Abstract
Cell-based immunotherapy, in which antigen-loaded antigen-presenting cells (APC) are used to elicit T cell responses, has become part of the search for alternative cancer and infectious disease treatments. Here, we report on the feasibility of using mRNA-electroporated CD40-activated B cells (CD40-B cells) as alternative APC for the ex vivo induction of antigen-specific CD8+ T cell responses. The potential of CD40-B cells as APC is reflected in their phenotypic analysis, showing a polyclonal, strongly activated B cell population with high expression of MHC and co-stimulatory molecules. Flow cytometric analysis of EGFP expression 24 h after EGFP mRNA-electroporation showed that CD40-B cells can be RNA transfected with high gene transfer efficiency. No difference in transfection efficiency or postelectroporation viability was observed between CD40-B cells and monocyte-derived dendritic cells (DC). Our first series of experiments show clearly that peptide-pulsed CD40-B cells are able to (re)activate both CD8+ and CD4+ T cells against influenza and cytomegalovirus (CMV) antigens. To demonstrate the ability of viral antigen mRNA-electroporated CD40-B cells to induce virus-specific CD8+ T cell responses, these antigen-loaded cells were co-cultured in vitro with autologous peripheral blood mononuclear cells (PBMC) for 7 days followed by analysis of T cell antigen-specificity. These experiments show that CD40-B cells electroporated with influenza M1 mRNA or with CMV pp65 mRNA are able to activate antigen-specific interferon (IFN)-gamma-producing CD8+ T cells. These findings demonstrate that mRNA-electroporated CD40-B cells can be used as alternative APC for the induction of antigen-specific (memory) CD8+ T cell responses, which might overcome some of the drawbacks inherent to DC immunotherapy protocols.
Keywords: CD40-activated B cells, dendritic cells, mRNA electroporation, immunotherapy
Introduction
Immunotherapy using antigen-loaded antigen-presenting cells (APC) has become an interesting development in the search for alternative treatment modalities concerning cancer and infectious diseases. All adoptive immunotherapy protocols used as alternative or adjuvant treatment require rapid, efficient and safe methods to generate antigen-specific T cells that can elicit cellular immunity.
Classically, cultured dendritic cells (DC) are loaded with antigens through RNA electroporation, pulsing, lipofection or viral transfection and used subsequently for T cell activation [1–3]. Cellular immunotherapy using antigen-loaded DC has already been tested in phase II and III clinical trials for the treatment of melanoma, prostate cancer, B cell lymphoma, cutaneous T cell lymphoma and metastatic renal cell carcinoma, which proves the possible value of cell-based vaccines for cancer immunotherapy [4–10]. Although DC are considered to be the most professional APC of the immune system, this approach suffers from some serious drawbacks: primary DC constitute only 0·1–0·5% of human peripheral blood mononuclear cells [11]. Culturing DC out of patients’ monocytes or CD34+ precursor cells is a more practical alternative [12], but requires multiple phlebotomies, leukapheresis or even bone marrow aspiration. Moreover, ex vivo expansion of DC from non-stem cell sources cannot be achieved. DC found in vivo cannot be considered as a homogeneous population of cells but consist rather of functionally disparate cell types with different immune stimulating capacities [13]. Thus, it also remains unclear how closely in vitro differentiated DC resemble DC found in tissues and lymphoid organs [14].
Earlier studies have shown that massive amounts of B cells can be obtained from small quantities of peripheral blood through activation by CD40 ligand, and are therefore a cost-effective source of autologous APC [15]. Moreover, several studies have demonstrated that these cells can induce ex vivo T cell responses against recall and neoantigens when loaded with antigen through pulsing with peptides or with tumour lysates, or when loaded through retroviral transduction [16–18].
Here, we provide evidence that not only DC but also CD40-activated B cells (CD40-B cells) can be loaded with antigen with high efficiency and viability by means of mRNA electroporation, a cytoplasm expression-based transfection technique which has many advantages over the classical peptide pulsing or viral transfection protocols [19]. Furthermore, we evaluated the ability of these alternative APC for the ex vivo induction of antigen-specific cellular immune responses. First, we show that both CD8+[cytomegalovirus (CMV) pp65/influenza matrix protein] and CD4+ (influenza haemagglutinin) T cell responses can be induced with major histocompatibility complex (MHC) class I and II-restricted peptide-pulsed CD40-B cells. The following data demonstrate clearly that mRNA-electroporated CD40-B cells (CMV pp65/influenza matrix protein) are highly efficient APC and can be considered as a valuable alternative to DC for the activation of antigen-specific CD8+ T cells in vitro.
Materials and methods
Cell lines
T2 cells (TAP-deficient, HLA-A2+, T×B hybrid) were kindly provided by Dr Pierre Van der Bruggen (Ludwig Institute for Cancer Research, Brussels, Belgium) and were cultured in complete medium consisting of Iscove's modified Dulbecco's medium (IMDM with 2 mm L-glutamine, Invitrogen, Merelbeke, Belgium) supplemented with 10 µg/ml gentamicin (Invitrogen, Merelbeke, Belgium), 1·25 µg/ml amphotericin B (Fungizone, Invitrogen) and 10% fetal calf serum (FCS; Sera Laboratory, Sussex, UK). 3T3 feeder cells stably transfected with CD40 ligand (T CD40L 3T3 feeder cells) were kindly provided by Dr K. Thielemans (Medical School of the Vrije Universiteit Brussel, Brussels, Belgium) and were cultured in IMDM medium (with 2 mm L-glutamine, Invitrogen), supplemented with 10 µg/ml gentamicin and 500 µg/ml G418 (Invitrogen). All cells were maintained in logarithmic phase growth at 37°C in a humidified atmosphere supplemented with 5% CO2.
