. Clin Infect Dis. 2004;38:342–7. doi: 10.1086/380646. [DOI] [PubMed] [Google Scholar]
- 8.Moretta A, Biassoni R, Bottino C, Mingari MC, Moretta A. Natural cytotoxicity receptors that trigger human NK-mediated cytolysis. Immunol Today. 2000;21:228–34. doi: 10.1016/s0167-5699(00)01596-6. [DOI] [PubMed] [Google Scholar]
- 9.Moretta A, Bottino C, Vitale M, et al. Receptors for HLA class I molecules in human natural killer cells. Annu Rev Immunol. 1996;14:619–48. doi: 10.1146/annurev.immunol.14.1.619. [DOI] [PubMed] [Google Scholar]
- 10.Pessino A, Sivori S, Bottino C, et al. Molecular cloning of NKp46: a novel member of the immunoglobulin superfamily involved in triggering of natural cytotoxicity. J Exp Med. 1998;188:953–60. doi: 10.1084/jem.188.5.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cantoni C, Bottino C, Vitale M, et al. NKp44, a triggering receptor involved in tumor cell lysis by activated human natural killer cells, is a novel member of the immunoglobulin superfamily. J Exp Med. 1999;189:787–95. doi: 10.1084/jem.189.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sato M, Ohashi J, Tsuchiya N, et al. Identification of novel single nucleotide substitutions in the NKp30 gene expressed in human natural killer cells. Tissue Antigens. 2001;58:255–8. doi: 10.1034/j.1399-0039.2001.580406.x. [DOI] [PubMed] [Google Scholar]
- 13.Sivori S, Pende D, Bottino C, et al. NKp46 is the major triggering receptor involved in the natural cytotoxicity of fresh or cultured human NK cells. Correlation between surface density of NKp46 and natural cytotoxicity against autologous, allogeneic or xenogeinic target cells. Eur J Immunol. 1999;29:1656–66. doi: 10.1002/(SICI)1521-4141(199905)29:05<1656::AID-IMMU1656>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 14.Pende D, Parolini S, Pessino A, et al. Identification and molecular characterization of NKp30, a novel triggering receptor involved in natural cytotoxicity mediated by human natural killer cells. J Exp Med. 1999;190:1505–16. doi: 10.1084/jem.190.10.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moretta L, Moretta A. Unravelling natural killer cell function: triggering and inhibitory human NK receptors. EMBO J. 2004;23:255–9. doi: 10.1038/sj.emboj.7600019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moretta A, Bottino C, Vitale M, et al. Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annu Rev Immunol. 2001;19:197–223. doi: 10.1146/annurev.immunol.19.1.197. [DOI] [PubMed] [Google Scholar]
- 17.Biron CA, Nguyen KB, Pien GC. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu Rev Immunol. 1999;17:189–220. doi: 10.1146/annurev.immunol.17.1.189. [DOI] [PubMed] [Google Scholar]
- 18.De Maria A, Biassoni R, Fogli M, et al. Identification, molecular cloning and functional characterization of NKp46 and NKp30 natural cytotoxicity receptors in Macaca fascicularis NK cells. Eur J Immunol. 2001;31:3546–56. doi: 10.1002/1521-4141(200112)31:12<3546::aid-immu3546>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 19.Zambello R, Falco M, Della Chiesa M, et al. Expression and function of KIR and natural cytotoxicity receptors in NK-type lymphoproliferative diseases of granular lymphocytes. Blood. 2003;102:1797–805. doi: 10.1182/blood-2002-12-3898. [DOI] [PubMed] [Google Scholar]
