Abstract
Dendritic cells (DCs) are potent antigen-presenting cells and can induce tumour- or pathogen-specific T cell responses. For adoptive immunotherapy purposes, immature DCs can be generated from adherent monocytes using granulocyte macrophage colony stimulating factor (GM-CSF) and interleukin (IL)-4, and further maturation is usually achieved by incubation with tumour necrosis factor (TNF)-α. However, TNF-α-stimulated DCs produce low levels of IL-12. In this study, we compared the effects of TNF-α, interferon (IFN)-γ, IL-1β or IFN-γ + IL-1β on the phenotypic and functional maturation of DCs. Our results show that IFN-γ, but not IL-1β, augmented the surface expression of CD80, CD83 and CD86 molecules without inducing IL-12 production from DCs. However, IL-1β, but not IFN-γ, induced IL-12 p40 production by DCs without enhancing phenotypic maturation. When combined, IFN-γ + IL-1β treatment profoundly up-regulated the expression of CD80, CD83, CD86 and major histocompatibility complex (MHC) class II antigens. Furthermore, IFN-γ + IL-1β-treated DCs produced larger amounts of IL-12 and induced stronger T cell proliferation and IFN-γ secretion in primary allogeneic mixed lymphocyte reaction (MLR) than did TNF-α-treated DCs. Our results show that IFN-γ + IL-1β induced human monocyte-derived DCs to differentiate into Th1-prone mature DCs.
Keywords: cytokines, dendritic cells, human, Th1/Th2
Introduction
Dendritic cells (DCs) are potent antigen-presenting cells (APCs [1,2]). Peripherally located DCs are generally immature and specialize in antigen capture with a low T cell stimulatory capacity. However, once immature DCs encounter the appropriate maturational signals, they are stimulated to migrate to the T cell-rich zone of secondary lymphoid organs [3–6] and to become potent T cell activators [2]. The capacity of DCs to stimulate T cells is dependent upon the up-regulation of co-stimulatory molecules such as CD80/CD86 and the secretion of a variety of cytokines such as interleukin (IL)-12 [7–9]. The pivotal role of DCs in the activation of naive T lymphocytes and the generation of primary T cell responses is now being explored in clinical trials of DC-based immunotherapy in humans [10–13]. Large numbers of human DCs can be generated from adherent peripheral blood monocytes ex vivo in the presence of granulocyte-monocyte colony stimulating factor (GM-CSF) and IL-4 [14]. These monocyte-derived DCs are differentiated further into the mature immunostimulatory DCs with various stimuli such as tumour necrosis factor (TNF)-α [14–16] or lipopolysaccharide [8]. The functional and phenotypic maturation of DCs is regulated dynamically and diversely by the different stimulatory agents. In the previous study, for example, we demonstrated that the TNF-α-induced maturation of DCs is regulated differentially from the lipopolysaccharide-induced maturational process [17].
In this study, we compared the effects of TNF-α, IFN-γ and IL-1β on the maturation of human monocyte-derived DCs. We demonstrated that the individual treatment of DCs with either IFN-γ or IL-1β did not induce a full maturation of DCs. However, dual treatment of DCs with IFN-γ + IL-1β induced significant phenotypic and functional maturation of DCs comparable to that obtained by TNF-α-treatment. Moreover, IFN-γ + IL-1β-treated DCs produced higher amounts of IL-12 and thus up-regulated the production of IFN-γ from allogeneic T cells than did TNF-α-treated DCs.
Materials and methods
Antibodies and immunoreactants
FITC-labelled anti-CD80 MoAb (mouse IgM, clone BB1) was purchased from PharMingen (San Diego, CA, USA). Phycoerythrin (PE)-labelled CD86 MoAb (mouse IgG2b, clone HA5·2B7), PE-labelled CD83 MoAb (mouse IgG2b, clone HB15A) were from Immunotech (Marseille, France) and ECD-labelled streptavidin was from Beckman Coulter (Tokyo, Japan). Biotin-conjugated HLA-DR MoAb (mouse IgG2a, clone CR3/43) was from Becton-Dickinson (Mountain View, CA, USA). FITC- and PE-labelled mouse IgG1, FITC- and PE-labelled mouse IgG2b, FITC-labelled mouse IgM (Immunotech) and biotin-conjugated mouse IgG2b (Immunotech) were used as isotype-matched controls. Mouse polyclonal IgG was obtained from Sigma (St Louis, MO, USA).