Source of primary cells
Peripheral blood mononuclear cells (PBMC) were obtained from healthy blood donors (donors A–D and F) or from haemochromatosis patients (donors E and G). All donors except donors E and G were CMV seropositive. Our study protocol was approved by the local Ethical Committee and informed consent was obtained from all participating subjects. Mononuclear cells were isolated by Ficoll-Hypaque gradient separation (LSM, ICN Biomedicals, Costa Mesa, CA, USA).
Generation of Epstein–Barr virus (EBV)-transformed B lymphoblastoid cell lines (B-LCL)
B-LCL were generated by infection of PBMC with the supernatant of the EBV-producing B95-8 monkey cell line (kindly provided by Dr Christine Van Broeckhoven, University of Antwerp, Belgium) [20]. Cell lines were cultured in IMDM supplemented with 10% FCS.
HLA typing of PBMC
To determine HLA-A2 expression, PBMC were first incubated with the supernatant of the BB7-2 hybridoma (anti-HLA-A2; ATCC), followed by staining with FITC-conjugated rabbit antimouse immunoglobulins (Dako, Heverlee, Belgium). To determine HLA-DR4 expression, PBMC were first incubated with anti-HLA-DR4 mouse IgG1 containing supernatant (Sanbio, Ijmuiden, the Netherlands), followed by staining with fluorescein isothyocyanate (FITC)-conjugated rabbit antimouse immunoglobulins. HLA-A2 and HLA-DR4 presence or absence was analysed by flow cytometry using a FACScan analytical flow cytometer (Becton Dickinson, Erembodegem, Belgium).
In vitro culture of DC
Immature monocyte-derived dendritic cells (iMo-DC) were cultured from peripheral blood monocytes as described by Romani et al. [21], with some minor modifications. Briefly, monocytes from PBMC were allowed to adhere into six-well plates in AIM-V medium (Invitrogen, Paisley, UK) for 2 h at 37°C. The non-adherent fraction was removed and adherent cells were cultured further for 6 days in IMDM supplemented with 10% FCS. One hundred ng/ml granulocyte-macrophage colony-stimulating factor (GM-CSF; Leucomax, Novartis Pharma, Basel, Switzerland) and 1000 U/ml interleukin (IL)-4 (R&D Systems, Minneapolis, MN, USA) were added to the cultures every 2–3 days starting from day 0. Maturation of iMo-DC was induced after day 6 by adding a maturation cocktail consisting of 2·5 ng/ml tumour necrosis factor (TNF)-α (Roche Molecular Biochemicals, Mannheim, Germany), 10−7 M prostaglandin E2 (PGE2) (Sigma, St Louis, MO, USA), 500 U/ml IL-6 (Biosource Europe, Nivelles, Belgium) and 100 U/ml IL-1 (Biosource, Camarillo, CA, USA).
In vitro culture of CD40-activated B cells
CD40-B cells were generated from PBMC by co-culturing whole PBMC at 2 × 106 cells/ml with irradiated (96 Gy) E-CD40L 3T3 feeder cells in IMDM supplemented with 10% FCS, 5 µg/ml insulin (Sigma, St Louis, MO, USA), 10 µg/ml gentamicin, 2 ng/ml IL-4 and 1·9 × 10−7m cyclosporin A (Novartis Pharma, Vilvoorde, Belgium) [15]. Cultured cells were transferred to irradiated new E-CD40L 3T3 feeder cells every 3 days. Before use in co-cultures, CD40-B cells were Ficoll-Hypaque density centrifuged and cryopreserved to remove non-viable cells and remaining E-CD40L 3T3 feeder cells.
Immunophenotyping of CD40-activated B cells
The following monoclonal antibodies were used for immunophenotyping of peripheral blood and CD40-B cells: (FITC)-conjugated anti-CD3, (FITC)-conjugated anti-CD10, (PE)-conjugated anti-CD19, (FITC)-conjugated anti-CD19, (PE)-conjugated anti-CD25, (FITC)-conjugated anti-CD79, (PE)-conjugated anti-CD80, (FITC)-conjugated anti-CD83, (PE)-conjugated anti-CD86, (FITC)-conjugated anti–kappa, (PE)-conjugated anti–lambda and (PE)-conjugated anti-HLA-DR (all from BD Bioscience, Erembodegem, Belgium). Non-reactive isotype-matched antibodies (BD Bioscience) were used as negative controls.
Freezing and thawing procedure
PBMC, CD40-B cells or mature DC were frozen in cryotubes (Nunc CryoTube Vials, Nalgene Nunc International, Roskilde, Denmark) at a concentration of 1–10 × 106 per ml in 90% FCS and 10% dimethylsulphoxide (DMSO) (Sigma). Cell suspensions were slowly frozen (−1°C/min) to −80°C by using a cryofreezing container (Nalgene Nunc International). Frozen cells were thawed quickly in a 37°C water bath, followed by the addition of 100 µg/ml DNaseI (Roche Diagnostics) and 50 µl/ml of a 3·79% MgSO4 solution for 10 min. Next, cells were centrifuged and resuspended at 0·5 × 106 per ml in IMDM for 15 min to remove residual DMSO. Finally, cells were washed once and resuspended in culture medium.