- 20.Nirmala R, Narayanan PR. Flow cytometry − a rapid tool to correlate functional activities of human peripheral blood lymphocytes with their corresponding phenotypes after in vitro stimulation. BMC Immunol. 2002;3:9–15. doi: 10.1186/1471-2172-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mavoungou E, Luty AJ, Kremsner PG. Natural killer (NK) cell-mediated cytolysis of Plasmodium falciparum-infected human red blood cells in vitro. Eur Cytokine Netw. 2003;14:134–42. [PubMed] [Google Scholar]
- 22.André P, Castriconi R, Espèli M, et al. Comparative analysis of human NK cell activation induced by NKG2D and natural cytotoxicity receptors. Eur J Immunol. 2004;34:961–71. doi: 10.1002/eji.200324705. [DOI] [PubMed] [Google Scholar]
- 23.Mandelboim O, Lieberman N, Lev M, et al. Recognition of haemagglutins on virus-infected cells by NKp46 activates lysis by human NK cells. Nature. 2001;409:1055–60. doi: 10.1038/35059110. [DOI] [PubMed] [Google Scholar]
- 24.Amon TI, Achdout H, Lieberman N, et al. The mechanism controlling the recognition of tumor- and virus-infected cells by NKp46. Blood. 2004;103:665–72. doi: 10.1182/blood-2003-05-1716. [DOI] [PubMed] [Google Scholar]
- 25.Moretta A, Biassoni R, Bottino C, et al. Major histocompatibility complex class I-specific receptors on human natural killer and T lymphocytes. Immunol Rev. 1997;155:105–17. doi: 10.1111/j.1600-065x.1997.tb00943.x. [DOI] [PubMed] [Google Scholar]
- 26.Olcese L, Cambiaggi A, Semenzato G, Bottino C, Moretta A, Vivier E. Human killer cell activatory receptors for MHC class I molecules are included in a multimeric complex expressed by natural killer cells. J Immunol. 1997;158:5083–6. [PubMed] [Google Scholar]
- 27.Diagne N, Rogier C, Sokhna CS, et al. Increased susceptibility to malaria during the early postpartum period. N Engl J Med. 2000;343:598–603. doi: 10.1056/NEJM200008313430901. [DOI] [PubMed] [Google Scholar]
- 28.Vleugels MPH, Eling WMC, Rolland R, De Graaf R. Cortisol and loss of malaria immunity in human pregnancy. Br J Obstet Gynaecol. 1987;94:758–64. doi: 10.1111/j.1471-0528.1987.tb03722.x. [DOI] [PubMed] [Google Scholar]
- 29.Mastorakos G, Ilias I. Maternal and fetal hypothalamic–pituitary–adrenal axes during pregnancy and postpartum. Ann NY Acad Sci. 2003;997:136–49. doi: 10.1196/annals.1290.016. [DOI] [PubMed] [Google Scholar]
- 30.Bouyou-Akotet MK, Mavoungou E. Cortisol and natural killer (NK) cell cytotoxicity in maternal malaria. Clin Infect Dis. 2004;39:147–8. [Google Scholar]
- 31.Sun R, Wei H, Zhang J, Li A, Zhan W, Tian Z. Recombinant human prolactin improves anti-tumour effects of murine natural killer cells in vitro and in vivo. Neuroimmunomodulation. 2003;10:169–76. doi: 10.1159/000067179. [DOI] [PubMed] [Google Scholar]
- 32.Castriconi R, Cantoni C, Della Chiesa M, et al. Transforming growth factor beta 1 inhibits expression of NKp30 and NKG2D receptors: consequences for the NK-mediated killing of dendritic cells. Proc Natl Acad Sci USA. 2003;100:4120–5. doi: 10.1073/pnas.0730640100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bottino C, Biassoni R, Millo R, Moretta L, Moretta A. The human natural cytotoxicity receptors (NCR) that induce HLA class I-independent NK cell triggering. Hum Immunol. 2000;1:1–6. doi: 10.1016/s0198-8859(99)00162-7. [DOI] [PubMed] [Google Scholar]
- 34.Vitale M, Bottino C, Sivori S, et al. NKp44, a novel triggering surface molecule specifically expressed by activated natural killer cells, is involved in non-major histocompatibility complex-restricted tumor cell lysis. J Exp Med. 1998;187:2065–72. doi: 10.1084/jem.187.12.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spaggiari GM, Carosio R, Pende D, et al. NK cell-mediated lysis of autologous antigen presenting cells is triggered by the engagement of the phosphatidylinositol 3-kinase upon ligation of the natural cytotoxicity receptors NKp30 and NKp46. Eur J Immunol. 2001;31:1656–65. doi: 10.1002/1521-4141(200106)31:6<1656::aid-immu1656>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 36.Fehniger TA, Caligiuri MA. Interleukin 15: biology and relevance to human disease. Blood. 2001;97:14–32. doi: 10.1182/blood.v97.1.14. [DOI] [PubMed] [Google Scholar]