Culture of DCs from buffy coats
Buffy coats were obtained from healthy volunteer donors according to institutional guidelines. Peripheral blood mononuclear cells (PBMCs) were prepared by density centrifugation using Ficoll-Paque (Amersham Biosciences, Sweden). PBMCs (50 × 106 cells) were incubated in 10 ml of RPMI-1640 medium supplemented with 5% heat-inactivated fetal calf serum (FCS), 2 mm l-glutamine, 100 U/ml penicillin and 100 µg/ml streptomycin (all media and supplements were from Gibco-BRL, Grand Island, NY, USA) and were incubated for 1 h at 37°C. Non-adherent cells were removed by extensive washing and the remaining adherent cells were recovered by scraping. The mean purity of the purified CD14+ cells was greater than 90%. Cells were subsequently cultured in six-well plates (3 × 106 cells/well) in 3 ml of RPMI-1640 medium supplemented with 10% heat-inactivated FCS, 10 mm HEPES, 1% non-essential amino acids, 1 mm sodium pyruvate, 2 mm l-glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin, 50 mm 2-ME (Sigma), 10 ng/ml GM-CSF (PeproTech, Rocky Hill, NJ, USA), and 50 ng/ml IL-4 (PeproTech) for 5 days. Cells were fed with 1·5 ml of fresh medium containing 20 ng/ml of GM-CSF and 100 ng/ml of IL-4 on days 2 and 4. To obtain mature DCs, immature DCs (4 × 105/ml) were stimulated with TNF-α (R&D), IFN-γ (PeproTech), IL-1β (PeproTech) or IFN-γ + IL-1β in 1 ml of medium containing 10 ng/ml GM-CSF, and 50 ng/ml IL-4 for 48 h.
Flow cytometric analysis
Cultured DCs were washed, resuspended at a concentration of 0·5–1 × 105 cells in 50 µl of cold phosphate-buffered saline (PBS) containing 0·1% sodium azide, 10 mg/ml bovine serum albumin (BSA) and 200 µg/ml mouse IgG (Sigma), and incubated for 15 min on ice. Subsequent staining with either labelled MoAb or appropriate isotypic controls was performed for 30 min on ice. Stained cells were washed, resuspended in 300 µl of cold PBS containing 0·1% sodium azide, 10 mg/ml BSA and 10 µg/ml 7-amino actinomycin D (Sigma) and analysed for three-colour immunofluorescence by flow cytometry (Coulter, Tokyo, Japan). Cellular debris was eliminated from the analysis using a gate on forward- and side-scatter. A viability gate was set using 7-amino-actinomycin D, which allows discrimination between viable, necrotic and apoptotic cells. A minimum of 104 cells was analysed for each sample. Results were processed using Flow Jo software (Treestar, San Carlos, CA, USA).
Allogeneic mixed lymphocyte reaction (MLR) and production of IFN-γ from allogeneic T cells
Allogeneic T cells were obtained from peripheral blood of healthy adults using a Ficoll-Paque gradient (Amersham Biosciences), adherence to plastic for 1 h at 37°C, and passage over a nylon wool column (Wako, Osaka, Japan). The purity of the CD3+ T cells in the recovered cells was always greater than 90%. CD3+ T cells were distributed at 1 × 105 cells per well into round-bottomed 96-well microplates, and were incubated for 5 days in the presence of graded numbers of irradiated (3000 rad, 137Cs source) mature DCs (pretreated with TNF-α, IFN-γ, IL-1β or IFN-γ + IL-1β) in 200 µl of medium containing 10% FCS. T cell proliferation was assessed after 8–14 h incorporation of [3H]thymidine (1 µCi/well; New England Nuclear, Boston, MA, USA) by standard procedures. The amount of IFN-γ in each supernatant of co-culture was measured using respective enzyme-linked immunosorbent assay (ELISA) kits (BioSource and Endogen, Woburn, MA, USA) according to the manufacturers’ protocols. The results are expressed as the mean of triplicate cultures. The s.e.m. of the results never exceeded 15%.