Plasmids and mRNA
Messenger (m)RNA encoding cytomegalovirus (CMV) pp65 protein was purchased from Curevac (Tübingen, Germany). The pGEM4Z/EGFP/A64 plasmid, used to prepare mRNA encoding the enhanced green fluorescent protein (EGFP), was kindly provided by Dr E. Gilboa (Duke University Medical Center, Durham, NC, USA) [22]. The pGEM4Z/M1/A64 plasmid was kindly provided by Dr A. Steinkasserer (University of Erlangen, Erlangen, Germany) [23]. These plasmids were propagated in Escherichia coli supercompetent cells (Stratagene, La Jolla, CA, USA) and purified on endotoxin-free Qiagen-tip 100 columns (Westburg, Leusden, the Netherlands). Next, plasmids were linearized with SpeI (MBI Fermentas, St Leon-Rot, Germany), purified using a polymerase chain reaction (PCR) purification kit (Westburg) and used as DNA templates for in vitro transcription (IVT). Transcription was carried out in a final 20 µl reaction mix at 37°C using T7 MessageMachine Kit (Ambion, Austin, TX, USA) to generate 5′-capped in vitro transcribed mRNA. Purification of mRNA was performed by DNaseI digestion, followed by LiCl precipitation, according to the manufacturer's instructions.
Synthetic peptides
An influenza virus-specific HLA-A*0201-restricted matrix protein M1 peptide (M1; amino acids (aa) 58–66, GILGFVFTL) was used for detection of M1-specific T cells. This peptide was purchased from Sigma-Genosys (Cambridge, UK). The CMV-specific HLA-A*0201-restricted pp65 (pp65; aa 495–503, NLVPMVATV) and the influenza A-specific HLA-DRβ*0401-restricted haemagglutinin (HA; aa 307–319, PKYVKQNTLKLAT) peptides were purchased from Eurogentec (Seraing, Belgium). All peptides were dissolved in DMSO to 10 mg/ml, diluted further to 1 mg/ml in serum-free IMDM and stored in aliquots at −80°C. 1–2 × 106 CD40-B cells cells were pulsed for 1–2 h at room temperature in the presence of β2-microglobulin (Sigma Aldrich, Bornem, Belgium) for class I peptides. The peptides were used at a final concentration of 20 µg/ml.
EGFP analysis
EGFP mRNA-electroporated CD40-B cells and DC were checked for EGFP expression following a previously described procedure [24]. Briefly, cells (1–5 × 106) were washed once in phosphate-buffered saline (PBS) supplemented with 1% FCS and resuspended in 0·5 ml of PBS supplemented with 1% bovine serum albumin (BSA) and 0·1% sodium azide. Ethidium bromide was added at a final concentration of 10 µg/ml directly prior to FCM analysis to assess cell viability.
mRNA electroporation
Electroporation of mRNA was performed as described previously with minor modifications [19]. Briefly, prior to electroporation 5–10 × 106 CD40-B cells or immature (i)Mo-DC were washed twice with Optimix Washing Solution (EquiBio, Ashford, Middlesex, UK) and resuspended in Optimix electroporation buffer (EquiBio). Subsequently, 0·2 ml of the cell suspension was mixed with 20 µg of IVT mRNA and electroporated in a standard 0·4 cm cuvette at 300 V and 150 µF using an Easyject Plus device (EquiBio). After electroporation, fresh complete medium (including cytokines for DC) was added to the cell suspension and cells were incubated further at 37°C in a humidified atmosphere supplemented with 5% CO2. All mock electroporations were performed according to this protocol but without the use of mRNA.
Induction of viral-specific CD8+ T cells with mRNA-electroporated APC
CD40-B cells and iMo-DC were electroporated with influenza matrix protein mRNA or with CMV pp65 mRNA and resuspended in IMDM supplemented with 5% human AB serum (Sigma-Aldrich, Belgium). Maturation of immature Mo-DC was started 1–2 h after electroporation by adding a maturation cocktail. Four to 12 h postelectroporation, antigen-loaded cells were used for stimulation of autologous PBMC or CD8+ T cells. Briefly, 5 × 106 CD40-B cells or mature Mo-DC were co-cultured with 20 × 106 autologous PBMC or CD8+ T cells in IMDM supplemented with 5% hAB serum, 2 ng/ml IL-4 and 10 ng/ml IL-7. CD8+ T cells were enriched using a negative selection cocktail and magnetic nanoparticles (EasySep CD8+ T cell enrichment cocktail, StemCell Technologies, Meylan, France) according to the manufacturer's instructions. On days 2 and 4 of the co-cultures, 20 U/ml IL-2 was added. After 7 days of culture, cells were restimulated for 3–6 h with peptide-loaded or unloaded T2 cells. The amount of interferon (IFN)-γ produced upon restimulation of the cultured PBMC was measured using an IFN-γ enzyme-linked immunosorbent assay (ELISA) (BioSource, Nivelles, Belgium) and an IFN-γ secretion assay (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer's instructions.
Statistical analysis
Results are expressed as mean ± standard deviation (s.d.) or ± standard error of the mean (s.e.m.) as indicated. Comparisons were validated using a two-sided Student's t-test. A P-value ≤0·05 was considered to be statistically significant.
