Abstract
In the rheumatoid synovium, deiminated (‘citrullinated’) forms of fibrin are the major targets of IgG autoantibodies to citrullinated proteins (ACPA), the most specific serological markers of rheumatoid arthritis (RA). To further the characterization of ACPA, we determined their subclass distribution. From a previously validated highly sensitive and specific enzyme-linked immunosorbent assay (ELISA) onto in vitro deiminated human fibrinogen − antihuman fibrin(ogen) autoantibodies (AhFibA)-ELISA − we derived and calibrated four ELISAs, using monoclonal antibodies to each of the four IgG subclasses, to determine the proportions of AhFibA subclasses in the sera. A series of 186 serum samples from RA patients was analysed. All AhFibA-positive sera contained IgG1-AhFibA, which reached the highest titres and accounted for more than 80% of AhFibA in three-quarters of the sera. One or two other subclasses were associated with IgG1 in 39% of the sera, IgG4-AhFibA being observed much more frequently and at higher titres than IgG3- or IgG2-AhFibA. IgG1 alone or IgG(1 + 4)-AhFibA were the AhFibA subclass profiles found in more than 80% of patients. AhFibA are mainly IgG1 and, to a lesser extent, IgG4. Such IgG subclass profiles may influence the effector phases of the immunological conflict between ACPA and deiminated fibrin that takes place specifically in the rheumatoid synovium and therefore may play a critical role in the self-maintenance of rheumatoid inflammation.
Keywords: autoantibodies, citrullinated antigen, fibrin(ogen), IgG subclass, rheumatoid arthritis (RA)
Introduction
Rheumatoid arthritis (RA), the most frequent autoimmune disease, is a chronic inflammatory rheumatism hallmarked by progressive and irreversible joint destruction. Among the numerous autoantibodies associated with RA, autoantibodies to ‘citrullinated’ (deiminated) proteins (ACPA) are now recognized as the most disease-specific. They were first described as two independent highly disease-specific IgG families, the antiperinuclear factor (APF) [1] and the so-called ‘antikeratin’ autoantibodies (AKA) [2]. However, work in our group showed that both APF and AKA belong to a single family of autoantibodies that target isoelectric variants of an epithelial protein, filaggrin, or of its precursor, profilaggrin [3]. These autoantibodies were then considered as ‘antifilaggrin autoantibodies (AFA)’. Further characterization of the epitopes recognized by AFA showed that post-translational modification of arginyl residues into citrullyl residues, i.e. deimination, was essential in the formation of the antigenic determinants of AFA [4,5], hence the present designation of ‘anticitrullinated proteins autoantibodies (ACPA)’. However, in spite of their clinical relevance, the potential role of ACPA in rheumatoid inflammation had still not been elucidated, leading us to investigate directly the synovial tissue, the main site of rheumatoid lesions. ACPA were first shown to be produced and concentrated in this tissue [6], then their major targets were identified as deiminated forms of the α- and β-chains of fibrin [7]. We thus proposed that the immunological conflict between deiminated fibrin and ACPA plays a major role in the self-maintenance of rheumatoid synovial inflammation. The immune complexes formed between ACPA and deiminated fibrin most probably activate effector functions, and then promote tissue inflammation, this inflammation leading in turn to plasma extravasation in the synovial tissue and thus to the formation of new fibrin deposits which, once deiminated, become the target of ACPA.
Several diagnostic immunoassays, based on the recognition of human or rat filaggrin or of citrulline-bearing peptides derived from human filaggrin, were developed [8–14] that, together with the classical ‘AKA’ and ‘APF’ indirect immunofluorescence tests, have allowed the collection of bioclinical data supporting the hypothesis of a critical role of ACPA in RA. In addition to having a high diagnostic specificity these autoantibodies appear early [15–17], even before the clinical onset of the disease [18–20], and are associated with the most severe forms of RA [21–24].
IgG subclass distribution depends on several biochemical and immunological parameters, such as the nature of the target antigen and the cytokine environment of B cells [25]. Moreover, the IgGs of the four subclasses have different properties and capacities to bind FcγR or to activate complement fractions [26,27]. The ways ACPA are involved in the pathophysiology of the disease thus depend on their subclass distribution. In the present study, this subclass distribution was determined using enzyme-linked immunosorbent assays (ELISAs) derived from an ELISA onto in vitro deiminated fibrinogen developed in our laboratory as a new assay for ACPA of very high diagnostic value (AhFibA-ELISA) [28]. We demonstrate that ACPA detected by these ELISAs − referred to herein as antihuman fibrin(ogen) autoantibodies (AhFibA) − correspond mainly to IgG1 and, to a lesser extent, to IgG4.
Patients and methods
Serum samples
We analysed a series of 186 serum samples from patients with RA attending the Rheumatology Departments of Purpan and Rangueil hospitals in Toulouse (females: 151, males: 35, median age 61 years, range 16–88 years). RA was diagnosed according to the revised criteria of the American College of Rheumatology [29]. The study was approved by the committee for protection of persons participating in biomedical research. Each person signed an informed consent.