Production of IL-12 p40, IL-12 p70 and IL-10 from DCs
The amount of TNF-α, IL-12 p40, IL-12 p70 and IL-10 in each supernatant was measured using respective ELISA kits (BioSource and Endogen, Woburn, MA, USA) according to the manufacturers’ protocols. The minimum detectable dose of TNF-α, IL-12 p40, IL-12 p70 and IL-10 were 1·7 pg/ml, 2·0 pg/ml, 0·5 pg/ml and 1·0 pg/ml, respectively.
Statistical analysis
Data were analysed using Student's t-test and a P-value less than 0·05 was considered to be statistically significant.
Results
The combination of IFN-γ and IL-1β induces mature surface phenotype of DCs
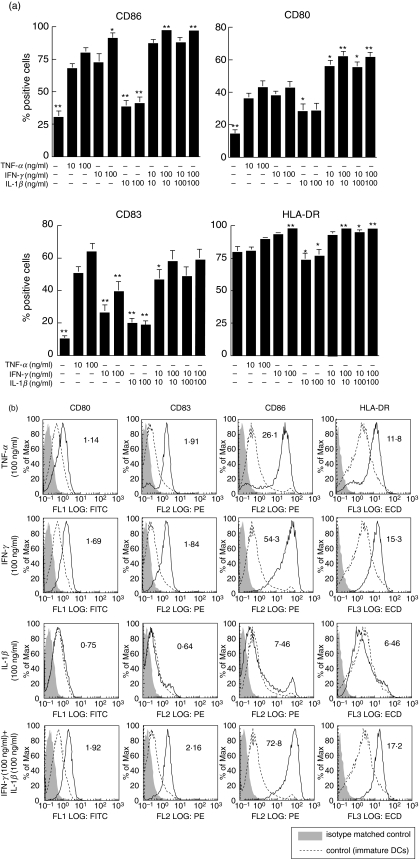
We examined whether IFN-γ or IL-1β stimulation induces a similar level of phenotypic activation of DCs as TNF-α (Fig. 1). Non-treated DCs expressed low levels of CD80, CD86 and HLA-DR along with an absence of membraneous CD83 [1,2]. Following treatment with TNF-α, the expression of CD80, CD86, HLA-DR and CD83 increased compared with the non-treated cells as described previously [18]. IFN-γ increased the surface expression of CD80, CD86, HLA-DR as much as to similar levels that of TNF-α treatment; however, the up-regulation of CD83 by IFN-γ was lower than the TNF-α treatment. In contrast, treatment with IL-1β showed a very weak stimulation of phenotypic maturation and did not enhance the surface expression of CD86, CD80, CD83 and HLA-DR significantly. When we used a combined IFN-γ and IL-1β treatment, however, the expression of these four molecules were enhanced, similar to that obtained by TNF-α.
Fig. 1.
The effects of TNF-α, IFN-γ, IL-1β or IFN-γ + IL-1β on the maturation of DCs. Peripheral blood monocytes were differentiated into immature DCs after treatment with GM-CSF and IL-4. At day 5, DCs were stimulated with TNF-α, IFN-γ, IL-1β or IFN-γ + IL-1β for 48 h. The surface expression of CD86, CD80, CD83 and MHC class II molecules were analysed by flow cytometry. (a) The results are shown as percentage of positive cells ± s.e.m. *P < 0·05 and **P < 0·01 compared with TNF-α (100 ng/ml) alone. (b) The solid line histograms are the phenotype of mature DCs stimulated with TNF-α, IFN-γ, IL-1β or IFN-γ + IL-1β, while the dotted line histograms are the phenotype of immature DCs. The shadow histograms are the phenotype of isotype matched controls. The numbers indicate the mean fluorescence intensity. These results are representative of seven independent experiments.