Results
CD40-activated B cells are easily expandable and show distinct co-stimulatory potential
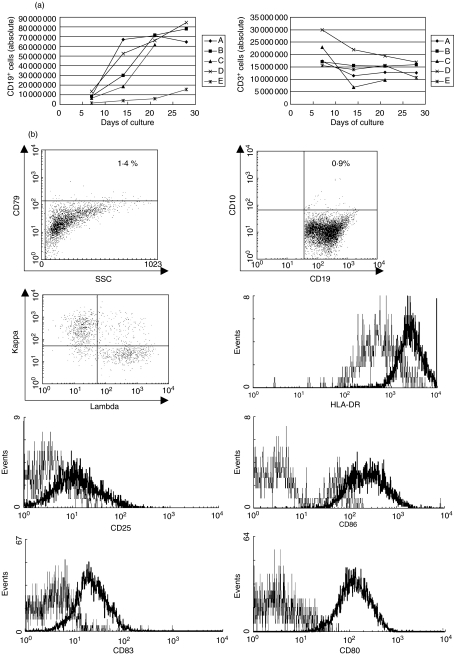
Starting from 72 × 106 whole PBMC, approximately 79·1 × 106 ± 8·6 × 106 (donors A–E, n = 5) CD40-activated B cells with >75% purity can be obtained after 20–28 days of culture (approximately eightfold increase of CD19+ B cells). Flow cytometric analysis of the final CD40-B cell preparations shows that less than 5% (CD45-negative) E-CD40L 3T3 cells are present in the total cell population. The gradual increase of CD19+ CD40-B cells and the simultaneous decrease of CD3+ T cells present in the cultures is depicted in Fig. 1a for the five CD40-B cell cultures used in this study (donors A–E). Phenotypical analysis of the cultured CD40-B cells shows a polyclonal (kappa/lambda ratio approx. = 1·4), highly activated (CD80+, CD83+, CD86+, CD25+, HLA-DR+) B cell population without any aberrant markers as CD10 or CD79 that are found commonly in B cell malignancies [25] (Fig. 1b, dot plots + bold histograms in histogram overlays, representative example of three independent experiments). Unstimulated B cells as found in PBMC did not express high levels of these activation markers (Fig. 1b, thin histograms in histogram overlays, representative example of three independent experiments). The high expression of several co-stimulatory molecules on CD40-B cells compared to naive B cells suggests that these CD40-B cells can play the role of potent antigen-presenting cells (APC) in the activation and co-stimulation of T cells.
Fig. 1.
Culture and phenotypical analysis of CD40-activated B cells. (a) Absolute amount of CD19+ and CD3+ T cells as measured in the CD40-B cell cultures of donors A–E. All cultures were started from 72 × 106 (donors A–D) or from 36 × 106 (donor E) density gradient-isolated PBMC. (b) Phenotypical analysis of CD40-B cells compared to naive B cells as found in a total PBMC population using flow cytometric analysis. All dot plots and histograms shown are based on the viable CD45+CD19+ cells present. Thin lines on histograms represent naive peripheral B cells, bold lines represent CD40-B cells. Results shown are representative of three individual experiments.
Peptide-pulsed CD40-activated B cells are able to stimulate antigen-specific CD4+ and CD8+ T cells
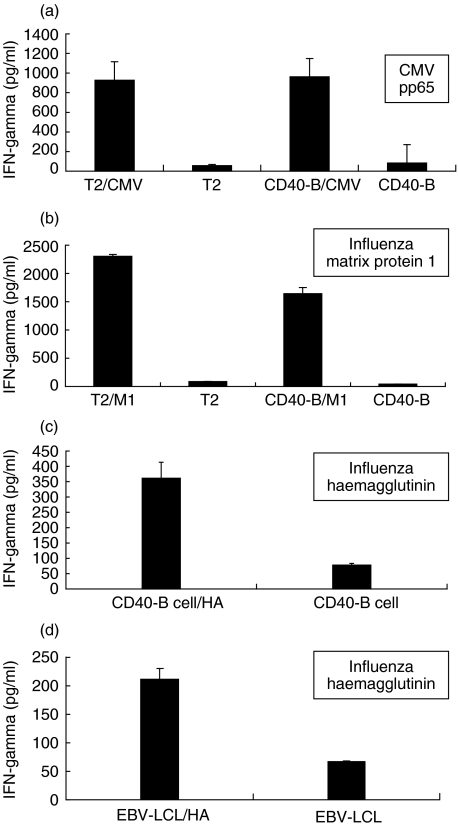
In order to demonstrate that CD40-B cells can be used as APC for T cell stimulation, PBMC were primed for 7 days with CMV pp65 mRNA-electroporated autologous mature DC. Next, activated PBMC were restimulated for 5 h with CMV pp65 peptide-loaded T2 cells or with autologous CMV pp65 peptide-loaded CD40-B cells as antigen-specific stimulators. Figure 2a (representative example for two independent experiments) demonstrates clearly that peptide-pulsed autologous CD40-B cells perform equally well compared to peptide-pulsed T2 cells as stimulator cells for antigen-specific IFN-γ production by T cells that were primed by DC (P = 0·72). Restimulation with unloaded T2 cells or with unloaded autologous CD40-B cells did not give rise to any significant IFN-γ production response (all P < 0·01 when compared to restimulation with loaded T2 or CD40-B cells), demonstrating the antigen-specific nature of the detected immune response. As a following step in the evaluation of CD40-B cells as APC, PBMC were primed for 7 days with influenza matrix protein M1 peptide-pulsed autologous CD40-B cells. After 7 days of co-culture, statistically significant IFN-γ production could be detected after restimulation with influenza M1 peptide-pulsed T2 cells and with influenza M1 peptide-pulsed CD40-B cells, while only background IFN-γ production was seen after restimulation with unloaded T2 cells or with unloaded autologous CD40-B cells (all P < 0·01) (Fig. 2b, representative example for two independent experiments). These results confirm that autologous peptide-pulsed CD40-B cells can act not only as stimulator cells for primed CD8+ T cells (Fig. 2a), but can also be used for the first stimulation of antigen-specific CD8+ T cells (Fig. 2b).
Fig. 2.