Antibodies and reagents
Goat Ig to human IgG (γ chain-specific) and mouse monoclonal antibodies (MoAb) to human IgG − anti-IgG (γ), JDC-10 − as well as to human IgG1 –anti-IgG1 (γ1), JDC-1–, IgG2 –anti-IgG2 (γ 2), HP6014–, IgG3 –anti-IgG3 (γ3), HP6050– and IgG4 –anti-IgG4 (γ 4), HP6025–, all conjugated to horseradish peroxidase (HRP), were purchased from Southern Biotechnology Associates (Birmingham, USA). An unlabelled mouse monoclonal antibody to human IgG1 (anti-IgG1 (γ1), NL16) and an HRP-labelled goat antimouse F(ab)′2 were purchased from Skybio (Bedfordshire, UK) and from Tago (Burlingame, CA, USA), respectively. HP6014, HP6050, HP6025 and NL16 correspond to clones validated by the Human Immunoglobulins Sub-Committee of the International Union of Immunological Societies supported by the World Health Organization [30]. All clones were specific for the corresponding subclass and JDC-10 recognized the four IgG subclasses with a similar avidity. Purified IgG of each subclass derived from human myelomas, and bearing either κ or λ light chains, were purchased from Sigma (St Louis, MO, USA).
In vitro deimination of human fibrinogen
Plasminogen-depleted human fibrinogen (95% pure, Calbiochem, Meudon, France) was purified further by affinity chromatography on a protein-G column (HiTrap® protein G, 1 ml, Amersham Biosciences, Orsay, France), as suggested by the manufacturer. Deimination was then performed with rabbit skeletal muscle PAD (Sigma, 7 U/mg fibrinogen) in 0·1 m Tris-HCl, pH 7·4, 10 mm CaCl2, 5 mm DTT for 2 h at 37°C.
AhFibA-ELISA
AhFibA-ELISA was developed and validated previously on several series of patients [16,28]. Briefly, microtitration plates (MaxiSorp, Nunc, Denmark) were coated overnight with human deiminated fibrinogen (5 µg/ml) diluted in phosphate-buffered saline (PBS), pH 7·4. The plates were blocked with PBS containing 2% bovine serum albumin and 100 µl of sera, diluted to 1 : 50 in 2 m NaCl PBS, were incubated for 1 h. After washing, HRP-labelled goat Ig to human IgG (γ chain-specific) were added, incubated for 1 h and washed again. All incubations and washing steps were performed at 4°C. Bound antibodies were detected with ortho-phenylene diamine dihydrochloride (Sigma, St. Louis, MO, USA). Plates were read using a Multiskan plate reader (Thermo Labsystem, Cergy-Pontoise, France). A serum was considered positive for AhFibA when the AhFibA titre reached at least the previously established 98·6% specificity diagnostic threshold [28].
Determination of the relative concentration of each AhFibA subclass
In preliminary experiments, optimal working dilutions were determined for the MoAb to human IgG and MoAbs for each of the four IgG subclasses. First, by ELISA onto in vitro deiminated fibrinogen and using successive dilutions of a pool of RA sera, we determined the dilution of the HRP-labelled JDC-10 anti-IgG(γ) MoAb giving a titration curve similar to that obtained with the HRP-labelled goat Ig to human IgG (γ chain-specific) used in the AhFibA-ELISA. The ELISA developed with JDC-10 (diluted to 1 : 3000) was called IgG-ELISA to differentiate it from the original AhFibA-ELISA. The JDC-10 MoAb was then used as a reference for the determination of the optimal working dilution of each of the four MoAb to IgG subclasses. Microtitration plates were coated with successive dilutions of an equimolar mix of myeloma IgGs of the four subclasses, each subclass being itself comprised of Ig with κ light chains (2/3) and λ light chains (1/3). Successive dilutions of each of the four HRP-labelled MoAb to IgG subclasses were tested and several titration curves were obtained. A titration curve was also obtained with JDC-10 diluted to 1 : 3000. Experiments were repeated at least fivefold, OD values were averaged and the titration curves were plotted against the IgG concentration detected by the corresponding MoAb (only one subclass for the MoAb to IgG subclasses, the sum of all subclasses for the titration curve of JDC-10). For each MoAb to IgG subclasses, the titration curve fitting to that obtained with JDC-10 defined the optimal dilution (Fig. 1). The determinations of optimal dilutions were necessary to circumvent the differences in the affinity of the MoAbs to the respective IgG subclasses and therefore to be able to calculate the proportion of each IgG subclass in the sera of patients. Optimal dilutions to use were 1 : 300, 1 : 1500, 1 : 1500 and 1 : 6000 for the anti-IgG1, -IgG2, -IgG3 and -IgG4 MoAbs, respectively. However, because a high background was obtained with the anti-IgG1 MoAb and we could not find any suitable commercially available HRP-labelled substitute, a three-layer ELISA was set up for this subclass. An unlabelled antihuman IgG1(γ1) mouse MoAb (NL16) and an HRP-labelled goat antimouse F(ab)′2 were used. Their optimal dilutions were 1 : 9000 and 1 : 2500, respectively (Fig. 1).
Fig. 1.
Titration of the MoAbs to IgG subclasses. The curves correspond to the optimal dilution (see Methods) of the anti-IgG1, -IgG2, -IgG3 or -IgG4 MoAb, i.e. the dilution giving the same OD for the same concentration of the corresponding detected IgG subclass. Curves are the means of at least five duplicate determinations, performed in independent experiments. Bars indicate standard error. Titration curves obtained with the unlabelled NL16 anti-IgG1 MoAb (1 : 9000) followed by HRP-labelled antimouse IgG F(ab)′2 (1 : 2500), with the HRP-labelled HP6014 anti-IgG2 MoAb (1 : 2500), the HRP-labelled HP6050 anti-IgG3 MoAb (1 : 1500) and the HRP-labelled HP6025 anti-IgG4 MoAb (1 : 6000) are indicated by arrows. The thick full line represents the reference curve obtained with the HRP-labelled JDC10 anti-IgG MoAb (1 : 3000).