IFN-γ + IL-1β-treated DCs produce higher amounts of IL-12 than TNF-α-treated DCs
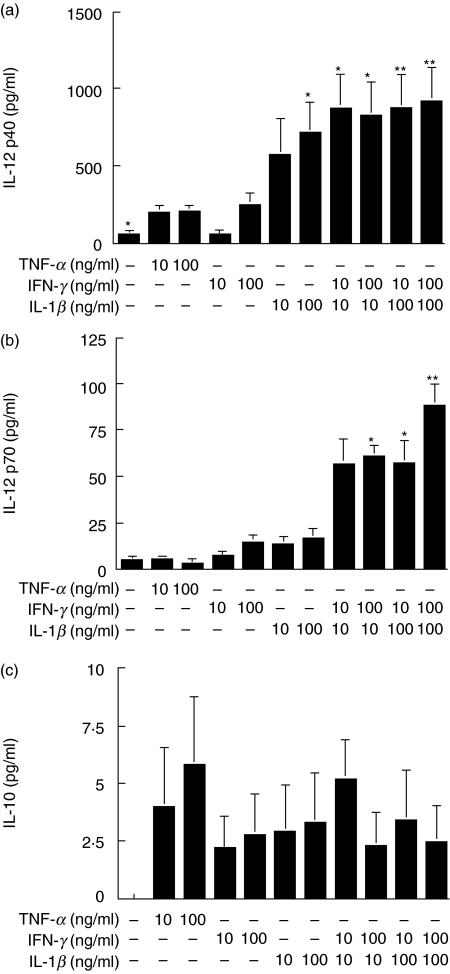
In order to investigate the functional properties of the treated DCs, we examined the cytokine production of DCs. During maturation, DCs secrete various cytokines such as IL-12 and IL-10, which induce the Th1 and Th2 response, respectively [2,9]. Although TNF-α induced a profound phenotypic maturation of DCs, induced IL-12 p40 and IL-12 p70 secretion was very weak (Fig. 2a, b). Similarly, IFN-γ did not induce high levels of IL-12 p40 and IL-12 p70 secretion. However, IL-1β stimulated DCs to produce large amounts of IL-12 p40, but not IL-12 p70. It is of note that both IL-12 p40 and IL-12 p70 secretion was synergistically up-regulated when DCs were treated with IFN-γ + IL-1β (Fig. 2a, b). In contrast, the secretion of IL-10 was marginally induced from DCs stimulated with either TNF-α, IFN-γ, IL-1β or IFN-γ + IL-1β (Fig. 2c). Although not statistically significant, TNF-α-treated DCs tended to produce larger amounts of IL-10 than DCs treated with either IFN-γ, IL-1β or IFN-γ + IL-1β (Fig. 2c).
Fig. 2.
The effects of TNF-α, IFN-γ, IL-1β or IFN-γ + IL-1β on the production of IL-12 and IL-10 from DCs. Peripheral blood monocytes were differentiated into immature DCs after treatment with GM-CSF and IL-4. At day 5, DCs were stimulated with TNF-α, IFN-γ, IL-1β or IFN-γ + IL-1β for 48 h. The production of IL-12 p40, IL-12 p70 and IL-10 was measured by ELISA. These data represent means ± s.e.m. of six independent experiments. *P < 0·05 and **P < 0·01 compared with TNF-α (100 ng/ml) stimulation alone.