Peptide-pulsed CD40-activated B cells can act as alternative antigen-presenting cells. (a) IFN-γ production of PBMC primed with CMV pp65 mRNA-electroporated mature DC after restimulation with unloaded or with CMV pp65 peptide-pulsed T2 or autologous CD40-B cells. (b) IFN-γ production of PBMC primed with influenza M1 peptide-pulsed CD40-B cells after restimulation with unloaded or with influenza M1 peptide-pulsed T2 or autologous CD40-B cells. (c) IFN-γ production of PBMC primed with influenza HA peptide-pulsed CD40-B cells after restimulation with unloaded or with influenza HA peptide-pulsed autologous CD40-B cells. (d) IFN-γ production of PBMC primed with influenza HA peptide-pulsed CD40-B cells after restimulation with unloaded or with influenza HA peptide-pulsed allogeneic EBV-LCL. Graphs represent results from IFN-γ ELISA assays (error bars indicate standard deviation) and are representative of two individual experiments.
To show that CD40-B cells could also induce antigen-specific CD4+ T cell responses, similar co-cultures were set up using influenza HA-peptide pulsed-CD40 B cells for the initial priming of the PBMC. Upon restimulation with influenza HA peptide-pulsed autologous CD40-B cells (Fig. 2c) or allogeneic EBV-LCL (Fig. 2d) after 7 days of co-culture, statistically significant HA-specific T cell responses could be observed compared to restimulation with unloaded autologous CD40-B cells or allogeneic EBV-LCL (Figs 2c, d all P < 0·01, representative examples for two independent experiments are shown). Flow cytometric secretion assays confirm that the IFN-γ is produced by antigen-specific CD4+ T cells (data not shown). These results demonstrate the ability of CD40-B cells to present antigens by MHC class II molecules in order to activate (memory) CD4+ T cells.
CD40-activated B cells can be mRNA-electroporated with high efficiency and viability
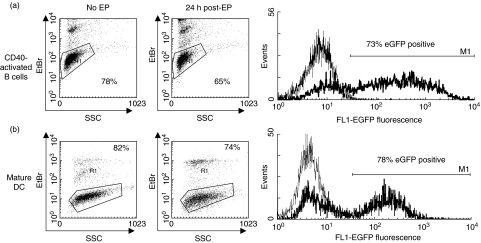
To demonstrate that CD40-B cells can be mRNA-electroporated with equal efficiency compared to DC, CD40-B cells and DC were cultured and electroporated with EGFP mRNA. Flow cytometric analysis of the EGFP expression 24 h postelectroporation showed that both cell types can be RNA electroporated with gene transfer rates up to 80% (representative EGFP histogram overlay in Fig. 3a for CD40-B cells and in Fig. 3b for DC). No difference in transfection efficiency was observed between CD40-B cells or DC (71·4 ± 3·0% (s.e.m.) for CD40-B cells versus 79·8 ± 4·4% (s.e.m.) for DC in five individual experiments, P = 0·153). Viability of these CD40-B cells after cryopreservation and thawing was high (up to 85%). No difference in viability 24 h postelectroporation was observed between CD40-B cells or DC (71·6 ± 3·3% (s.e.m.) for CD40-B cells versus 70·6 ± 2·1% (s.e.m.) for DC in five individual experiments, P = 0·826) (representative viability dot plots in Fig. 3a for non-electroporated and EGFP mRNA-electroporated CD40-B cells and in Fig. 3b for non-electroporated and EGFP mRNA-electroporated DC). Moreover, mock or EGFP mRNA-electroporation did not have any effect on the phenotype of the CD40-B cells (data not shown).
Fig. 3.
CD40-activated B cells can be mRNA-electroporated with high efficiency and viability. (a) Viability of non-electroporated and EGFP mRNA-electroporated CD40-B cells as determined by ethidium bromide (EtBr) staining (dot plots). Flow cytometric analysis of EGFP expression in CD40-activated B cells; thin lines represent mock-electroporated CD40-B cells, bold lines represent EGFP mRNA-electroporated CD40-B cells (histogram). (b) Viability of not electroporated and EGFP mRNA-electroporated mature DC as determined by ethidium bromide staining (dot plots). Flow cytometric analysis of EGFP expression in mature DC; thin lines represent mock-electroporated mature DC, bold lines represent EGFP mRNA-electroporated mature DC (histogram). Data are representative of five individual experiments.