Finally, using each secondary and, when applicable, tertiary antibody at the above established optimal dilution, we measured the titres of each of the four AhFibA IgG subclasses in the sera of patients, by ELISA onto in vitro deiminated fibrinogen. The corresponding ELISAs are referred to as IgG1-, IgG2-, IgG3- or IgG4-ELISA. The autoantibodies measured by these assays and the IgG-ELISA are referred to as IgG1-, IgG2-, IgG3-, IgG4 and IgG-AhFibA, respectively. Serum samples were tested four times and the four results were averaged. For each determination, the same 1 : 50 serum dilution was tested in the five ELISAs. For each ELISA, a pool of positive RA sera was used as a reference to correct interassay variations. The titre was considered to be the difference between the mean OD value obtained with the serum and that obtained with the secondary (and, if applicable, tertiary) antibodies only (blanks). Using the previously established titration curves, similar for all subclasses, the proportions of each AhFibA subclass were calculated in each serum.
Statistical analyses
Data analyses were performed using statistica for Windows (StatSoft, Tulsa, OK, USA). Multiple comparisons of median differences were performed by Kruskal–Wallis anova. When this test was significant, pairwise comparisons were made by the Mann–Whitney U-test. P-value adjustment for multiple comparisons used the Holm (sequential Bonferroni) correction method. Correlations were sought by computing Spearman*s rank correlation coefficients. A test was considered significant for P ≤ 0·05.
Results
Correlations between the titres obtained in the IgG-, IgG1-, IgG2-, IgG3- and IgG4-ELISA and those obtained in the AhFibA-ELISA
For the 186 RA sera, we first compared the titres obtained with the IgG-ELISA (using the JDC-10 anti-IgG MoAb) with those obtained in the reference AhFibA-ELISA (Fig. 2). These titres were strongly and highly significantly correlated (r = 0·97, P < 0·001).
Fig. 2.
Distributions of titres obtained with the reference AhFibA-ELISA and those obtained with the IgG-, IgG1-, IgG2-, IgG3- and IgG4-ELISAs on the whole population of patients. For each serum, titres obtained in the (a) IgG-, (b) IgG1-, (c) IgG2-, (d) IgG3- and (e) IgG4-ELISAs were plotted against the titre obtained in the original AhFibA-ELISA.
Then, an equally strong correlation was observed between the IgG1-AhFibA titres and the AhFibA-ELISA titres (r = 0·94, P < 0·001).
The IgG2-, IgG3- and IgG4-AhFibA titres were also significantly, although more weakly, correlated with those of the AhFibA-ELISA (r = 0·76, 0·66 and 0·70, respectively, with P < 0·001 for each coefficient). When compared pairwise, no significant differences were found between these three correlation coefficients. However, each of these correlations was significantly weaker not only than the correlation between the IgG-ELISA and the AhFibA-ELISA, but also than that between the IgG1-ELISA and the AhFibA-ELISA (P < 0·001 in each case).
As expected, as the IgG-ELISA and AhFibA-ELISA titres were correlated perfectly, similar results were observed when comparing the IgG1- to IgG4-AhFibA titres to the IgG-AhFibA titres (r = 0·98, 0·78, 0·70, 0·75 for the correlation between IgG-ELISA and IgG1- to four ELISA titres, respectively).
Titre distributions in the IgG- and IgG1- to IgG4-ELISA
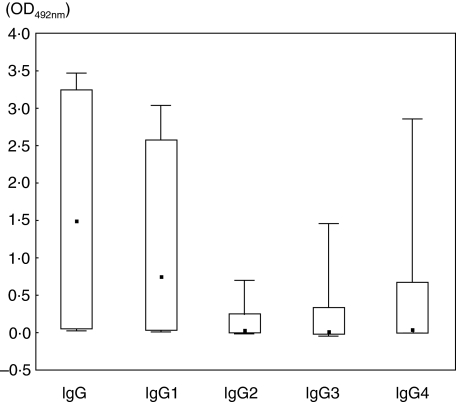
The box-and-whisker representation in Fig. 3 shows that IgG1-AhFibA titres covered the entire range of measurable ODs (median 0·727, range 0·008–3·028), reaching values almost as high as those reached by the IgG-AhFibA titres (median, 1·473, range 0·020–3·456). In addition, for a few sera, IgG4-AhFibA also reached high titres (range from −0·011 to 2·846), but their median was only 0·020. IgG3-AhFibA titres ranged from −0·050 to 1·448 (median 0·024) and IgG2-AhFibA titres from –0·018 to 0·695 (median 0·019).
Fig. 3.
Box-plot of the titre distributions in the IgG-, IgG1-, IgG2-, IgG3- and IgG4-ELISAs. Boxes contain 90% of the OD values obtained, bars extend to the extreme values. The median is indicated by a square.
As expected, given their range and median values, the IgG1-AhFibA titres were significantly higher than the titres of IgG2-, IgG3- and even IgG4-AhFibA (P < 10−6). In addition, the IgG4-AhFibA titres were significantly higher than the titres of IgG2- and IgG3-AhFibA (P < 10−3), whereas no significant difference was observed between the IgG2- and the IgG3-AhFibA titres (P = 0·90).