IFN-γ + IL-1β -treated DCs are efficient APCs in primary allogeneic MLR
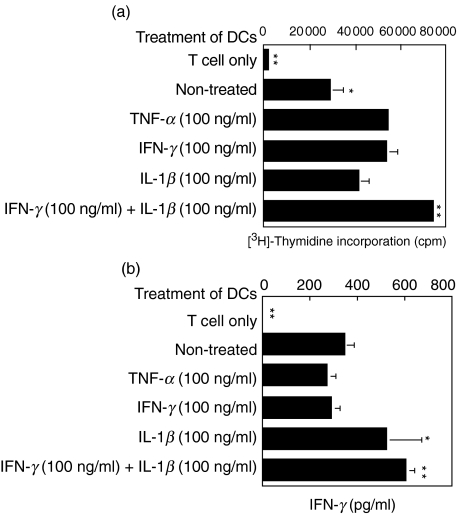
Because the combined stimulation with IFN-γ + IL-1β synergistically augmented IL-12 production as well as the surface expression of co-stimulatory molecules, we examined the immunostimulatory function of DCs by measuring the proliferation and the IFN-γ production of T cells in allogeneic MLR. TNF-α-treated DCs strongly enhanced the proliferation of allogeneic T cells when compared to non-treated DCs (Fig. 3). IFN-γ-treated DCs stimulated T cell proliferation, similar to TNF-α treatment. IL-1β-treated DCs also enhanced the proliferation of T cells when compared to non-treated DCs; however, the enhancement was weaker than that of TNF-α- or IFN-γ-treated DCs. In contrast, IFN-γ + IL-1β treatment up-regulated the immunostimulatory function of DCs additively (Fig. 3a).
Fig. 3.
The effects of TNF-α, IFN-γ, IL-1β or IFN-γ + IL-1β on the allostimulatory function of DCs. Peripheral blood monocytes were differentiated into immature DCs after treatment with GM-CSF and IL-4. At day 5, DCs were stimulated with TNF-α, IFN-γ, IL-1β or IFN-γ + IL-1β, and were further co-cultured with allogeneic T cells for 5 days in triplicate. The proliferation of T cells was measured by [3H]-thymidine incorporation assay (a; n = 3). We simultaneously assessed the IFN-γ production from T cells (b; n = 5). Data are shown as mean values. The production of IFN-γ was measured by ELISA. *P < 0·05 and **P < 0·01 compared with TNF-α (100 ng/ml) stimulation alone.
In addition to the proliferation assay, we simultaneously assessed the IFN-γ production from T cells in the allogeneic MLR. Although TNF-α- or IFN-γ-treated DCs enhanced the proliferation of T cells greater than did non-treated DCs, neither TNF-α- nor IFN-γ-treated DCs were able to up-regulate the production of IFN-γ from allogeneic T cells (Fig. 3b). It was of great interest that the production of IFN-γ from T cells was augmented slightly by IL-1β-treated DCs and consistently by IFN-γ + IL-1β.
Discussion
DCs are central in the initiation and regulation of the immune response, and are capable of activating naive T cells as well as directing lymphocytes towards Th1 or Th2 differentiation pathways [1,2]. Due to their potent capacity to stimulate resting T cells, DCs are the candidate cell type for immunotherapy against various tumours [19,20]. Human DCs are generated from peripheral blood monocytes in the presence of GM-CSF and IL-4 [14]. These immature DCs express low levels of co-stimulatory molecules and their immunostimulatory function is weak. It is well known that further stimulation of DCs with TNF-α strongly augments the expression of co-stimulatory molecules and the immunostimulatory function of DCs [18]. In the present study, we compared the effects of IFN-γ, IL-1β and IFN-γ + IL-1β on the maturation of DCs. Although IFN-γ or IL-1β only partially induced the maturation of DCs, IFN-γ + IL-1β-treated DCs expressed high levels of CD80, CD83 and CD86, and exhibited very potent immunostimulatory function, similar to TNF-α-treated DCs. Moreover, it should be emphasized that IFN-γ + IL-1β-treated DCs produced larger amounts of IL-12 than did TNF-α-treated DCs, which allowed T cells to secrete larger amounts of IFN-γ in the allogeneic MLR. In contrast, DCs