CD40-activated B cells electroporated with mRNA coding for viral antigens can induce viral-specific CD8+ T cell responses
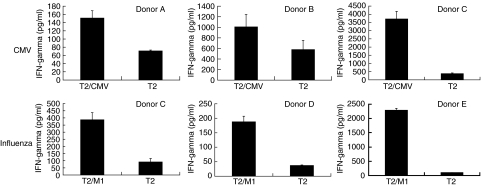
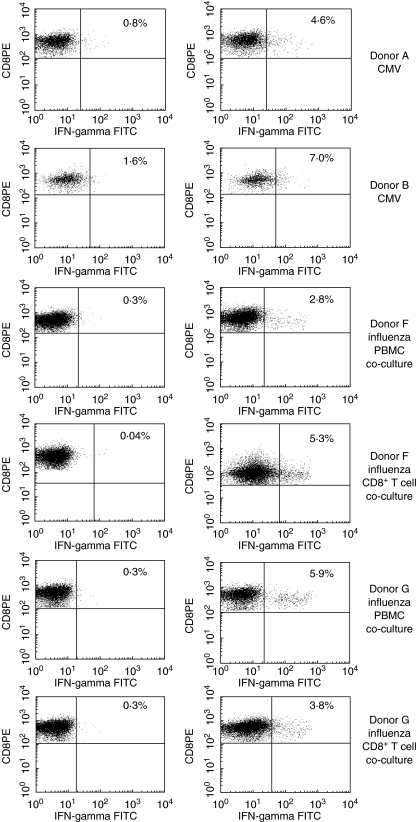
To demonstrate the ability of mRNA-electroporated CD40-B cells to activate CMV or influenza-specific CD8+ T cell responses, mRNA-electroporated CD40-B cells were co-cultured with autologous PBMC in a T cell : CD40-B cell ratio of 4 : 1. After 1 week of co-culture, cells were restimulated for 3–6 h with CMV or influenza peptide-pulsed T2 cells or with unloaded T2 as control stimulators. Upon restimulation of PBMC cultures that were stimulated with CD40-B cells electroporated with CMV pp65 or influenza M1 mRNA, antigen-specific IFN-γ release could be detected against CMV or influenza peptide-pulsed T2 cells (Fig. 4, top row: three independent experiments for CMV-specific T cells, bottom row: three independent experiments for influenza-specific T cells), while IFN-γ release against unloaded T2 cells was significantly lower (P < 0·01 in all experiments shown). Also, using the IFN-γ secretion assay, an alternative detection method for antigen-specific T cells, we were able to demonstrate that the observed CMV- and influenza-specific IFN-γ release was produced by CD8+ T cells (Fig. 5). All donors had a higher percentage IFN-γ producing CD8+ T cells after restimulation with the peptide-loaded T2 cells compared to unloaded T2 cells. Co-cultures set up with CD8+ T cells instead of PBMC also yielded comparable antigen-specific CD8+ T cell responses, which rules out the influence of other APC in the PBMC population (Fig. 5). Minor differences in the frequency of antigen-specific CD8+ T cells may be due to the presence of CD4+ helper or regulatory T cells in the PBMC cell population [26,27]. Irradiating the CD40-B cells (at 30 Gy) prior to mRNA electroporation also did not have any influence on the antigen-presenting capacities of these cells (data not shown). In summary, these experiments demonstrate that mRNA-electroporated CD40-B cells can be used as alternative APC for inducing antigen-specific (memory) CD8+ T cell responses.
Fig. 4.
CD40-activated B cells transfected with mRNA coding for viral antigens can induce viral-specific T cell responses. (Top row) IFN-γ release of PBMC from donors A–C primed with CMV pp65 mRNA-electroporated autologous CD40-B cells after restimulation with CMV pp65 peptide-pulsed (T2/CMV) or with unloaded T2 cells (T2). (Bottom row) IFN-γ release of PBMC from donors C–E primed with influenza M1 matrix mRNA-electroporated autologous CD40-B cells after restimulation with influenza M1 peptide-pulsed (T2/M1) or with unloaded T2 cells (T2). Graphs represent results from the IFN-γ ELISA assays (error bars indicate standard deviation).
Fig. 5.
IFN-γ secretion by antigen-specific CD8+ T cells after ex vivo induction of viral antigen-specific cellular immune responses by mRNA-electroporated autologous CD40-activated B cells. Antigen-specific cellular immune responses against influenza M1 and/or CMV pp65 after restimulation of primed PBMC with T2 cells (left dot plots) or with T2 cells pulsed with the relevant peptide (right dot plots). Donors A, B and F were typed CMV seropositive. The figures represent the percentage viable IFN-γ positive CD3+CD8+ T cells.
Discussion
Earlier studies have already shown that CD40-expanded B cells can be loaded with antigens by means of peptide pulsing or retroviral transduction [17,18]. Our present findings not only indicate that CD40-B cells are to be considered as a highly activated cell population with distinct phenotypical characteristics, but also that these cells can be transfected with high efficiency by RNA electroporation. Our results further demonstrate that peptide-pulsed CD40-B cells are able to present antigens to both CD8+ and CD4+ T cells with subsequent (re)activation of the antigen-specific immune response. Furthermore, we found that mRNA-loaded CD40-B cells present antigens efficiently and are able to boost a CD8+ T lymphocyte response to recall antigens such as the influenza matrix M1 and the cytomegalovirus pp65 proteins. Moreover, the antigen-specific responses induced by the mRNA-electroporated autologous CD40-B cells were clearly MHC class I-restricted, as evident from the T2-based peptide restimulation assays. These results confirm and extend the findings by Coughlin et al., who recently reported the induction of influenza and melanoma antigen-specific immune responses by RNA-transfected CD40-B cells [28]. The biological variability of the observed cellular immune response against the influenza matrix protein M1 in the several blood donors could be due to different precursor frequencies of T cells directed against influenza circulating in the blood of the donors tested. This may also be the case for the CMV responses observed in the CMV seropositive donors. However, low CMV-specific CD8+ T cell immune responses against the HLA-A*0201 restricted pp65 CMV-peptide could also be explained by the relative dominance of HLA-B*07 restricted CD8+ T cell responses in people sharing HLA-A*02 and HLA-B*07 alleles [29].
The induction of CD4+ T cell responses through mRNA-electroporated CD40-B cells would require the use of mRNA coding for a secreted antigenic protein that can taken up by dendritic cells for MHC class II processing or the artificial linking of the mRNA antigen sequence to endosomal targeting sequences, such as the invariant chain (Ii) fragment, or to lysosomal targeting sequences of lysosomal proteins, such as lysosomal-associated membrane protein-1 (LAMP) or DC-LAMP [30]. Both strategies remain to be investigated for CD40-B cells. These results underscore that CD40-B cells can act as alternative APC for the ex vivo induction of T cell immune responses, as was shown previously for mRNA-loaded DC [19,31]. RNA-loaded APC form an excellent tool for future vaccination strategies, as RNA-based loading of APC overcomes the problem of HLA restriction which limits the use of peptide pulsing. Through RNA electroporation, APC can be loaded with the full-length antigen, enabling presentation of multiple epitopes without the need for prior characterization of immunogenic epitopes. This approach also minimizes the risk for generation of tumour antigen loss variants or virus escape variants. Moreover, RNA electroporation is a more feasible and safe method to apply in a clinical setting compared to using viral vectors for transfection of antigens into APC.