Subclass profiles of AhFibA
Among the 186 RA sera tested, 141 were positive in the AhFibA-ELISA [28]. For each of them, the titration curves established in preliminary experiments allowed us to calculate, from the titres obtained in the IgG1- to 4-ELISAs, the proportion of each AhFibA subclass (see Fig. 1 and Methods). IgG1-AhFibA accounted for more than 80% of AhFibA in 73% (103/141) of the sera. Moreover, in all sera but one, IgG1-AhFibA accounted for more than 40% of AhFibA. A similarly high proportion of the total AhFibA was never reached for IgG2-AhFibA. It was reached only once for IgG3-AhFibA (one serum with 67% of IgG3 and 31% of IgG1) and three times for IgG4-AhFibA, but the three corresponding sera contained an equal proportion of IgG1- and IgG4-AhFibA (IgG1-AhFibA: 53%, 41%, 47% and IgG4-AhFibA: 44%, 47%, 43%, respectively).
To establish the IgG subclass profile of each patient, we considered that a subclass was represented when it reached at least 10% of the total AhFibA. As shown in Fig. 4, all profiles contained IgG1-AhFibA. Among the 55/141 (39%) in which another subclass was present, IgG4-AhFibA were the most frequently associated subclass (35/55, 63·6%), much more frequent than IgG3-, found in 15/55 sera (27·3%) and IgG2-AhFibA, found in 11/55 sera (20%).
Fig. 4.
IgG subclass profiles of AhFibA. For each of the 141 AhFibA-ELISA positive sera, subclass proportions were calculated using titration curves established as described in the Patients and methods section. The histogram bars represent the proportion and number of patients with the subclass profiles described below. (+): subclass accounting for more than 10% of total AhFibA; (–): subclass accounting for less than 10% of total AhFibA.
Two main profiles emerged from this analysis, IgG1-AhFibA alone or IgG1-AhFibA in combination with IgG4-AhFibA, observed for 61% (86/141) and 21·3% (30/141) of the sera, respectively. Therefore, taken together, these two profiles accounted for more than 80% of the observed AhFibA subclass distributions. IgG3- and IgG2-AhFibA were far less associated to IgG1-AhFibA than IgG4-AhFibA and the combination of several subclasses to IgG1-AhFibA is exceptional (Fig. 4).
In conclusion, AhFibA are mainly IgG1, this subclass being highly represented in all sera, and IgG4, found in roughly one-quarter of the sera (24·8%, 35/141).
Discussion
To analyse the subclass distribution of IgG autoantibodies to citrullinated (deiminated) proteins, we developed ELISAs using in vitro deiminated human fibrinogen as immunosorbent. As all the tests were quantitative and adjusted to be comparative for each serum, we could establish the proportion of each AhFibA subclass. In 1990, our group determined, by indirect immunofluorescence, the subclass distribution of AKA in a short series of 31 AKA-positive RA sera [31]. In this initial work, IgG1-, 2-, 3- and 4-AKA were detected in 27 (87%), 6 (19%), 4 (13%) and 11 (35%) of the sera, respectively. Two predominant profiles were distinguished, IgG1-AKA alone (31·3%) and IgG(1 + 4)-AKA (25·8%). In the present study, using convenient ELISAs which allowed the investigation of a much larger series of samples, we confirmed our previous results as we distinguished the same two predominant profiles: IgG1-AhFibA alone (61%) and IgG(1 + 4)-AhFibA (21·3%). We detected IgG1 in 100% of the AhFibA-positive sera versus 86% in the previous study, a difference that can be explained by the higher sensitivity of the ELISA compared to indirect immunofluorescence, allowing the measurement of AhFibA subclasses even in sera with very low AhFibA-titres. Moreover, although chosen arbitrarily, the 10% threshold used to consider a subclass as represented in a serum appeared to provide a good picture of the subclass profiles. When the 20% threshold was chosen, the two main profiles remained: IgG1 (87%) and IgG(1 + 4) (8·5%).
In RA, concentrations of total IgG and of each IgG subclass were shown to be in a normal range in spite of some differences between treated and untreated patients [31,32]. In particular, IgG1 is the most represented subclass (7·2–12 g/l depending on the cohorts), whereas IgG4 concentration is very low (0·3–0·6 g/l) [31,32]. Thus, if one cannot exclude that IgG1-AhFibA preponderance reflects the high level of total IgG1, one can exclude that the augmentation of IgG4-AhFibA reflects only an increase of the total IgG4. Consistently, Cohen et al. demonstrated the absence, in RA sera, of an IgG4 increase for antibodies to exogenous antigens such as tetanus toxoid [33].
The mechanisms underlying IgG production influence their subclass distribution. Indeed, class switch recombination is regulated by several factors, including the biochemical properties of the target antigen or cytokines in the B cell environment. The predominance of IgG1-AhFibA is consistent with the protein nature of the antigen. In addition, even though T cells specific for deiminated fibrin have not yet been described, AhFibA subclasses may, at least in part, be determined by the cytokines released from antigen-specific T helper (Th) lymphocytes, in the environment of B cells. Two Th cell subsets are classically defined. Whereas IgG1 is the main subclass associated to the Th1 response, IgG4 synthesis requires IL4, and is therefore observed rather in a Th2 context. In RA, very low levels of cytokines are detected in synovial fluid or on cultures of unstimulated synovial T cells. However, on in vitro stimulated T cells an increase of interferon (IFN)-γ and decrease of IL4 were observed in synovial fluid versus peripheral blood [34,35]. IFN-γ was also found increased in the peripheral blood, synovial fluid and synovial tissue compared to other arthritides [36,37]. Thus a Th1/Th2 imbalance may exist in the rheumatoid synovium in favour of Th1. The presence of IgG1-AhFibA in all the sera, where they account for almost all AhFibA, is consistent with the generally accepted Th1 status of RA.