treated with IFN-γ + IL-1β did not produce larger amounts of IL-10 than TNF-α-treated DCs. One of the most important roles of DCs in establishing protective immunity is to drive the expansion and commitment of naive T cells toward either the Th1 or Th2 lineage [2]. The mechanisms of these events are partially dependent on the pattern of cytokines secreted by DCs into the local microenvironment [21]. IL-12 has been shown to generate a polarization of the immune response toward the Th1 pathway in vivo. IL-12 is also a potent inducer of IFN-γ and TNF-α production by both natural killer (NK) cells and T cells [22], and these cytokines are critical to the development of cell-mediated immune responses [23], which is crucial for the induction of antitumour immunity. IL-12 is a heterodimer formed by a 35-kDa light chain (known as p35) and a 40-kDa heavy chain (known as p40) [22]. The two genes encoding p40 and p35 are unrelated and located on separate chromosomes (5q31–33 and 3p12–q13.2, respectively, in humans; and chromosomes 11 and 6, respectively, in mice) [24]. In addition, the transcription of the p40 and p35 genes is regulated differentially [25]. Both genes encoding IL-12 need to be expressed co-ordinately in the same cells to produce the biologically active heterodimer [26]. In the absence of IL-12 p35, p40 is secreted as a monomer or a homodimer, whereas p35 can be secreted only when associated with p40 [27]. IL-12 p40 is often secreted in large excess over the p70 heterodimer [28]. For both murine and human homodimeric p40 (p40)2 is able to act as an antagonist of IL-12 by binding to the one subunit of the IL-12 receptor [29,30]. However, because of its low affinity to the human IL-12R, human (p40)2 has only a minor ability to antagonize IL-12 functions compared to murine (p40)2[ 31].
In our study, IL-1β stimulated DCs to produce large amounts of IL-12 p40, but not bioactive IL-12 p70. In spite of this cytokine profile, IL-1β-treated DCs augmented IFN-γ production from T cells. Thus, there is a possibility that the production of IL-12 p40 by DC may help the generation of a Th1 response in human. The detailed mechanism was unclear at the moment but it might be due to the human/murine species difference.
IL-10-treated DCs are not only less efficient at stimulating T cell responses but also can induce a state of Ag-specific tolerance [32–34]. In our study, the phenotypic maturation is accompanied by production of high levels of IL-12 p70 and low levels of IL-10 in IFN-γ + IL-1β-treated DCs. This cytokine profile generates a polarization of the immune response toward the Th1 pathway.
Mosca et al. reported that a combination of CD40L + IFN-γ induced an up-regulation of CD80 and CD83 expression in human monocyte-derived DCs. Using an intracellular cytokine assay, they also demonstrated that only a subset of DCs that express high levels of CD80 and CD8 generated large amounts of IL-12 [35]. Wesa et al. showed that CD40L + IL-1β treatment induced high levels of CD54, CD86 and HLA-DR in human monocyte-derived DCs and that IL-1β was a maturation factor for DCs [36]. In accordance with these previous reports, the present study demonstrated further that the combined treatment with IFN-γ + IL-1β clearly enhanced the phenotypic and functional maturation of human DCs with Th1-prone characteristics.