Although the ability of mRNA-loaded CD40-B cells to activate T cells in vivo remains to be proved, the expression of chemokine receptors as CCR7 suggests that following injection they have the ability to migrate to regional lymph nodes [32]. Moreover, the possible downside of using non-human cells such as the murine fibroblast cell line 3T3 to activate B cells can be overcome by using soluble trimeric CD40 ligand [16]. Importantly, given the massive expansion capacity of CD40-B cells, in strong contrast with in vitro-generated DC, they consitute an expandable source of APC (starting from less than 100 ml of peripheral blood) for multiple vaccinations in an active immunotherapy setting or for multiple restimulations ex vivo of antigen-specific T cell populations in an adoptive setting.
In conclusion, our findings demonstrate that not only peptide pulsing but also mRNA electroporation of CD40-B cells lead to effective CD8+ T cell activation. Concomitant pulsing of the CD40-B cells with MHC class II-restricted peptides might be advantageous when a T helper response is required for effective priming of naive CD8+ T cells. Because CD40-B cells offer many advantages over monocyte-derived DC, we strongly believe that our findings with mRNA-electroporated CD40-B cells could contribute to the eventual development of a workable immunotherapy. Moreover, this RNA/APC strategy can be applied not only in infectious diseases but also in the fields of oncology and haematology.
Acknowledgments
This work was supported by grants G.0456·03 and G.0313·01 of the Fund for Scientific Research − Flanders, Belgium (FWO-Vlaanderen). G.A.V.d.B. is holder of a PhD fellowship from the FWO-Vlaanderen. V.F.I.V.T. is a postdoctoral fellow of the FWO-Vlaanderen.
References
- 1.Nair SK, Boczkowski D, Morse M, Cumming RI, Lyerly HK, Gilboa E. Induction of primary carcinoembryonic antigen (CEA)-specific cytotoxic T lymphocytes in vitro using human dendritic cells transfected with RNA. Nat Biotechnol. 1998;16:364–9. doi: 10.1038/nbt0498-364. [DOI] [PubMed] [Google Scholar]
- 2.Zhou Y, Bosch ML, Salgaller ML. Current methods for loading dendritic cells with tumor antigen for induction of antitumor immunity. J Immunother. 2002;25:289–303. doi: 10.1097/00002371-200207000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Rubio V, Stuge TB, Singh N, et al. Ex vivo identification, isolation and analysis of tumor-cytolytic T cells. Nat Med. 2003;9:1377–82. doi: 10.1038/nm942. [DOI] [PubMed] [Google Scholar]
- 4.Thurner B, Haendle I, Röder C, et al. Vaccination with Mage-3A1 peptide-pulsed mature, monocyte-derived dendritic cells expands specific cytotoxic T cells and induces regression of some metastases in advanced stage IV melanoma. J Exp Med. 1999;11:1669–78. doi: 10.1084/jem.190.11.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rini BI. Technology evaluation. APC-8015, Dendreon. Curr Opin Mol Ther. 2002;4:76–9. [PubMed] [Google Scholar]
- 6.Heiser A, Coleman D, Dannull J, et al. Autologous dendritic cells transfected with prostate-specific antigen RNA stimulate CTL responses against metastatic prostate tumors. J Clin Invest. 2002;109:409–17. doi: 10.1172/JCI14364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsu FJ, Benike C, Fagnoni F, et al. Vaccination of patients with B-cell lymphoma with peptide-or tumor lysate-pulsed dendritic cells. Nat Med. 1998;4:328–32. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]
- 8.Maier T, Tun-Kyi A, Tassis A, et al. Vaccination of cutaneous T cell lymphoma patients using intranodal injection of autologous tumor lysate pulsed dendritic cells. Blood. 2003;102:2338–44. doi: 10.1182/blood-2002-08-2455. [DOI] [PubMed] [Google Scholar]
- 9.Kugler A, Stuhler G, Walden P, et al. Regression of human metastatic renal cell carcinoma after vaccination with tumor cell-dendritic cell hybrids. Nat Med. 2000;6:332–6. doi: 10.1038/73193. [DOI] [PubMed] [Google Scholar]
- 10.Banchereau J, Palucka AK, Dhodapkar M, et al. Immune and clinical responses in patients with metastatic melanoma to CD34(+) progenitor-derived dendritic cell vaccine. Cancer Res. 2001;61:6451–8. [PubMed] [Google Scholar]
- 11.Steinman RM, Witmer-Pack MD, Inaba K. Dendritic cells: antigen presentation, accessory function and clinical relevance. Adv Exp Med Biol. 1993;329:1–9. doi: 10.1007/978-1-4615-2930-9_1. [DOI] [PubMed] [Google Scholar]
- 12.Girolomoni G, Ricciardi Castagnoli P. Dendritic cells hold promise for immunotherapy. Immunol Today. 1997;18:102–4. doi: 10.1016/s0167-5699(97)01030-x. [DOI] [PubMed] [Google Scholar]
- 13.Banchereau J, Steiman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 14.Cerundolo V, Hermans IF, Salio M. Dendritic cells: a journey from laboratory to clinic. Nature Immunol. 2004;5:7–10. doi: 10.1038/ni0104-7. [DOI] [PubMed] [Google Scholar]