IgG4 was the second most frequently observed subclass, sometimes at high titres. This is unexpected in a strict Th1 context. Gerli et al. suggested that IL4 synthesis may be prevalent in early RA [38] but, because our series of patients is constituted of well-established RA, this cannot explain our observation. On the other hand, some treatments reported to increase the Th2/Th1 ratio [32,39] may have influenced the generation of IgG4-AhFibA in those patients.
IL4 not originating from T cells could also account for the presence of IgG4-AhFibA. Indeed, other cells (mast cells, NK cells, etc.) are able to secrete IL4 that, if produced in the vicinity of B cells, may induce class switch to IgG4. Interestingly, besides AhFibA producing B cells [6], mast cells were described in RA synovium [40]. Consistently, in a mouse model of polarized Th1 immunity, IL4 production by mast cells could promote the switch to IgG1 (human IgG4 counterpart) characteristic of a Th2 response [41]. Thus, bystander IL4 stimulation of B cells, independently of T cells, may explain the switch to IgG4.
Rheumatoid factors (RF) are not only found in the serum of most patients with RA but also in the synovial fluid and tissue. Interestingly, Cohen et al. [33] observed the same unexpected profile for RF of the IgG class, its major subclasses being IgG1 and, to a lesser extent, IgG4. Such results give weight to the cytokinic environnement of B cells. Moreover, the effector mechanisms activated by both autoantibodies will very probably co-operate to amplify the inflammation.
Whatever the mechanisms responsible for their synthesis may be, activation of AhFibA effector functions may be responsible for the synovial self-maintenance of inflammation. Because IgG effector functions differ according to the IgG subclass, the subclass profile of AhFibA is important in the role they may play in RA pathophysiology. Consistently, subclass specificity was shown to be crucial for inflammation or protection against pathogens. For example, in a Cryptococcus neoformans infection model, the protection efficacy of MoAb of identical specificity via IgG receptors that is Fcγ receptors (FcγR) was shown to depend on their subclass [42,43].
In the FcγR family, each type of receptor (FcγRI, II and III) has a restricted binding potential and can display overlapping but also distinct roles in immune functions. Interaction between both partners (FcγR and Ig) which depends on IgG subclasses and on FcγR types and expression levels will thus influence the in vivo immune response. Concerning the receptor FcγRIIIa (CD16), it has been shown that the FcγRIIIa-158 V allele of the FcγRIIIa gene, which encodes a receptor with a higher affinity for IgG compared to the FcγRIIIa-158F allele [44], was associated to an increased susceptibility for RA in some Caucasian populations [45,46]. The predominance of AhFibA-IgG1 in RA sera is perfectly consistent with a downstream involvement of FcγRIIIa as IgG1 binds to this receptor with the highest affinity [47].
In addition to the interaction of IgG with FcγR, their interaction with complement and thus activation of the complement cascade constitutes another potential effector mechanism of the IgG1-AhFibA. Indeed, several complement fractions (including C1 which efficiently binds IgG1, C3, C5b-9) are expressed in the rheumatoid synovial tissue [48,49].
Finally, animal models support the hypothesis of the involvement of both effector functions (complement and FcγR) in RA. For example, in antigen-induced arthritis in mice, the degree of expression of the FcRγ chain, common to Fcγ-RI and -RIIIa, is related to arthritis severity [50]. In the K/B×N mouse model of arthritis, disease induction by autoantibodies to glucose 6-phosphate isomerase [51] requires both functional complement and FcγRIII pathways, recipient strains lacking either FcγRIII or C5 being resistant [52].
Concerning IgG4, the second most observed AhFibA subclass, their effector mechanisms are less understood. However, their role in RA is not excluded. Indeed, the pathogenic role of autoreactive IgG4 is suspected in several autoimmune diseases, particularly dermatological bullous diseases. IgG4 towards the major autoantigens of such diseases were reported to be associated to clinical features: to disease progression and severity in Pemphigus vulgaris[53,54], to progression from a preclinical to a clinical form in P. foliaceus[55], and to a long disease duration in bullous pemphigoid [56]. Finally, IgG1 in mice (the human IgG4 counterpart) may trigger immune effector functions via FcγRIII [42], supporting our hypothesis that a particular interaction between AhFibA subclasse(s) and particular type(s) of IgG receptors may be critical in the self-maintenance of inflammation.
In conclusion, the major AhFibA subclass is IgG1, IgG4 being the second most represented isotype. This result could contribute to the understanding of the pathophysiological role played by AhFibA via the immunological conflict with deiminated fibrin that takes place specifically in the rheumatoid synovium. Further unravelling of the involvement of specific AhFibA–FcγR interaction in RA is essential.
Acknowledgments
We thank Professor B. Fournié (Rheumatology Department, Hôpital Purpan, Toulouse), Professor B. Mazières and Professor A. Cantagrel (Rheumatology Department, Hôpital Rangueil, Toulouse), for providing patient data and sera. The skilful technical assistance of Josiane Granié and Valérie Sert is gratefully acknowledged. This work was supported by grants from the ‘Université Paul Sabatier-Toulouse III’, the ‘Institut National de la Santé et de la Recherche Médicale’ (INSERM), the ‘Centre National de la Recherche Scientifique’ (CNRS) and by a postdoctoral fellowship from the INSERM to Sabine Chapuy-Regaud.