References
- 1.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–96. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 2.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 3.Saeki H, Moore AM, Brown MJ, Hwang ST. Cutting edge: secondary lymphoid-tissue chemokine (SLC) and CC chemokine receptor 7 (CCR7) participate in the emigration pathway of mature dendritic cells from the skin to regional lymph nodes. J Immunol. 1999;162:2472–5. [PubMed] [Google Scholar]
- 4.Sallusto F, Schaerli P, Loetscher P, et al. Rapid and coordinated switch in chemokine receptor expression during dendritic cell maturation. Eur J Immunol. 1998;28:2760–9. doi: 10.1002/(SICI)1521-4141(199809)28:09<2760::AID-IMMU2760>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 5.Sozzani S, Allavena P, D’Amico G, et al. Differential regulation of chemokine receptors during dendritic cell maturation: a model for their trafficking properties. J Immunol. 1998;161:1083–6. [PubMed] [Google Scholar]
- 6.Dieu MC, Vanbervliet B, Vicari A, et al. Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J Exp Med. 1998;188:373–86. doi: 10.1084/jem.188.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J Exp Med. 1996;184:747–52. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verhasselt V, Buelens C, Willems F, De Groote D, Haeffner-Cavaillon N, Goldman M. Bacterial lipopolysaccharide stimulates the production of cytokines and the expression of costimulatory molecules by human peripheral blood dendritic cells: evidence for a soluble CD14-dependent pathway. J Immunol. 1997;158:2919–25. [PubMed] [Google Scholar]
- 9.Rissoan MC, Soumelis V, Kadowaki N, et al. Reciprocal control of T helper cell and dendritic cell differentiation. Science. 1999;283:1183–6. doi: 10.1126/science.283.5405.1183. [DOI] [PubMed] [Google Scholar]
- 10.Panelli MC, Wunderlich J, Jeffries J, et al. Phase 1 study in patients with metastatic melanoma of immunization with dendritic cells presenting epitopes derived from the melanoma-associated antigens MART-1 and gp100. J Immunother. 2000;23:487–98. doi: 10.1097/00002371-200007000-00013. [DOI] [PubMed] [Google Scholar]
- 11.Thurner B, Haendle I, Röder C, et al. Vaccination with Mage-3A1 peptide-pulsed mature, monocyte-derived dendritic cells expands specific cytotoxic T cells and induces regression of some metastases in advanced stage IV melanoma. J Exp Med. 1999;190:1669–78. doi: 10.1084/jem.190.11.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schuler-Thurner B, Dieckmann D, Keikavoussi P, et al. Mage-3 and influenza-matrix peptide-specific cytotoxic T cells are inducible in terminal stage HLA-A2.1+ melanoma patients by mature monocyte-derived dendritic cells. J Immunol. 2000;165:3492–6. doi: 10.4049/jimmunol.165.6.3492. [DOI] [PubMed] [Google Scholar]
- 13.Fong L, Engleman EG. Dendritic cells in cancer immunotherapy. Annu Rev Immunol. 2000;18:245–73. doi: 10.1146/annurev.immunol.18.1.245. [DOI] [PubMed] [Google Scholar]
- 14.Sallusto FA, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor. J Exp Med. 1994;179:1109–18. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bender A, Sapp M, Schuler G, Steinman RM, Bhardwaj N. Improved method for the generation of dendritic cells from nonproliferating progenitors in human blood. J Immunol Meth. 1996;196:121–35. doi: 10.1016/0022-1759(96)00079-8. [DOI] [PubMed] [Google Scholar]
- 16.Luft T, Pang KC, Thomas E, et al. Type I IFNs enhance the terminal differentiation of dendritic cells. J Immunol. 1998;161:1947–53. [PubMed] [Google Scholar]
- 17.Uchi H, Arrighi JF, Aubry JP, Furue M, Hauser C. The sesquiterpene lactone parthenolide inhibits LPS- but not TNF-alpha-induced maturation of human monocyte-derived dendritic cells by inhibition of the p38 mitogen-activated protein kinase pathway. J Allergy Clin Immunol. 2002;110:269–76. doi: 10.1067/mai.2002.126381. [DOI] [PubMed] [Google Scholar]