- 15.Schultze JL, Michalak S, Seamon MJ, et al. CD40-activated human B cells: an alternative source of highly efficient antigen presenting cells to generate autologous antigen-specific T cells for adoptive immunotherapy. J Clin Invest. 1997;100:2757–65. doi: 10.1172/JCI119822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Von Bergwel T, Baildon MS, Vonderheide RH, et al. Human primary and memory cytotoxic T lymphocyte responses are efficiently induced by means of CD40-activated B cells as antigen-presenting cells: potential for clinical application. Blood. 2002;99:3319–25. doi: 10.1182/blood.v99.9.3319. [DOI] [PubMed] [Google Scholar]
- 17.Kondo E, Topp MS, Kiem HP, et al. Efficient generation of antigen-specific cytotoxic T cells using retrovirally transduced CD40-activated B cells. J Immunol. 2002;169:2164–71. doi: 10.4049/jimmunol.169.4.2164. [DOI] [PubMed] [Google Scholar]
- 18.Lapointe R, Bellemare-Pelletier A, Housseau F, Thibodeau J, Hwu J. CD40-stimulated B lymphocytes pulsed with tumour antigens are effective antigen-presenting cells that can generate specific T cells. Cancer Res. 2003;63:2836–43. [PubMed] [Google Scholar]
- 19.Van Tendeloo VF, Ponsaerts P, Lardon F, et al. Highly efficient gene delivery by mRNA electroporation in human hematopoietic cells: superiority to lipofection and passive pulsing of mRNA and to electroporation of plasmid cDNA for tumour antigen loading of dendritic cells. Blood. 2001;98:49–56. doi: 10.1182/blood.v98.1.49. [DOI] [PubMed] [Google Scholar]
- 20.Miller G, Lipman M. Release of infectious Epstein–Barr virus by transformed marmoset leukocytes. Proc Natl Acad Sci USA. 1973;70:190–4. doi: 10.1073/pnas.70.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Romani N, Reider D, Heuer M, et al. Generation of mature dendritic cells from human blood. An improved method with special regard to clinical applicability. J Immunol Meth. 1996;196:137–51. doi: 10.1016/0022-1759(96)00078-6. [DOI] [PubMed] [Google Scholar]
- 22.Boczkowski D, Nair SK, Snyder D, Gilboa E. Dendritic cells pulsed with RNA are potent antigen-presenting cells in vitro and in vivo. J Exp Med. 1996;184:465–72. doi: 10.1084/jem.184.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strobel I, Berchtold S, Gotze A, Schulze U, Schuler G, Steinkasserer A. Human dendritic cells transfected with either RNA or DNA encoding influenza matrix protein M1 differ in their ability to stimulate cytotoxic T lymphocytes. Gene Ther. 2000;7:2028–35. doi: 10.1038/sj.gt.3301326. [DOI] [PubMed] [Google Scholar]
- 24.Ponsaerts P, Van Tendeloo VF, Cools N, et al. mRNA-electroporated mature dendritic cells retain transgene expression, phenotypical properties and stimulatory capacity after cryopreservation. Leukemia. 2002;16:1324–30. doi: 10.1038/sj.leu.2402511. [DOI] [PubMed] [Google Scholar]
- 25.Harris NL. Mature B-cell neoplasms. In: Jaffe ES, Harris NL, Stein H, Vardiman JW, editors. WHO classification of tumours − pathology, genetics of tumours of haematopoietic and lymphoid tissues. Lyon: IARC Press; 2001. pp. 119–85. [Google Scholar]
- 26.Salkowitz JR, Sieg SF, Harding CV, Lederman MM. In vitro human memory CD8 T cell expansion in response to cytomegalovirus requires CD4+ T cell help. J Infect Dis. 2004;189:971–83. doi: 10.1086/382032. [DOI] [PubMed] [Google Scholar]
- 27.Riberdy JM, Christensen JP, Branum K, Doherty PC. Diminished primary and secondary influenza virus-specific CD8(+) T cell responses in CD4-depleted Ig(−/−) mice. J Virol. 2000;74:9762–5. doi: 10.1128/jvi.74.20.9762-9765.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coughlin CM, Vance BA, Grupp SA, Vonderheide RH. RNA-transfected CD40-activated B cells induce functional T cell responses against viral and tumor antigen targets: implications for pediatric immunotherapy. Blood. 2004;103:2046–54. doi: 10.1182/blood-2003-07-2379. [DOI] [PubMed] [Google Scholar]
- 29.Lacey SF, Villacres MC, La Rosa C, et al. Relative dominance of HLA-B*07 restricted CD8+ T lymphocyte immune responses to human cytomegalovirus pp65 in persons sharing HLA-A*02 and HLA-B*07 alleles. Hum Immunol. 2003;64:440–52. doi: 10.1016/s0198-8859(03)00028-4. [DOI] [PubMed] [Google Scholar]
- 30.Bonehill A, Heirman C, Tuyaerts S, et al. Messenger RNA-electroporated dendritic cells presenting MAGE-A3 simultaneously in HLA class I and class II molecules. J Immunol. 2004;172:6649–57. doi: 10.4049/jimmunol.172.11.6649. [DOI] [PubMed] [Google Scholar]
- 31.Ponsaerts P, Van Tendeloo V, Berneman ZN. Cancer immunotherapy using RNA-loaded dendritic cells. Clin Exp Immunol. 2003;134:378–84. doi: 10.1046/j.1365-2249.2003.02286.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roy MP, Kim CH, Butcher EC. Cytokine control of memory B cell homing machinery. J Immunol. 2002;169:1676–82. doi: 10.4049/jimmunol.169.4.1676. [DOI] [PubMed] [Google Scholar]