References
- 1.Nienhuis RLFME. A new serum factor in patients with rheumatoid arthritis. Ann Rheum Dis. 1964;23:302–5. doi: 10.1136/ard.23.4.302. (with the technical assistance of C. Smids) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Young BJ, Mallya RK, Leslie RD, Clark CJ, Hamblin TJ. Anti-keratin antibodies in rheumatoid arthritis. Br Med J. 1979;2:97–9. doi: 10.1136/bmj.2.6182.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sebbag M, Simon M, Vincent C, et al. The antiperinuclear factor and the so-called antikeratin antibodies are the same rheumatoid arthritis-specific autoantibodies. J Clin Invest. 1995;95:2672–9. doi: 10.1172/JCI117969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schellekens GA, de Jong BA, van den Hoogen FH, van de Putte LB, van Venrooij WJ. Citrulline is an essential constituent of antigenic determinants recognized by rheumatoid arthritis-specific autoantibodies. J Clin Invest. 1998;101:273–81. doi: 10.1172/JCI1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Girbal-Neuhauser E, Durieux JJ, Arnaud M, et al. The epitopes targeted by the rheumatoid arthritis-associated antifilaggrin autoantibodies are posttranslationally generated on various sites of (pro)filaggrin by deimination of arginine residues. J Immunol. 1999;162:585–94. [PubMed] [Google Scholar]
- 6.Masson-Bessière C, Sebbag M, Durieux JJ, et al. In the rheumatoid pannus, anti-filaggrin autoantibodies are produced by local plasma cells and constitute a higher proportion of IgG than in synovial fluid and serum. Clin Exp Immunol. 2000;119:544–52. doi: 10.1046/j.1365-2249.2000.01171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Masson-Bessière C, Sebbag M, Girbal-Neuhauser E, et al. The major synovial targets of the rheumatoid arthritis-specific antifilaggrin autoantibodies are deiminated forms of the alpha- and beta-chains of fibrin. J Immunol. 2001;166:4177–84. doi: 10.4049/jimmunol.166.6.4177. [DOI] [PubMed] [Google Scholar]
- 8.Gomès-Daudrix V, Sebbag M, Girbal E, et al. Immunoblotting detection of so-called ‘antikeratin antibodies’: a new assay for the diagnosis of rheumatoid arthritis. Ann Rheum Dis. 1994;53:735–42. doi: 10.1136/ard.53.11.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vincent C, Simon M, Sebbag M, et al. Immunoblotting detection of autoantibodies to human epidermis filaggrin: a new diagnostic test for rheumatoid arthritis. J Rheumatol. 1998;25:838–46. [PubMed] [Google Scholar]
- 10.Palosuo T, Lukka M, Alenius H, et al. Purification of filaggrin from human epidermis and measurement of antifilaggrin autoantibodies in sera from patients with rheumatoid arthritis by an enzyme-linked immunosorbent assay. Int Arch Allergy Immunol. 1998;115:294–302. doi: 10.1159/000069460. [DOI] [PubMed] [Google Scholar]
- 11.Schellekens GA, Visser H, de Jong BA, et al. The diagnostic properties of rheumatoid arthritis antibodies recognizing a cyclic citrullinated peptide. Arthritis Rheum. 2000;43:155–63. doi: 10.1002/1529-0131(200001)43:1<155::AID-ANR20>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 12.Nogueira L, Sebbag M, Vincent C, et al. Performance of two ELISAs for antifilaggrin autoantibodies, using either affinity purified or deiminated recombinant human filaggrin, in the diagnosis of rheumatoid arthritis. Ann Rheum Dis. 2001;60:882–7. [PMC free article] [PubMed] [Google Scholar]
- 13.Vincent C, Nogueira L, Sebbag M, et al. Detection of antibodies to deiminated recombinant rat filaggrin by enzyme-linked immunosorbent assay: a highly effective test for the diagnosis of rheumatoid arthritis. Arthritis Rheum. 2002;46:2051–8. doi: 10.1002/art.10436. [DOI] [PubMed] [Google Scholar]
- 14.Union A, Meheus L, Humbel RL, et al. Identification of citrullinated rheumatoid arthritis-specific epitopes in natural filaggrin relevant for antifilaggrin autoantibody detection by line immunoassay. Arthritis Rheum. 2002;46:1185–95. doi: 10.1002/art.10229. [DOI] [PubMed] [Google Scholar]
- 15.Paimela L, Palosuo T, Aho K, et al. Association of autoantibodies to filaggrin with an active disease in early rheumatoid arthritis. Ann Rheum Dis. 2001;60:32–5. doi: 10.1136/ard.60.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nogueira L, Chapuy-Regaud S, Constantin A, et al. Autoantibodies to deiminated fibrinogen are the most efficient serological criterion for early rheumatoid arthritis diagnosis. Arthritis Res Ther. 2003;5:18. [Abstract] [Google Scholar]
- 17.van Gaalen FA, Linn-Rasker SP, van Venrooij WJ, et al. Autoantibodies to cyclic citrullinated peptides predict progression to rheumatoid arthritis in patients with undifferentiated arthritis: a prospective cohort study. Arthritis Rheum. 2004;50:709–15. doi: 10.1002/art.20044. [DOI] [PubMed] [Google Scholar]
- 18.Kurki P, Aho K, Palosuo T, Heliovaara M. Immunopathology of rheumatoid arthritis. Antikeratin antibodies precede the clinical disease. Arthritis Rheum. 1992;35:914–17. doi: 10.1002/art.1780350810. [DOI] [PubMed] [Google Scholar]