- 18.Nelson EL, Strobl S, Subleski J, Prieto D, Kopp WC, Nelson PJ. Cycling of human dendritic cell effector phenotypes in response to TNF-alpha: modification of the current ‘maturation’ paradigm and implications for in vivo immunoregulation. FASEB J. 1999;13:2021–30. doi: 10.1096/fasebj.13.14.2021. [DOI] [PubMed] [Google Scholar]
- 19.Grabbe S, Beissert S, Schwarz T, Granstein RD. Dendritic cells as initiators of tumor immune responses − a possible strategy for tumor immunotherapy. Immunol Today. 1995;16:117–21. doi: 10.1016/0167-5699(95)80125-1. [DOI] [PubMed] [Google Scholar]
- 20.Schuler G, Steinman RM. Dendritic cells as adjuvants for immune-mediated resistance to tumors. J Exp Med. 1997;186:1183–7. doi: 10.1084/jem.186.8.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O'Garra A. Cytokines induce the development of functionally heterogeneous T helper cell subsets. Immunity. 1998;8:275–83. doi: 10.1016/s1074-7613(00)80533-6. [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi M, Fitz L, Ryan M, et al. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J Exp Med. 1989;170:827–46. doi: 10.1084/jem.170.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmitt E, Hoehn P, Huels C, et al. T helper type 1 development of naive CD4+ T cells requires the co-ordinate action of IL-12 and IFN- and is inhibited by TGF-β. Eur J Immunol. 1994;24:793–8. doi: 10.1002/eji.1830240403. [DOI] [PubMed] [Google Scholar]
- 24.Trinchieri G. Interleukin-12: a cytokine at the interface of inflammation and immunity. Adv Immunol. 1998;70:83–243. doi: 10.1016/s0065-2776(08)60387-9. [DOI] [PubMed] [Google Scholar]
- 25.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–46. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 26.Wolf SF, Temple PA, Kobayashi M, et al. Cloning of cDNA for natural killer cell stimulatory factor, a heterodimeric cytokine with multiple biologic effects on T and natural killer cells. J Immunol. 1991;146:3074–81. [PubMed] [Google Scholar]
- 27.Ma X, Trinchieri G. Regulation of interleukin-12 production in antigen-presenting cells. Adv Immunol. 2001;79:55–92. doi: 10.1016/s0065-2776(01)79002-5. [DOI] [PubMed] [Google Scholar]
- 28.D’Andrea A, Rengaraju M, Valiante NM, et al. Production of natural-killer cell stimulatory factor (interleukin 12) by peripheral-blood mononuclear cells. J Exp Med. 1992;176:1387–98. doi: 10.1084/jem.176.5.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gillessen S, Carvajal D, Ling P, et al. Mouse interleukin-12 (IL-12) p40 homodimer: a potent IL-12 antagonist. Eur J Immunol. 1995;25:200–6. doi: 10.1002/eji.1830250133. [DOI] [PubMed] [Google Scholar]
- 30.Ling P, Gately MK, Gubler U, et al. Human IL-12 p40 homodimer binds to the IL-12 receptor but does not mediate biologic activity. J Immunol. 1995;154:116–27. [PubMed] [Google Scholar]
- 31.Germann T, Rude E, Mattner F, Gately MK. The IL-12 p40 homodimer as a specific antagonist of the IL-12 heterodimer. Immunol Today. 1995;16:500–1. doi: 10.1016/0167-5699(95)80035-2. [DOI] [PubMed] [Google Scholar]
- 32.Enk AH, Angeloni VL, Udey MC, Katz SI. Inhibition of Langerhans cell antigen-presenting function by IL-10. J Immunol. 1993;151:2390–8. [PubMed] [Google Scholar]
- 33.Enk AH, Saloga J, Becker D, Mohamadzadeh M, Knop J. Induction of hapten-specific tolerance by interleukin-10 in vivo. J Exp Med. 1994;179:1397–402. doi: 10.1084/jem.179.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steinbrink K, Jonuleit H, Müller G, Schuler G, Knop J, Enk AH. Interleukin-10-treated human dendritic cells induce a melanoma-antigen-specific anergy in CD8+ T cells resulting in a failure to lyse tumor cells. Blood. 1999;93:1634–42. [PubMed] [Google Scholar]
- 35.Mosca PJ, Hobeika AC, Clay TM, et al. A subset of human monocyte-derived dendritic cells expresses high levels of interleukin-12 in response to combined CD40 ligand and interferon-gamma treatment. Blood. 2000;96:3499–504. [PubMed] [Google Scholar]
- 36.Wesa AK, Galy A. IL-1 beta induces dendritic cells to produce IL-12. Int Immunol. 2001;13:1053–61. doi: 10.1093/intimm/13.8.1053. [DOI] [PubMed] [Google Scholar]