- 37.Masera RG, Staurenghi A, Sartori ML. Natural killer cell activity in the peripheral blood of patients with Cushing's syndrome. Eur J Endocrinol. 1999;140:299–306. doi: 10.1530/eje.0.1400299. [DOI] [PubMed] [Google Scholar]
- 38.Kadioglu P, Acbay O, Demir G. The effect of prolactin and bromocriptin on human peripheral immune status. J Endocrinol Invest. 2001;24:147–51. doi: 10.1007/BF03343834. [DOI] [PubMed] [Google Scholar]
- 39.Ritz J, Trinchieri G, Lanier LL. NK-cell antigens: section report. In: Schlossman SF, Boumsell L, Giks W, et al., editors. Leukocyte typing V. Oxford: Oxford University Press; 1995. p. 1367. [Google Scholar]
- 40.Nagler A, Lanier LL, Cwirla S, Phillips JH. Comparative studies of human FcRII-positive and negative natural killer cells. J Immunol. 1989;143:3183–91. [PubMed] [Google Scholar]
- 41.Nagler A, Lanier LL, Philips JH. Constitutive expression of high affinity interleukin 2 receptors on human CD16-natural killer cells in vivo. J Exp Med. 1990;171:1527–33. doi: 10.1084/jem.171.5.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Warren HS, Kinnear BF, Kastelein RL, Lanier LL. Analysis of the costimulatory role of IL-2 and IL-15 in initiating proliferation of resting (CD56dim) human NK cells. J Immunol. 1996;156:3254–9. [PubMed] [Google Scholar]
- 43.Vera-Lastra O, Jara LJ, Espinoza LR. Prolactin and autoimmunity. Autoimmun Rev. 2002;1:360–4. doi: 10.1016/s1568-9972(02)00081-2. [DOI] [PubMed] [Google Scholar]
- 44.Ihle JN, Witthuhn BA, Quelle FW, Yamamoto K, Silvennoinen O. Signaling through the hematopoietic cytokine receptors. Annu Rev Immunol. 1995;13:369–98. doi: 10.1146/annurev.iy.13.040195.002101. [DOI] [PubMed] [Google Scholar]
- 45.Miyazaki T, Kawahara A, Fujii H, et al. Functional activation of Jak1 and Jak3 by selective association with IL-2 receptor subunits. Science. 1994;266:1045–7. doi: 10.1126/science.7973659. [DOI] [PubMed] [Google Scholar]
- 46.Lin JX, Migone TS, Tsang M, et al. The role of shared receptor motifs and common Stat proteins in the generation of cytokine pleiotropy and redundancy by IL-2, IL-4, IL-7, IL-13, and IL-15. Immunity. 1995;4:331–9. doi: 10.1016/1074-7613(95)90141-8. [DOI] [PubMed] [Google Scholar]
- 47.Orago AS, Facer CA. Cytotoxicity of human natural killer (NK) cell subsets for Plasmodium falciparum erythrocytic schizonts: stimulation by cytokines and inhibition by neomycin. Clin Exp Immunol. 1991;86:22–9. doi: 10.1111/j.1365-2249.1991.tb05768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Artavanis-Tsakonas K, Eleme K, McQueen K, Parham P, Davis D, Riley EM. Activation of a subset of human natural killer cells upon contact with Plasmodium falciparum-infected erythrocytes. J Immunol. 2003;171:5396–405. doi: 10.4049/jimmunol.171.10.5396. [DOI] [PubMed] [Google Scholar]
- 49.De Maria A, Fogli M, Costa P, et al. The impaired NK cell cytolytic function in viremic HIV-1 infection is associated with a reduced surface expression of natural cytotoxicity receptors (NKp46, NKp30 and NKp44) Eur J Immunol. 2003;33:2410–18. doi: 10.1002/eji.200324141. [DOI] [PubMed] [Google Scholar]