- 19.Berthelot JM, Maugars Y, Castagne A, Audrain M, Prost A. Antiperinuclear factors are present in polyarthritis before ACR criteria for rheumatoid arthritis are fulfilled. Ann Rheum Dis. 1997;56:123–5. doi: 10.1136/ard.56.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nielen MM, van Schaardenburg D, Reesink HW, et al. Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors. Arthritis Rheum. 2004;50:380–6. doi: 10.1002/art.20018. [DOI] [PubMed] [Google Scholar]
- 21.Kroot EJ, de Jong BA, van Leeuwen MA, et al. The prognostic value of anti-cyclic citrullinated peptide antibody in patients with recent-onset rheumatoid arthritis. Arthritis Rheum. 2000;43:1831–5. doi: 10.1002/1529-0131(200008)43:8<1831::AID-ANR19>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 22.Forslin K, Vincent C, Serre G, Svensson B. Antifilaggrin antibodies in early rheumatoid arthritis may predict radiological progression. Scand J Rheumatol. 2001;30:221–4. doi: 10.1080/030097401316909567. [DOI] [PubMed] [Google Scholar]
- 23.Vencovsky J, Machacek S, Sedova L, et al. Autoantibodies can be prognostic markers of an erosive disease in early rheumatoid arthritis. Ann Rheum Dis. 2003;62:427–30. doi: 10.1136/ard.62.5.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Rycke L, Peene I, Hoffman IEA, et al. Rheumatoid factor and anti-citrullinated protein antibodies in rheumatoid arthritis: diagnostic value, associations with radiological progression rate, and extra-articular manifestations. Ann Rheum Dis. 2004;63:1587–93. doi: 10.1136/ard.2003.017574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stavnezer J. Immunoglobulin class switching. Curr Opin Immunol. 1996;8:199–205. doi: 10.1016/s0952-7915(96)80058-6. [DOI] [PubMed] [Google Scholar]
- 26.Salmon JE, Pricop L. Human receptors for immunoglobulin G: key elements in the pathogenesis of rheumatic disease. Arthritis Rheum. 2001;44:739–50. doi: 10.1002/1529-0131(200104)44:4<739::AID-ANR129>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 27.Linton SM, Morgan BP. Complement activation and inhibition in experimental models of arthritis. Mol Immunol. 1999;36:905–14. doi: 10.1016/s0161-5890(99)00113-3. [DOI] [PubMed] [Google Scholar]
- 28.Nogueira L, Sebbag M, Chapuy-Regaud S, et al. Autoantibodies to deiminated fibrinogen are the most efficient serological criterion for the diagnosis of rheumatoid arthritis. Arthritis Res. 2002;4:A30. [Abstract] [Google Scholar]
- 29.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 30.Jefferis R, Reimer CB, Skvaril F, et al. Evaluation of monoclonal antibodies having specificity for human IgG sub-classes: results of an IUIS/WHO collaborative study. Immunol Lett. 1985;10:223–52. doi: 10.1016/0165-2478(85)90082-3. [DOI] [PubMed] [Google Scholar]
- 31.Vincent C, Serre G, Basile JP, et al. Subclass distribution of IgG antibodies to the rat oesophagus stratum corneum (so-called anti-keratin antibodies) in rheumatoid arthritis. Clin Exp Immunol. 1990;81:83–9. doi: 10.1111/j.1365-2249.1990.tb05295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kiely PD, Helbert MR, Miles J, Oliveira DB. Immunosuppressant effect of gold on IgG subclasses and IgE; evidence for sparing of Th2 responses. Clin Exp Immunol. 2000;120:369–74. doi: 10.1046/j.1365-2249.2000.01207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cohen PL, Cheek RL, Hadler JA, Yount WJ, Eisenberg RA. The subclass distribution of human IgG rheumatoid factor. J Immunol. 1987;139:1466–71. [PubMed] [Google Scholar]
- 34.Kusaba M, Honda J, Fukuda T, Oizumi K. Analysis of type 1 and type 2 T cells in synovial fluid and peripheral blood of patients with rheumatoid arthritis. J Rheumatol. 1998;25:1466–71. [PubMed] [Google Scholar]
- 35.Isomaki P, Luukkainen R, Lassila O, Toivanen P, Punnonen J. Synovial fluid T cells from patients with rheumatoid arthritis are refractory to the T helper type 2 differentiation-inducing effects of interleukin-4. Immunology. 1999;96:358–64. doi: 10.1046/j.1365-2567.1999.00712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Canete JD, Martinez SE, Farres J, et al. Differential Th1/Th2 cytokine patterns in chronic arthritis: interferon gamma is highly expressed in synovium of rheumatoid arthritis compared with seronegative spondyloarthropathies. Ann Rheum Dis. 2000;59:263–8. doi: 10.1136/ard.59.4.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van der Graaff WL, Prins AP, Niers TM, Dijkmans BA, van Lier RA. Quantitation of interferon gamma- and interleukin-4-producing T cells in synovial fluid and peripheral blood of arthritis patients. Rheumatology (Oxford) 1999;38:214–20. doi: 10.1093/rheumatology/38.3.214. [DOI] [PubMed] [Google Scholar]
- 38.Gerli R, Bistoni O, Russano A, et al. In vivo activated T cells in rheumatoid synovitis. Analysis of Th1- and Th2-type cytokine production at clonal level in different stages of disease. Clin Exp Immunol. 2002;129:549–55. doi: 10.1046/j.1365-2249.2002.01913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Constantin A, Loubet-Lescoulie P, Lambert N, et al. Antiinflammatory and immunoregulatory action of methotrexate in the treatment of rheumatoid arthritis: evidence of increased interleukin-4 and interleukin-10 gene expression demonstrated in vitro by competitive reverse transcriptase-polymerase chain reaction. Arthritis Rheum. 1998;41:48–57. doi: 10.1002/1529-0131(199801)41:1<48::AID-ART7>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 40.Crisp AJ, Chapman CM, Kirkham SE, Schiller AL, Krane SM. Articular mastocytosis in rheumatoid arthritis. Arthritis Rheum. 1984;27:845–51. doi: 10.1002/art.1780270802. [DOI] [PubMed] [Google Scholar]
- 41.Karulin AY, Hesse MD, Yip HC, Lehmann PV. Indirect IL-4 pathway in type 1 immunity. J Immunol. 2002;168:545–53. doi: 10.4049/jimmunol.168.2.545. [DOI] [PubMed] [Google Scholar]
- 42.Yuan R, Clynes R, Oh J, Ravetch JV, Scharff MD. Antibody-mediated modulation of Cryptococcus neoformans infection is dependent on distinct Fc receptor functions and IgG subclasses. J Exp Med. 1998;187:641–8. doi: 10.1084/jem.187.4.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yuan RR, Spira G, Oh J, Paizi M, Casadevall A, Scharff MD. Isotype switching increases efficacy of antibody protection against Cryptococcus neoformans infection in mice. Infect Immun. 1998;66:1057–62. doi: 10.1128/iai.66.3.1057-1062.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koene HR, Kleijer M, Algra J, von Roos D, dem Borne AE, de Haas M. Fc gammaRIIIa-158V/F polymorphism influences the binding of IgG by natural killer cell Fc gammaRIIIa, independently of the Fc gammaRIIIa-48L/R/H phenotype. Blood. 1997;90:1109–14. [PubMed] [Google Scholar]
- 45.Morgan AW, Keyte VH, Babbage SJ, et al. FcgammaRIIIA-158V and rheumatoid arthritis: a confirmation study. Rheumatology (Oxford) 2003;42:528–33. doi: 10.1093/rheumatology/keg169. [DOI] [PubMed] [Google Scholar]
- 46.Radstake TR, Petit E, Pierlot C, van de Putte LB, Cornelis F, Barrera P. Role of Fcgamma receptors IIA IIIA, and IIIB in susceptibility to rheumatoid arthritis. J Rheumatol. 2003;30:926–33. [PubMed] [Google Scholar]
- 47.Dijstelbloem HM, van de Winkel JG, Kallenberg CG. Inflammation in autoimmunity: receptors for IgG revisited. Trends Immunol. 2001;22:510–16. doi: 10.1016/s1471-4906(01)02014-2. [DOI] [PubMed] [Google Scholar]
- 48.Gulati P, Guc D, Lemercier C, Lappin D, Whaley K. Expression of the components and regulatory proteins of the classical pathway of complement in normal and diseased synovium. Rheumatol Int. 1994;14:13–19. doi: 10.1007/BF00302666. [DOI] [PubMed] [Google Scholar]
- 49.Neumann E, Barnum SR, Tarner IH, et al. Local production of complement proteins in rheumatoid arthritis synovium. Arthritis Rheum. 2002;46:934–45. doi: 10.1002/art.10183. [DOI] [PubMed] [Google Scholar]
- 50.van Lent PL, van Vuuren AJ, Blom AB, et al. Role of Fc receptor gamma chain in inflammation and cartilage damage during experimental antigen-induced arthritis. Arthritis Rheum. 2000;43:740–52. doi: 10.1002/1529-0131(200004)43:4<740::AID-ANR4>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 51.Korganow AS, Ji H, Mangialaio S, et al. From systemic T cell self-reactivity to organ-specific autoimmune disease via immunoglobulins. Immunity. 1999;10:451–61. doi: 10.1016/s1074-7613(00)80045-x. [DOI] [PubMed] [Google Scholar]
- 52.Ji H, Ohmura K, Mahmood U, et al. Arthritis critically dependent on innate immune system players. Immunity. 2002;16:157–68. doi: 10.1016/s1074-7613(02)00275-3. [DOI] [PubMed] [Google Scholar]
- 53.Bhol K, Natarajan K, Nagarwalla N, Mohimen A, Aoki V, Ahmed AR. Correlation of peptide specificity and IgG subclass with pathogenic and nonpathogenic autoantibodies in pemphigus vulgaris: a model for autoimmunity. Proc Natl Acad Sci USA. 1995;92:5239–43. doi: 10.1073/pnas.92.11.5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kricheli D, David M, Frusic-Zlotkin M, et al. The distribution of Pemphigus vulgaris-IgG subclasses and their reactivity with desmoglein 3 and 1 in pemphigus patients and their first-degree relatives. Br J Dermatol. 2000;143:337–42. doi: 10.1046/j.1365-2133.2000.03659.x. [DOI] [PubMed] [Google Scholar]
- 55.Warren SJ, Arteaga LA, Rivitti EA, et al. The role of subclass switching in the pathogenesis of endemic Pemphigus foliaceus. J Invest Dermatol. 2003;120:104–8. doi: 10.1046/j.1523-1747.2003.12017.x. [DOI] [PubMed] [Google Scholar]
- 56.Laffitte E, Skaria M, Jaunin F, et al. Autoantibodies to the extracellular and intracellular domain of bullous pemphigoid 180, the putative key autoantigen in bullous pemphigoid, belong predominantly to the IgG1 and IgG4 subclasses. Br J Dermatol. 2001;144:760–8. doi: 10.1046/j.1365-2133.2001.04130.x. [DOI] [PubMed] [Google Scholar]